Abstract
Oceanic uptake of anthropogenic carbon dioxide results in decrease in seawater pH and increase in temperature. In this study, we demonstrated the synergistic effects of elevated seawater temperature and declined seawater pH on gene expression patterns of aspein, calmodulin, nacrein, she-7-F10 and hsp70 in the pearl oyster Pinctada fucata. Under ‘business-as-usual’ scenarios, four treatments were examined: (1) ambient pH (8.10) and ambient temperature (27°C) (control condition), (2) ambient pH and elevated temperature (+3°C), (3) declined pH (7.70) and ambient temperature, (4) declined pH and elevated temperature. The results showed that under warming and acidic seawater conditions, expression of aspein and calmodulin showed no significant differences among different time point in condition 8.10 T. But the levels of aspein and calmodulin in conditions 8.10 T+3, 7.70 T and 7.70 T+3, and levels of nacrein, she-7-F10 in all the four treatments changed significantly. Low pH and pH×temperature interaction influenced the expression of aspein and calmodulin significantly after hours 48 and 96. Significant effects of low pH and pH×temperature interaction on the expression of nacrein were observed at hour 96. The expression level of she-7-F10 was affected significantly by pH after hours 48 and 96. The expression of hsp70 was significantly affected by temperature, pH, temperature×pH interaction at hour 6, and by temperature×pH interaction at hour 24. This study suggested that declined pH and pH×temperature interaction induced down regulation of calcification related genes, and the interaction between declined seawater pH and elevated temperature caused up regulation of hsp70 in P. facata. These results demonstrate that the declined seawater pH and elevated temperature will impact the physiological process, and potentially the adaptability of P. fucata to future warming and acidified ocean.
Introduction
Increasing concentrations of CO2 in the atmosphere are causing the ocean to become warmer and acidify [1]. Global surface temperatures rose by 0.76°C and global seawater pH decreased by 0.1 unit due to increasing CO2 emissions since the industrial revolution [2]. Under ‘business as usual’ scenarios, the ocean is predicted to increase in sea surface temperature by 1–4°C and decrease in ocean pH by 0.3–0.4 units by the year 2100 [3], [4]. Ocean absorbing of emitted CO2 lead to profound changes in the seawater carbonate chemistry with decrease in calcite, aragonite saturation state and seawater carbonate ions. These changes have been identified as a great threat to marine organisms, particularly to calcifying organisms [5], [6].
Effects of ocean acidification on calcification of marine organisms have been a focus in recent studies [7]. Decreasing in seawater pH has negative effects on calcification rate of organisms like coral Stylophora pistillata [8], echinoderm Amphiura filiformis [9] and molluscs Limacina helicina and Crassostrea gigas [10], [11]. However, other studies reported that calcification rate showed no significant change in Mytilus edulis, and increased in Littorina littorea and Sepia officinalis when exposed to low seawater pH [12], [13]. Hence, marine organisms' response to carbonate system variations is diverse.
It is predicted that the oceans will warm and acidify simultaneously. Therefore, studies that include both decreased seawater pH and increased temperature will provide a more realistic assessment of marine organism's responses to future environmental change, than studies limited to a single factor [14]. In studies examining the synergistic impacts of declined seawater pH and elevated temperature on marine organisms, Reynaud et al. [15] found no reduction in calcification in the coral S. pistallata when reared at reduced seawater pH but a 50% reduction in calcification when reared at declined seawater pH and elevated temperature. Metzger et al. [16] showed that the crab Cancer pagurus was more sensitive to increased temperature under low pH conditions. Byrne et al. [17] demonstrated that exposure of the abalone Haliotis coccoradiata and sea urchin Heliocidaris erythrogramma to warming (+2°C to 4°C) and acidification (pH 7.6–7.8) resulted in unshelled larvae and abnormal juveniles. Martin et al. [18] reported that the death of algae Lithophyllum cabiochae was observed only under elevated temperature and was two- to threefold higher under elevated pCO2. They also found that net calcification of L. cabiochae decreased by 50% when both temperature and pCO2 were elevated while no effect was found under elevated temperature and lower pH alone.
To date, studies into the consequences of interactive effects of warming and acidification on animals has been limited to early development or calcification. But more research is needed to understand the core physiological mechanisms behind calcification to assess better the sensitivity of these organisms to ocean warming and acidification. Organisms regulate a number of cellular processes during stressful events, and the regulation and modulation of gene expression can be one of the most rapid and sensitive responses to environmental stress [19], [20]. Assessing the expression pattern of genes responsible for calcification under ocean warming and acidify conditions may be a way to assess the potential impacts of climate change on marine organisms.
Pressure to reduce CO2 emissions in response to the threat of climate change has led to governments seeking new options for carbon capture and storage (CCS). Several approaches have been suggested to mitigate the future atmospheric CO2 concentration. The most important are ocean sequestration of carbon dioxide [21]. Ocean sequestration may directly affect marine life in the selected dumping sites. Immersion in CO2-laden, acidic seawater from CO2 injection poses physiological challenges to marine animals that respond by tolerance, compensation, or death. Little is known about molecular mechanism of marine organisms to tolerate seawater acidification [22].
