Abstract
As first noted by Aristotle in honeybee workers, many insect pollinators show a preference to visit flowers of just one species during a foraging trip. This “flower constancy” probably benefits plants, because pollen is more likely to be deposited on conspecific stigmas. But it is less clear why insects should ignore rewarding alternative flowers. Many researchers have argued that flower constancy is caused by constraints imposed by insect nervous systems rather than because flower constancy is itself an efficient foraging method. We argue that this view is unsatisfactory because it both fails to explain why foragers flexibly adjust the degree of flower constancy and does not explain why foragers of closely related species show different degrees of constancy. While limitations of the nervous system exist and are likely to influence flower constancy to some degree, the observed behavioural flexibility suggests that flower constancy is a successful foraging strategy given the insect’s own information about different foraging options.
Keywords: Flower constancy, honeybee, foraging, learning, pollination
Background
Over 2000 y ago Aristotle (340BC) observed that honeybee (Apis mellifera) workers visit flowers of only one flower type during a foraging trip.1 This is known as flower constancy and has been shown to occur in a wide range of insect pollinators.1-8 Flower constancy is beneficial for plants because it prevents pollen loss to allospecific plants and stigma blocking with heterospecific pollen.4,6,9,10 On a longer time scale, it may also have important consequences in plant evolution and speciation.5
But what benefits do the pollinators receive? Indeed, there may be circumstances in which constancy clearly has a cost. A flower constant honeybee foraging in a field with several interspersed plant species in bloom (Fig. 1A) might lose out on energetically superior opportunities if she focuses on just one plant species. In certain experimental situations honeybee foragers become constant to a floral type that offers lower rewards than a simultaneously available alternative.11-14 The bees show “spontaneous” flower constancy for a color, irrespective of the energetic value of the rewards offered by the flowers of the other color.
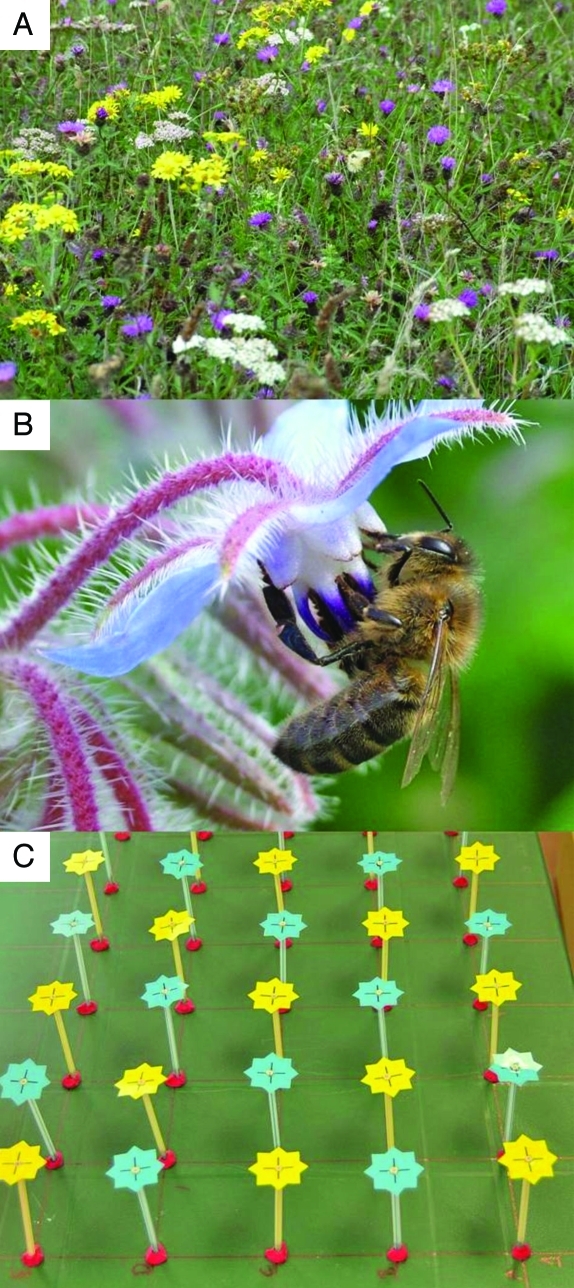
Figure 1.