The pearl oyster Pinctada fucata is distributed along the southern coasts of China and Japan. It is a species of economic importance for pearl production, approximately a quarter of pearl production (about US$160 million) is from the cultured P. fucata [23]. It also plays an important role in the food web as well as in the cycling of carbon and calcium carbonate (CaCO3) [24]. Pearl formation and shell growth is a highly controlled biomineralization process [25], [26]. Shell matrix proteins such as Aspein, Nacrein, Calmodulin and She-7-F10 are believed to play a key role in the CaCO3 crystal polymorphism (calcite and aragonite) and the microstructures of pearl and shell layers [27]. The aspein, encoding an unusually acidic protein, might be a key factor for the formation of the calcitic prismatic layer of P. fucata [28]. Calmodulin is a ubiquitous intracellular mediator of calcium signaling and some recent studies showed that calmodulin play an important role in the regulation of the uptake and transport of calcium in biomineralization [29]. Nacrein was believed to mediate both HCO3 − and Ca2+ concentrations, and thus is deeply involved in CaCO3 crystallization [30]. Nacrein has been used as a marker to evaluate cellular metabolism during biomineralization. Its production increases with increasing calcium concentration and means that a higher biomineralization rate is occurring [31]. She-7-F10 shared high levels of identity with shell matrix structural proteins and was thought to be involved in the process of shell biomineralization [32]. Hsp70 comprise a group of highly conserved proteins that have general protective function in all living organisms [33]. It has been reported that P. fucata has the ability to express Hsp70 in response to stressful stimuli [34].
This study investigates the synergistic effects of seawater warming and declined pH on gene expression patterns of calmodulin, nacrein, aspein, she-7-F10 and hsp70 in P. fucata to provide the first hand molecular evidence to evaluate the mechanisms for marine mollusc to response to elevated pCO2 seawater and temperature.
Results
Total alkalinity (TA) and salinity showed no clear change between treatments throughout the experiment. However, pH, pCO2, saturation states for aragonite and calcite differed clearly between different pH levels (pH 8.10 vs. 7.70) throughout the experiment (Table 1). The natural temperature in Daya Bay ranged from 26.5 to 27.5 during the experiment. There showed no tank effects among different tanks in each treatment (p>0.1).
Table 1. Parameters of the carbonate system in each treatment.
| 8.10 T | T | Sal | pH | TA | pCO2 | DIC | Ωara | Ωcal |
| 0 h | 27±0.1 | 33.36±0.31 | 8.13±0.02 | 2187.04±36.04 | 421.51±22.42 | 1912.22±20.17 | 3.14±0.20 | 4.75±0.21 |
| 48 h | 27±0.2 | 33.71±0.12 | 8.14±0.01 | 2189.36±41.29 | 422.16±34.81 | 1914.75±18.37 | 3.15±0.09 | 4.77±0.16 |
| 96 h | 27±0.1 | 32.88±0.24 | 8.14±0.02 | 2183.68±37.92 | 421.24±29.17 | 1915.63±30.13 | 3.15±0.13 | 4.76±0.19 |
| 7.70 T | ||||||||
| 0 h | 27±0.2 | 32.87±0.45 | 7.70±0.01 | 2184.11±43.17 | 1424.40±37.31 | 2119.23±24.25 | 1.27±0.07 | 1.92±0.12 |
| 48 h | 27±0.3 | 33.12±0.17 | 7.69±0.01 | 2185.73±29.88 | 1425.48±25.72 | 2116.44±23.56 | 1.27±0.09 | 1.92±0.17 |
| 96 h | 27±0.1 | 33.26±0.15 | 7.70±0.03 | 2187.39±37.27 | 1462.75±34.19 | 2120.57±28.93 | 1.24±0.12 | 1.88±0.11 |
| 8.10 T+3 | ||||||||
| 0 h | 30±0.1 | 33.28±0.22 | 8.14±0.02 | 2182.76±36.55 | 427.93±31.55 | 1868.31±19.08 | 3.43±0.14 | 5.16±0.11 |
| 48 h | 30±0.2 | 33.31±0.40 | 8.14±0.01 | 2180.93±23.61 | 439.89±27.18 | 1864.52±26.17 | 3.37±0.13 | 5.04±0.13 |
| 96 h | 30±0.1 | 32.68±0.51 | 8.13±0.03 | 2187.26±29.43 | 428.86±23.79 | 1870.24±23.72 | 3.44±0.15 | 5.15±0.22 |
| 7.70 T+3 | ||||||||
| 0 h | 30±0.1 | 33.30±0.39 | 7.70±0.01 | 2189.56±25.16 | 1459.84±32.63 | 2106.77±34.15 | 1.39±0.09 | 2.09±0.14 |
| 48 h | 30±0.2 | 33.21±0.46 | 7.71±0.01 | 2184.11±33.47 | 1419.84±26.48 | 2107.62±27.82 | 1.42±0.12 | 2.12±0.13 |
| 96 h | 30±0.2 | 32.46±0.37 | 7.68±0.02 | 2187.68±21.59 | 1422.21±33.69 | 2107.80±21.37 | 1.42±0.17 | 2.13±0.11 |
T: temperature (°C), Sal: salinity, TA: total alkalinity (µmol/kg), pCO2: CO2 partial pressure (μatm),
DIC: dissolved inorganic carbon (µmol/kg), Ωara: aragonite saturation state, Ωcal: calcite saturation state.
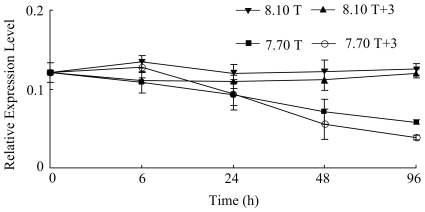
The relative expression level of aspein fluctuated gradually during hours 0–96 in condition 8.10 T and showed no significant difference among different time point (p>0.1). In conditions 8.10 T+3, 7.70 T and 7.70 T+3, the levels decreased significantly to minimum values on hour 96 (p<0.1) (Fig. 1, Table 2). The expression of aspein was significantly affected by pH and pH×temperature interaction at hours 48 and 96 (p<0.1) (Table 3). The expression was significantly lower in condition 7.70 T and 7.70 T+3 than in condition 8.10 T and 8.10 T+3 at hours 48 and 96 (Fig. 1).
Figure 1. Real-time PCR analysis of expression of aspein in response to elevated temperature and declined pH.
Table 2. Results of statistical tests performed to test the differences of aspein, calmodulin, nacrein, she-7-F10 and hsp70 in Pinctada fucata among different time point.
| Gene | Group | 0 h | 6 h | 24 h | 48 h | 96 h |
| aspein | 8.10 T | 96.04±8.14a | 97.1±10.08a | 100.65±10.32a | 97.93±7.34a | 100.73±5.47a |
| 8.10 T+3 | 96.04±8.14a | 104.40±8.52a | 91.90±7.50ab | 90.51±3.65ab | 86.73±2.42b | |
| 7.70 T | 96.04±8.14a | 101.30±12.02a | 94.90±6.06a | 79.55±4.36b | 70.48±2.58c | |
| 7.70 T+3 | 96.04±8.14a | 91.65±6.59a | 86.40±9.96b | 73.71±3.09c | 60.71±3.78d | |
| calmodulin | 8.10 T | 0.12±0.01a | 0.13±0.01a | 0.12±0.01a | 0.12±0.02a | 0.13±0.01a |
| 8.10 T+3 | 0.12±0.01a | 0.11±0.01a | 0.11±0.01a | 0.11±0.01a | 0.12±0.01a | |
| 7.70 T | 0.12±0.01a | 0.11±0.01a | 0.09±0.01b | 0.07±0.01c | 0.06±0.01d | |
| 7.70 T+3 | 0.12±0.01a | 0.13±0.01a | 0.09±0.01b | 0.06±0.01c | 0.04±0.01d | |
| nacrein | 8.10 T | 6.02±0.50a | 5.78±0.22b | 6.26±0.72c | 5.88±0.49b | 5.85±0.37b |
| 8.10 T+3 | 6.02±0.50a | 6.23±0.28b | 5.64±0.12c | 4.88±0.26d | 4.66±0.30e | |
| 7.70 T | 6.02±0.50a | 5.94±0.42a | 5.20±0.31b | 4.19±0.38c | 2.48±0.22d | |
| 7.70 T+3 | 6.02±0.50a | 5.55±0.52b | 4.84±0.37c | 3.89±0.37d | 1.96±0.11e | |
| she-7-10 | 8.10 T | 2.61±0.24a | 2.69±0.20a | 2.29±0.27b | 2.31±0.12b | 2.46±0.07c |
| 8.10 T+3 | 2.61±0.24a | 2.53±0.21a | 2.47±0.10a | 2.00±0.16b | 1.98±0.11b | |
| 7.70 T | 2.61±0.24a | 2.21±0.15b | 2.10±0.24b | 1.67±0.12c | 0.79±0.05d | |
| 7.70 T+3 | 2.61±0.24a | 2.02±0.17b | 1.81±0.18c | 1.81±0.07c | 0.21±0.01d | |
| hsp70 | 8.10 T | 1.30±0.16a | 2.25±0.14b | 2.26±0.17b | 1.79±0.08c | 1.90±0.11d |
| 8.10 T+3 | 1.30±0.16a | 2.99±0.31b | 2.89±0.26b | 2.20±0.13c | 1.42±0.10a | |
| 7.70 T | 1.30±0.16a | 3.80±0.33b | 3.34±0.27c | 2.37±0.23d | 1.22±0.09a | |
| 7.70 T+3 | 1.30±0.16a | 4.30±0.37b | 4.20±0.18c | 2.77±0.19d | 1.03±0.08a |
Means not sharing the same superscript in each line are significantly different (p<0.1).
Table 3. Results of statistical tests performed to test the effects of pH and temperature on gene expression patterns of aspein, calmodulin, nacrein, she-7-F10 and hsp70 in Pinctada fucata.