(A) A field with wild flowers in East Sussex, UK. (B) A honeybee (Apis mellifera) foraging on borage (Borago officinalis) (Photos by F.L.W. Ratnieks). (C) Experimental setup of artificial blue and yellow flowers to study flower constancy in honeybees (Photo by C. Grüter).
Theories of flower constancy
Several hypothesis have been put forward to explain flower constancy in insect pollinators, many of which are not mutually exclusive (for review see refs.4-6,10). Very popular is the idea that flower constancy is caused by nervous system limitations.5,6,10,13-15 For example, the “interference hypothesis” argues that learning how to forage on a new flower type interferes with memories about how to forage on the current or previous type.4-7,10 Therefore, pollinators would do best to stick to just one type of flower. Although short-term memories (STM) are particularly prone to interference,5,16 this hypothesis no longer has many supporters as convincing empirical evidence that interference causes flower constancy is lacking.4,6 Related to this is the “search image hypothesis,”4,10,17 which states that flower constancy is favored because it allows foragers to develop a search image for a particular flower type which in turn helps the insect to efficiently locate flowers. The underlying assumption is that an animal can only have a search image for one flower type at a time, which temporarily inhibits the detection of other types.18 However, there is little evidence for the formation of search images in pollinators.6
The “learning investment hypothesis” argues that flower constancy is the best strategy because switching from one flower species to a new one would lead to a period of poor efficiency as insects need to learn how to extract pollen or nectar from this new species.5,9,10 While learning how to extract nectar of complex flowers indeed involves a learning phase of reduced efficiency,19,20 these time costs seem to be too low to be an important cause of flower constancy.5 An additional problem for this hypothesis is that pollinators exploiting flowers with simple morphologies and easily accessible rewards also show flower constancy.8
The “resource “ or “work partitioning hypothesis” takes a different angle.5,14,21 It is argued that flower constancy in social pollinators, such as honeybees, is a strategy to avoid intra-colonial competition for flowers. However, given the impressive foraging range (up to approx. Thirteen km in honeybees and 4 km in bumble bees, respectively) and the high colony densities of social bees in many areas, competition among foragers within a colony is presumably much weaker than competition between colonies.22-24 Furthermore, the prediction that colony size and the foraging strategy of a species are associated with the degree of flower constancy21 is not supported by a comparative study using Trigona stingless bees.2 An additional problem for this hypothesis is that some solitary pollinators are also flower constant.7,8
The hypotheses above are all unsatisfactory in one or another way as explanations for flower constancy in insect pollinators, and at best each might explain flower constancy in particular circumstances. Although there could be multiple causes of flower constancy the hypotheses above are largely unable to explain two important observations. First, individual insect foragers show considerable behavioral flexibility and quickly respond to changes of costs and benefits of being flower constant (Table 1). Second, closely related species, such as Apis mellifera and A. cerana, show different flower choice behaviors in similar experimental situations.21 Although limitations in the nervous system of pollinators certainly exist, we argue that flower constancy is less a limitation and more an adaptive behavior in its own right that can quickly be adjusted depending on the information a forager has about the energetic value of the flower species being visited.
Table 1. Factors affecting the degree of flower constancy in eusocial bees, Apidae.