| 6 h | 24 h | 48 h | 96 h | ||||||||||
| Dunn-Sidak test | Dunn-Sidak test | Dunn-Sidak test | Dunn-Sidak test | ||||||||||
| Source of variation | df | F | P | (1) (2) (3) (4) | F | P | (1) (2) (3) (4) | F | P | (1) (2) (3) (4) | F | P | (1) (2) (3) (4) |
| aspein | |||||||||||||
| pH | 1 | 1.123 | 0.320 | 5.524 | 0.336 | 7.017 | 0.029* | a a b b | 1.047 | 0.047* | a a b b | ||
| Tem | 1 | 2.072 | 0.188 | 2.842 | 0.181 | 4.822 | 0.112 | 3.151 | 0.114 | ||||
| Tem*pH | 1 | 2.130 | 0.183 | 7.820 | 0.111 | 6.779 | 0.051* | a a b b | 3.208 | 0.053* | a a b b | ||
| calmodulin | |||||||||||||
| pH | 1 | 5.230 | 0.148 | 0.953 | 0.358 | 3.027 | 0.013* | a a b b | 0.011 | 0 .001* | a a b b | ||
| Tem | 1 | 5.518 | 0.151 | 58.026 | 0.120 | 10.046 | 0.120 | 25.980 | 0.918 | ||||
| Tem*pH | 1 | 0.419 | 0.536 | 10.460 | 0.260 | 3.648 | 0.093* | a a b b | 1.467 | 0.062* | a a b b | ||
| nacrein | |||||||||||||
| pH | 1 | 2.904 | 0.127 | 0.274 | 0.615 | 2.321 | 0.166 | 0.056 | 0.024* | a a b b | |||
| Tem | 1 | 0.577 | 0.469 | 46.744 | 0.973 | 2.691 | 0.140 | 22.591 | 0.819 | ||||
| Tem*pH | 1 | 0.978 | 0.352 | 2.330 | 0.209 | 0.234 | 0.641 | 1.864 | 0.052* | a a b b | |||
| she-7-F10 | |||||||||||||
| pH | 1 | 0.022 | 0.885 | 0.063 | 0.808 | 11.986 | 0.079* | a a b b | 3.730 | 0.010* | a a b b | ||
| Tem | 1 | 0.179 | 0.684 | 0.533 | 0.486 | 1.300 | 0.287 | 11.293 | 0.134 | ||||
| Tem*pH | 1 | 0.254 | 0.628 | 0.488 | 0.505 | 0.758 | 0.409 | 0.268 | 0.618 | ||||
| hsp70 | |||||||||||||
| pH | 1 | 3.765 | 0.088* | a a b b | 10.662 | 0.910 | 1.570 | 0.246 | 1.887 | 0.207 | |||
| Tem | 1 | 1.351 | 0.058* | a b a b | 2.927 | 0.216 | 3.450 | 0.100 | 8.740 | 0.179 | |||
| Tem*pH | 1 | 0.881 | 0.029* | a b b b | 3.227 | 0.011* | a b b b | 7.030 | 0.375 | 1.975 | 0.198 |
Two-way repeated measures analysis of variance (ANOVA) significant at the p<0.1 level is indicated by asterisks. Results of multiple (Dunn-Sidak test) comparisons are given.
Means not sharing the same superscript are significantly different (p<0.1). (1) 8.10 T, (2) 8.10 T+3, (3) 7.70 T, (4) 7.70 T+3.
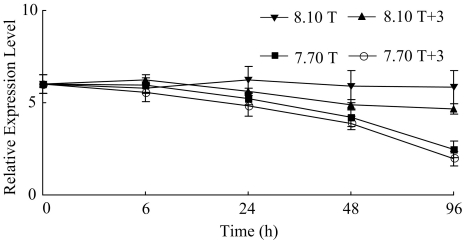
The level of calmodulin did not change markedly during hours 0–96 in 8.10 T and 8.10 T+3 (p>0.1). In conditions 7.70 T+3 and 7.70 T, the level decreased significantly during hours 24–96 (p<0.1) (Table 2). The expression of calmodulin was affected significantly by pH and pH×temperature interaction at hours 48 and 96 (p<0.1) (Fig. 2). Post hoc analysis indicated that the expression of calmodulin showed significantly difference between condition 8.10 T, 8.10 T+3 and 7.70 T+3, 7.70 T (Table 3).
Figure 2. Real-time PCR analysis of expression of calmodulin in response to elevated temperature and declined pH.
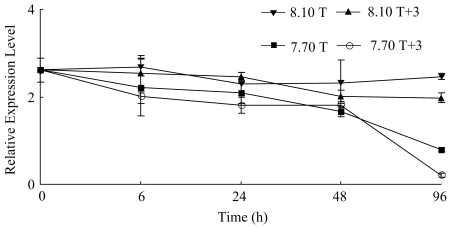
The expression level of nacrein at hours 24, 48 and 96 showed significantly difference from the level of time 0 in all the four treatments (p<0.1) (Table 2). ANOVA demonstrated a significant effect of pH and pH×temperature interaction on the expression of the nacrein gene on hour 96 (p<0.1) (Fig. 3). Significant difference was observed between condition 8.10 T, 8.10 T+3 and 7.70 T+3, 7.70 T (Table 3). The variations in the expression level of she-7-F10 in the four treatments during the experiment were similar to those of nacrein (p<0.1) (Table 2). The expression level of she-7-F10 was affected significantly by pH at hours 48 and 96 (p<0.1) (Fig. 4). The levels of expression were significantly different between conditions 8.10 T, 8.10 T+3 and conditions 7.70 T, 7.70 T+3 (Table 3).
Figure 3. Real-time PCR analysis of expression of nacrein in response to elevated temperature and declined pH.
Figure 4. Real-time PCR analysis of expression of she-7-F10 in response to elevated temperature and declined pH.
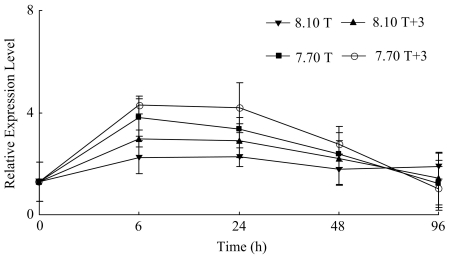
The expression level of hsp70 increased significantly to a higher level on hours 6 and 24 in condition 8.10 T, 8.10 T+3, 7.70 T and 7.70 T+3, and then decreased markedly (p<0.1) (Table 2). The expression of hsp70 was significantly affected by temperature, pH, temperature×pH interaction on hour 6, and by temperature×pH interaction on hour 24 (p<0.1) (Fig. 5, Table 3).
Figure 5. Real-time PCR analysis of expression of hsp70 in response to elevated temperature and declined pH.
Discussion
In the present study, environmental parameters that could affect the comparison between treatments were monitored. The gentle bubbling protocol maintained the seawater carbonate buffer system in a relatively stable condition in each treatment throughout the experiment. The seawater was saturated with respect to aragonite and calcite in each treatment. This demonstrates that the high seawater renewal rate used, successfully prevented any “aquarium” effect. We are, therefore, confident that P. fucata responses were only caused by pH and temperature treatment.
This study examined expression changes in genes of aspein, calmodulin, nacrein and she-7-F10 in P. fucata associated with calcification under low pH and high temperature conditions. Shell and pearl formation of P. fucata needs a large amount of calcium and biomineralization implies the transport and control of calcium ions [35]. The present study showed that pH was the driving factor influencing levels of mRNA transcript for aspein, calmodulin and nacrein, whereas temperature aggravated the sensitivity of P. fucata to pH. The expression level of she-7-F10 was affected significantly by pH only, suggesting that different genes respond differently to elevated temperature and declined pH. The down regulation of aspein, calmodulin, nacrein and she-7-F10 was in accordance with Todgham and Hofmann [36], who found down regulation of calcification genes when sea urchin larvae of early prism stage were exposed to pCO2 values of 543 μatm. In contrast to these results, CO2 induced seawater acidification down to pH of 7.5 and 7.25 caused a compensatory increase in transcript levels of a range of calcification genes (msp130, SM30) in 3 day old Paracentrotus lividus larvae [37]. Zippay and Hofmann [38] showed that decreased pH did not affect the expression pattern of two shell formation genes at any of the abalone larval stages. The differences in results suggested that given no differences in methodology that responses may be due to adaptive capacity of a species to changing climatic conditions which differed between populations with a large geographic distribution.
The decrease in expression level of aspein, calmodulin, nacrein and she-7-F10 may be linked to the decrease in calcification rate. The high sensitivity of these genes to declined pH was consistent with the general consensus on the negative relationship between pCO2 and calcification of marine bivalves [39]. If the elevated temperature and declined pH are maintained for longer, this decrease could trigger a cascading effect on shell growth and pearl formation of P. fucata. Considering the down regulation of calcification related genes observed in this study, it is possible that disturbances in the balance between the coordinated production of mineral and organic matrix could affect the composition and mechanical properties of the shell and pearl. This will further increase the ratio of low-quality pearls. When investigating the microstructure of larval spicules, Clark et al [40]. noted eroded surface structures in two out of four sea urchin species at CO2 acidified seawater of pH 7.70.
Living systems have evolved a variety of strategies to respond to external or internal environmental challenges. While these responses are often behavioral or metabolic, a powerful mechanism widely employed to maintain cellular homeostasis under stress is the adjustment of gene expression [36]. Bivalves will be affected by ocean warming and acidification predicted under climate change scenarios, and only those endowed with sufficient defense mechanisms will be able to survive. Expression of hsp70 is frequently used as a component of physiological mechanisms through which bivalves cope with environmental challenges [41]. The present study showed that in the warming and acidified seawater conditions, activation of the heat shock response occurs in P. fucata. It was worth noting that combined exposure to elevated temperature and declined pH resulted in up regulation of hsp70, suggesting that elevated pCO2 aggravated the sensitivity of P. fucata to temperature. The interactive effects of temperature and pCO2 had already been reported in other studies. Anthony et al. reported higher reductions in algal calcification rate at high temperature (28–29°C; −190%) than at low temperature (25–26°C; −130%) under elevated pCO2 (1000–1400 ppm) relative to control conditions [42]. Reynaud et al. [15] reported that elevated pCO2 had no effects on coral calcification rates at 25°C, but caused a significant calcification decrease at 28°C. The up regulation of hsp70 seemed to be consistent with the suggestion of O'Donnell et al. [43] that the gradual accumulation of hsp70 might acted as a buffer against subsequent heat stress and support increased stress protection in gradually warming and acidified environments. The heat shock response was an energy consuming process. A shift to anaerobiosis as a result of thermally induced hypoxia in marine bivalves will caused metabolic depression and consequently a reduction in ATP turnover. If this was the case, there might be an effect on the expression of hsp70. Liu et al. [44] found that the clearance, respiration, and excretion rates of P. fucata decreased under low pH conditions. Thus, decrease in the expression of hsp70 on hours 48 and 96 in the present study, may be related to decrease in the energy budget to meet the energy demand for hsp70 synthesis. However, the response of hsp70 to low pH and high temperature over long-term scales is still unknown and need further studies.
This study examined the short-term acute responses of P. fucata to seawater acidification. This leaded to some limitations for the results of the experiment. In the context of the results of this study, it is likely that this physiological stress was a result of the acute shock of transfer to treatment pH and temperature levels, and not evidence of physiological stress caused by long-term seawater acidification. These results provide fundamental information for the response of marine organism to CCS. However, further studies are needed to evaluate responses to elevated pCO2 and seawater temperature of marine organisms over longer time-scales.
In summary, our study showed that acidified and warming seawater resulted in a significant down regulation of calcification related genes, and the interaction between declined pH and elevated temperature caused up regulation of hsp70 in P. fucata. The potential economic implications of this study for aquaculture industries include reduced growth rate of the pearl oyster and reduction in yield of the pearl. Our approach provides molecular elements on the response of P. fucata to CCS and projected climate change.