| Factor | Species | Reference |
|---|---|---|
| Reward quality |
Apis mellifera Apis cerana Trigona dorsalis |
14
,
26
,
27
,
37
,
38
21 39 |
| Reward quantity |
Apis mellifera Bombus spp |
26
,
28
,
40
41 |
| Number of previous rewards |
Apis mellifera |
26
|
| Color difference between flowers |
Apis mellifera Bombus ephippiatus Bombus spp |
10
,
13
,
14
,
25
10 41 |
| Floral dissimilarities (number of differing traits) |
Trigona dorsalis Oxytrigona mellicolor Bombus impatiens |
39
39 15 , 42 |
| Flower handling time |
Apis mellifera |
25
,
37
|
| Distance between flowers |
Apis mellifera Bombus spp |
14
,
43
,
44
41 |
| Local enhancement (presence of other bees on flowers) | Oxytrigona mellicolor | 39 |
Behavioral flexibility within species
Worker European honeybees (Apis mellifera) (Fig. 1B) show a very high degree of flower constancy and several studies have argued that this is “spontaneous,” that is caused by constraints of the nervous system rather than being an efficient strategy of food collection.11-14,25 It has been argued that, due to sensory constraints, bees would become constant if two flower types have colors that are perceived as substantially different. That is, if the alternative colors are highly distinct in bee color space. However, a recent study26 suggests that the reported inability of honeybees to adjust their flower choice according to differences in the rewards experienced was caused by the use of unnaturally large nectar (sucrose solution) rewards per flower (see also refs.27,28 for a similar argument). The use of realistic reward volumes is important because natural selection is likely to result in discrimination by bees in the natural range only.26,28 Ecologically realistic rewards showed that honeybee foragers were flexible and quickly adjusted their level of constancy, in a mixed patch of otherwise identical blue and yellow artificial flowers (Fig. 1C), according to the quality and quantity of the energetic rewards given to them on one or two training flowers. Lower rewards in both volume, concentration and number lead to lower levels of constancy, that is a stronger tendency to land on the alternative flower type.26 At the highest levels of reward the level of constancy reached a plateau. These results fit well with several other studies that demonstrate how different foraging bee species adjust their constancy according to various parameters that affect the energetic costs and benefits of being flower constant, such as flower handling time and interfloral distance (Table 1).
Flower constancy and information costs
Even when foraging in a habitat with several rewarding flower species available, flower constancy can be the best strategy for an insect given informational uncertainties regarding the alternatives. If the insect is collecting from a relatively profitable flower species, many alternative flower species will be less profitable. In a field with dozens of flower species5(see also Figure 1A) a bee would have to sample many species to find a better one. In addition, she would have to sample many flowers of each species to acquire reliable information about the rewards of the alternative species if individual flowers of one species vary in their energetic value.5 Thus acquiring the information necessary to compare species may be costly in terms of time and energy. This “costly-information hypothesis”5 predicts that insects should be flower constant if the average reward of a flower species is above a certain threshold, but should increasingly invest into sampling alternatives as the reward goes down. Thus, this hypothesis can explain both the high levels of flower constancy that occur when an insect is visiting a profitable food source and the behavioral flexibility that occurs if the reward from a given flower type is low (Table 1). In the experiments where bees showed “spontaneous constancy” to colors,11-14 the rewards may simply have been above the flower constancy threshold.
Differences between species
If constraints caused by limitations of the nervous system were mainly responsible for flower constancy,13,14,25 closely related species should behave similarly. For example, photoreceptor spectral sensitivity in the European honeybee and the Asian honeybee (A. cerana) can be assumed to be similar.29 However, A. cerana foragers are overall less flower constant than A. mellifera and seem unaffected by how distinct color pairs are in the bee color space.21 Other eusocial bee species also seem to be less flower constant than the European honeybee.1,2,5 What causes these interspecific differences? Several hypotheses have been suggested. The “resource/work partitioning hypothesis” and its problems have been mentioned above. The “communication hypothesis” predicts that species that communicate information concerning high quality food sources to nestmates will be more flower constant than social species without communication or which are non-social.5,26 Honeybees communicate the location and the odour of high quality food sources, inside the nest by means of the waggle dance.22,30,31 Additionally, they provide information about the sugar concentration, nectar flow-rate and flower type when they offer small food samples to other bees inside the nest.32-35 By dancing more for higher quality food sources foragers are effectively filtering information as part of their communication system,36 thereby lowering the incentive of recruited foragers to sample other food sources. Although attractive, there is currently little empirical support for this hypothesis. A. cerana also performs waggle dances but is less flower constant21 than A. mellifera. Furthermore, in a comparative study of Trigona stingless bees, species with a solitary foraging strategy were as flower constant as species with strong recruitment systems.2 Other factors like differences between foraging habitats (tropical vs. temperate), competition, and resource defense, could affect the tendency of a species to be flower constant. Comparative studies under similar or controlled ecological conditions are needed to gain further information about flower constancy levels of insect pollinators in order to link flower constancy to the characteristics of foraging habitats and the foraging ecology.