Materials and Methods
Seawater acidification and experimental design
One declined pH level (7.70), one ambient pH level (current level of 8.10), one elevated temperature (+3°C) and one ambient temperature (27°C) were selected for the study, based on projections by IPCC [2], [3] for the year 2100. Three replicates were set up for each temperature×pH treatment. Seawater was collected from Daya Bay Station, Chinese Academy of Sciences, on the southern coast of China (23°31′–24°50′N and 113°29′–114°49′E, average natural seawater pH 8.10±0.03, salinity 33±0.5‰). Water temperatures in elevated temperature treatment were maintained to 30±0.5°C using external chillers or unmanipulated for the ambient control. Temperatures were measured three times daily with a mercury thermometer. The pH in the acid seawater group (pH 7.70) was regulated by bubbling CO2 gas into the seawater until the desired pH was reached. The pH of each level in the experimental chamber was checked three times daily before and after water exchange to ensure stability throughout the experiment using a pH meter (PHS-3E Rex Instrument) calibrated with NBS standard buffers. With this high frequency of pH checks, we were able to sustain the targeted pH values with only a small range of variation over the course of the study (8.10±0.05 and 7.70±0.05). TA in each experimental tank was determined by potentiometric titration [45], [46] at hours 0, 48 and 96 before water exchange. The saturation states for aragonite, calcite and pCO2 values were determined from TA, pHNBS and salinity data using CO2SYS [47] with the constants supplied by Mehrbach et al. [48] refitted by Dickson and Millero [49] and the KSO4 dissociation constant from Dickson [50].
Animal collection and acclimation
Animals of similar sizes (shell height 48.92±1.41 mm) for the experiment were collected from the major pearl oyster growing area in Daya Bay Station (23°31′–24°50′N and 113°29′–114°49′E) (temperature 27±0.5°C, pH 8.10±0.05). This sampling procedure was done to ensure that the effects of ocean warming and acidification on gene expression patterns were reflective of the response of pearl oyster in their main area of distribution. The animals were selected and cleaned off epibionts, then acclimated in one 500 L aquarium at ambient seawater temperature (27±0.5°C) and pH (8.10±0.05) for one week prior to experimentation. This temperature was optimal for growth of P. fucata on the southern coast of China. They were fed daily with Platymonas subcordiformis at the satiation feed rate. Excess food and feces were removed by siphoning from the bottom of the aquarium, and fresh filtered seawater (salinity 33±0.5) was added into the aquarium every day.
After the acclimation period, animals were randomly assigned in twelve75-L aquariums (about 50 individual per aquarium) and maintained in four conditions: (1) ambient pH (8.10) and ambient temperature (27°C) (8.10 T, control condition), (2) ambient pH and elevated temperature (8.10 T+3), (3) declined pH (7.70) and ambient temperature (7.70 T), and (4) declined pH and elevated temperature (7.70 T+3). Each condition has three replicates. The header tanks were continuously bubbled with air to aid mixing and to maintain dissolved oxygen (DO)>90%. The experiment lasted for four days. The animals were fed daily with Platymonas subcordiformis. To ensure that similar conditions prevailed in each aquarium, except for the carbonate chemistry, water changes were performed daily in the aquariums. Seawater pH (7.70) of the low pH treatment was manipulated by bubbling CO2 gas into 100 L header tank. Water from the header tank was gravity-fed to replicate aquariums of pH 7.70. There was an individual header tank for each experimental aquarium. Water temperature was warmed to the required temperature (+3°C), or unmanipulated for the ambient control. To ensure no substantial change in pH and temperature occurred within experimental aquariums, pH and temperature in each aquarium were measured immediately after water changes. The pear oysters from all groups were sampled on hours 0, 6, 24, 48 and 96. On each sampling time point, 5 new individuals were randomly selected from each aquarium.
Molecular analyses
Total RNA was extracted from the mantle tissue of P. fucata using the Mollusc RNA Kit (Omega Bio-Tek, Inc, Georgia, America) according to the manufacturer's instructions. The RNA quantity was analyzed on Thermo NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc, Wilmington, America) and quality was checked by gel electrophoresis. Then, high-quality total RNA was reverse transcribed to cDNA using the PrimeScript™ reagent kit (TaKaRa) following the manufacturer's instructions.
Quantitative real-time PCR reactions were carried out on Roche LightCycler480 thermal cycler (Roche Applied Science, Germany). The amplifications were performed in triplicate in a total volume of 10 µl containing 5 µl of 2× SYBR Premix Ex Taq™ (TaKaRa), 2 µl of diluted cDNA, 0.2 µl of each primer (10 µM) and 2.6 µl of double-distilled water. The cycle conditions were as follows: 1 cycle of 95°C for 10 s, 45 cycles of 95°C for 5 s, 60°C for 25 s, and 80°C for 1 s, 1 cycle of 95°C for 1 s, 65°C for 15 s, and 60°C for 1 s, 1 cycle of 40°C for 1 s. The specificity of the PCR amplification was verified from the melting curve. The housekeeping gene gapdh was selected as references for the calculation of relative expression levels of the genes. Primers sequences of calmodulin, nacrein, aspein, she-7-F10, hsp70 and gapdh in P. fucata were outlined in Table 4.
Table 4. Primers sequences of genes used in real-time PCR analysis.
| Gene | Acession no. | Primer sequence (5′-3′) |
| she-7-F10: F | EU177506 | GAGGTCTCGGTGGAGTAAGTGG |
| she-7-F10: R | AACACTGCCGAGTCCTCCTAAT | |
| aspein: F | AB094512 | TTCATTTCGCTCTTTCAACCAG |
| aspein: R | GCATCCGAAGAACAAAGTTTTT | |
| calmodulin: F | AY341376 | TGACGGTGACGGACAGGTTA |
| calmodulin: R | GTTTGACAAAATTGCGTTTATGAC | |
| nacrein: F | D83523 | TGTTCATCTAACACCGGAGATG |
| nacrein: R | TGAAGAACCCTTCTTGACACCT | |
| hsp70: F | EU822509 | TTTGACTTGGGAGGAGGAACC |
| hsp70: R | CTCACCATTCTGTTGTCAAAGTCC | |
| gapdh: F | AB205404 | TCTGCTGATGCTCCTATGTTTG |
| gapdh: R | CGTTGATTATCTTGGCGAGTG |
Statistical analysis
To avoid pseudoreplication, all variables measured were averaged for 5 pearl oysters in each aquarium, and these aquarium means were used in all statistical analyses. All data were tested for homoscedasticity and normality and were log-transformed if necessary. The dependent variables (expression levels of the genes) at each time point were analyzed by two-way repeated measures ANOVA with temperature and pH as fixed factors. Dunn-Sidak tests followed ANOVA were used to assess differences among treatments and among different time point. Tank was analyzed as a factor by one-way ANOVA to assess tank effects. Significance was set at p<0.1 for all tests. The software SPSS 16.0 was used for analyses.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study is supported by national Natural Science Foundation of China (41006090), the Joint Program of NSFC-Guangdong (U0831001) and the funds of knowledge innovation program of Chinese Academy of Sciences (KZCX2-YW-Q07-03 and ZCX2-EW-Q21). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:C09S04. [Google Scholar]
- 2.IPCC (Intergovernmental Panel on Climate Change) The 4th Assessment Report of the IPCC. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 3.Solomon S, Qin D, Manning M. Climate change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 4.Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 5.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 6.Doney SC, Fabry VJ, Feely RA. Ocean acidification: the other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 7.Dupont S, Thorndyke M. Impact of CO2-driven ocean acidification on invertebrates early life-history – What we know, what we need to know and what we can do. Biogeosciences Disscuss. 2009;6:3109–3131. [Google Scholar]
- 8.Marubini F, Ferrier-Pages C, Furla P, Allemand D. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs. 2008;27:491–499. [Google Scholar]
- 9.Wood HL, Spicer JI, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B. 2008;275:1767–1773. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comeau S, Gorsky G, Jeffree R, Teyssié JL, Gattuso JP. Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosciences. 2009;6:1877–1882. [Google Scholar]
- 11.Gazeau F, Quiblier C, Jansen JM, Gattuso J-P, Middelburg JJ, et al. Impact of elevated CO2 on shellfish calcification. Geophys Res Lett. 2007;34:L07603. [Google Scholar]
- 12.Findlay HS, Wood HL, Kendall MA, Spicer JI, Twitchett RJ, et al. Calcification, a physiological process to be considered in the context of the whole organism. Biogeosciences Discuss. 2009;6:2267–2284. [Google Scholar]
- 13.Gutowska MA, Melzner F, Pörtner HO, Meier S. Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Mar Biol. 2010;157:1653–1663. [Google Scholar]
- 14.Gooding RA, Harley CDG, Tang E. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc Natl Acad Sci. 2009;106:9316–9321. doi: 10.1073/pnas.0811143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagés C, Jaubert J, et al. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Global Change Biol. 2003;9:1660–1668. [Google Scholar]
- 16.Metzger R, Sartoris FJ, Langenbuch M, Pörtner HO. Influence of elevated CO2 concentrations on thermal tolerance of the edible crab Cancer pagurus. J Thermal Biol. 2007;32:144–151. [Google Scholar]
- 17.Byrne M, Ho M, Wong E, Soars NA, Selvakumaraswamy P, et al. Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean. Proc R Soc B. 2010:1–8. doi: 10.1098/rspb.2010.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin SE, Gattuso J-P. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Global Change Biol. 2009;15:2089–2100. [Google Scholar]
- 19.Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, et al. Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol. 2008;18:1501–1507. doi: 10.1016/j.cub.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Place SP, O'Donnell MJ, Hofmann GE. Gene expression in the intertidal mussel Mytilus californianus: physiological response to environmental factors on a biogeographic scale. Mar Ecol Prog Ser. 2008;356:1–14. [Google Scholar]
- 21.Ohsumi T. Introduction: what is the ocean sequestration of carbon dioxide? J Oceanogr. 2004;60:693–694. [Google Scholar]
- 22.Turley C, Blackford JC, Widdicombe S, Lowe D, Nightingale PD, et al. Reviewing the impact of increased atmospheric CO2 on oceanic pH and the marine ecosystem. In: Schellnhuber HJ, Cramer W, Nakicenovic N, Wigley T, Yohe G, editors. Avoiding Dangerous Climate Change, 8. Cambridge University Press, Cambridge; 2006. pp. 65–70. [Google Scholar]
- 23.Southgate PC, Lucas JS. The Pearl Oyster. Oxford: Elsevier; 2008. 573 [Google Scholar]
- 24.He MX, Huang LM, Shi JH, Jiang YP. Variability of ribosomal DNA ITS-2 and its utility in detecting genetic relatedness of pearl oyster. Mar Biotechnol. 2005;15:40–45. doi: 10.1007/s10126-004-0003-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang LJ, He MX. Quantitative expression of shell matrix protein genes and their correlations with shell traits in the pearl oyster Pinctada fucata. Aquaculture. 2011;314:73–79. [Google Scholar]
- 26.Wang N, Kinoshita S, Riho C, Maeyama K, Nagai K, et al. Quantitative expression analysis of nacreous shell matrix protein genes in the process of pearl biogenesis. Comp Biochem Phys B. 2009;154:346–350. doi: 10.1016/j.cbpb.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Marin F, Luquet G, Marie B, Medakovic D. Molluscan shell proteins: primary structure, origin, and evolution. Curr Top Dev Biol. 2007;80:209–276. doi: 10.1016/S0070-2153(07)80006-8. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi T, Endo K. Biphasic and dually coordinated expression of the genes encoding major shell matrix proteins in the pearl oyster Pinctada fucata. Mar Biotechnol. 2006;8:52–61. doi: 10.1007/s10126-005-5037-x. [DOI] [PubMed] [Google Scholar]
- 29.Zayzafoon M, Fulzele K, McDonald JM. Calmodulin and calmodulin dependent kinase IIalpha regulate osteoblast differentiation by controlling c-fos expression. J Biol Chem. 2005;280:7049–7059. doi: 10.1074/jbc.M412680200. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto H, Miyoshi F, Kohno J. The carbonic anhydrase domain protein nacrein is expressed in the epithelial cells of the mantle and acts as a negative regulator in calcification in the mollusc Pinctada fucata. Zool Sci. 2005;22:311–315. doi: 10.2108/zsj.22.311. [DOI] [PubMed] [Google Scholar]
- 31.Gong NP, Li Q, Huang J, Fang Z, Zhang GY, et al. Culture of outer epithelial cells from mantle tissue to study shell matrix protein secretion for biomineralization. Cell Tissue Res. 2008;333:493–501. doi: 10.1007/s00441-008-0609-5. [DOI] [PubMed] [Google Scholar]
- 32.Guan YY, Huang LM, He MX. Construction of cDNA subtractive library from pearl oyster (Pinctada fucata Gould) with red color shell by SSH. Chin J Oceanol Limnol. 2011;29:616–622. [Google Scholar]
- 33.Hofmann GE. Ecologically relevant variation in induction and function of heat shock proteins in marine organisms. Am Zool. 1999;39:889–900. [Google Scholar]
- 34.Wang ZL, Wu ZH, Jian JC, Lu YS. Cloning and expression of heat shock protein 70 gene in the haemocytes of pearl oyster (Pinctada fucata, Gould 1850) responding to bacterial challenge. Fish Shellfish Immun. 2009;26:639–645. doi: 10.1016/j.fsi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, Blair HC, Peng Y, Zaidi N, Adebanjo OA, et al. Calcineurin regulates bone formation by the osteoblast. Proc Natl Acad Sci. 2005;102:17130–17135. doi: 10.1073/pnas.0508480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todgham AE, Hofmann GE. Transcriptomic response of sea urchin larvae Strogylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol. 2009;212:2579–2594. doi: 10.1242/jeb.032540. [DOI] [PubMed] [Google Scholar]
- 37.Martin S, Richier S, Pedrotti M-L, Dupont S, Castejon C, et al. Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2 driven ocean acidification. J Exp Biol. 2011;214:1357–1368. doi: 10.1242/jeb.051169. [DOI] [PubMed] [Google Scholar]
- 38.Zippay M, Hofmann GE. Effect of pH on gene expression and thermal tolerance of early life history stages of red abalone (Haliotis Rufescens). J Shellfish Res. 2010;29:429–439. [Google Scholar]
- 39.Berge JA, Bjerkeng B, Pettersen O, Schanning MT, Oxnevad S. Effects of increased sea water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere. 2006;62:681–687. doi: 10.1016/j.chemosphere.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 40.Clark D, Lamare M, Barker M. Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among tropical, temperate, and a polar species. Mar Biol. 2009;156:1125–1137. [Google Scholar]
- 41.Cummings V, Hewitt J, Van Rooyen A, Currie K, Beard S, et al. Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS ONE. 2011;6:e16069. doi: 10.1371/journal.pone.0016069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Donnell MJ, Hammond LM, Hofmann GE. Predicted impact of ocean acidification on a marine invertebrate: elevated CO2 alters response to thermal stress in sea urchin larvae. Mar Biol. 2009;156:439–446. [Google Scholar]
- 44.Liu WG, Lin JS, He MX. Seawater acidification has species-specific effects on the metabolic rate of three bivalves from the southern coast of China. Chin J Oceanol Limnol. 2011 In press. [Google Scholar]
- 45.Bradshaw AL, Brewer PG, Shafer DK, Williams RT. Measurements of total carbon dioxide and alkalinity by potentiometric titration in the GEOSECS program. Earth Planet Sci 15 Lett. 1981;55:99–115. [Google Scholar]
- 46.Dickson AG, Afghan JD, Anderson GC. Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity. Mar Chem. 2003;80:185–197. [Google Scholar]
- 47.Pierrot D, Lewis E, Wallace DWR. MS Excel program developed for CO2 system calculations. 2006. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN.
- 48.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RN. Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- 49.Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res. 1987;34:1733–1743. [Google Scholar]
- 50.Dickson AG. Thermodynamics of the dissociation of boric-acid in potassium-chloride solutions form 273.15-K to 318.15 K. J Chem Thermodynam. 1990;22:113–127. [Google Scholar]