In summary, there is currently no convincing explanation for the observed differences in flower constancy among bee species. However, there is good evidence that flower constancy in bees is an adaptive behavior and is not merely a result of nervous system constraints. The “costly-information hypothesis” is consistent with experimental results, for example those that show that honeybees are flower constant if the reward per flower is high but are less constant if the rewards are low.26
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/16972
References
- 1.Grant V. The flower constancy of bees. Bot Rev. 1950;16:379–98. doi: 10.1007/BF02869992. [DOI] [Google Scholar]
- 2.Slaa EJ, Cevaal A, Sommeijer MJ. Floral constancy in Trigona stingless bees foraging on artificial flower patches: a comparative study. J Apic Res. 1998;37:191–8. [Google Scholar]
- 3.Free JB. The flower constancy of bumblebees. J Anim Ecol. 1970;39:395–402. doi: 10.2307/2978. [DOI] [Google Scholar]
- 4.Goulson D. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol. 1999;2:185–209. doi: 10.1078/1433-8319-00070. [DOI] [Google Scholar]
- 5.Chittka L, Thomson JD, Waser NM. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86:361–77. doi: 10.1007/s001140050636. [DOI] [Google Scholar]
- 6.Gegear RJ, Laverty TM. The effect of variation among floral traits on the flower constancy of pollinators. In: Chittka L, Thomson JD, eds. Cognitive Ecology of Pollination: Animal Behavior and Floral Evolution. Cambridge: Cambridge University Press, 2001: 1-20. [Google Scholar]
- 7.Goulson D, Stout J, Hawson SA. Can flower constancy in nectar butterflies be explained by Darwin's interference hypothesis? Oecologia. 1997;112:225–31. doi: 10.1007/s004420050304. [DOI] [PubMed] [Google Scholar]
- 8.Goulson D, Wright NP. Flower constancy in the hoverfies Episyrphus balteatus (Degeer) and Syrphus ribesii (L.) (Syrphidae) Behav Ecol. 1998;9:213–9. doi: 10.1093/beheco/9.3.213. [DOI] [Google Scholar]
- 9.Darwin C. Cross and self fertilization in the vegetable kingdom. London: Murray, 1876. [Google Scholar]
- 10.Waser NM. Flower constancy: definition, cause, and measurement. Am Nat. 1986;127:593–603. doi: 10.1086/284507. [DOI] [Google Scholar]
- 11.Wells H, Wells PH. Honey bee foraging ecology: optimal diet, minimal uncertainty or individual constancy? J Anim Ecol. 1983;52:829–36. doi: 10.2307/4457. [DOI] [Google Scholar]
- 12.Wells H, Wells PH. Optimal diet, minimal uncertainty and individual constancy in the foraging of honey bees, Apis mellifera. J Anim Ecol. 1986;55:881–91. doi: 10.2307/4422. [DOI] [Google Scholar]
- 13.Hill PSM, Wells PH, Wells H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim Behav. 1997;54:615–27. doi: 10.1006/anbe.1996.0467. [DOI] [PubMed] [Google Scholar]
- 14.Hill PSM, Hollis J, Wells H. Foraging decisions in nectarivores: unexpected interactions between flower constancy and energetic rewards. Anim Behav. 2001;62:729–37. doi: 10.1006/anbe.2001.1775. [DOI] [Google Scholar]
- 15.Gegear RJ, Laverty TM. Flower constancy in bumblebees: a test of the trait variability hypothesis. Anim Behav. 2005;69:939–49. doi: 10.1016/j.anbehav.2004.06.029. [DOI] [Google Scholar]
- 16.Menzel R. Behavioural access to short-term memory in bees. Nature. 1979;281:368–9. doi: 10.1038/281368a0. [DOI] [PubMed] [Google Scholar]
- 17.Goulson D. Are insects flower constant because they use search images to find flowers? Oikos. 2000;88:547–52. doi: 10.1034/j.1600-0706.2000.880311.x. [DOI] [Google Scholar]
- 18.Shettleworth SJ. Cognition, Evolution, and Behavior. Oxford: Oxford University Press, 2010. [Google Scholar]
- 19.Laverty TM. Bumble bee learning and flower morphology. Anim Behav. 1994;47:531–45. doi: 10.1006/anbe.1994.1077. [DOI] [Google Scholar]
- 20.Chittka L, Thomson JD. Sensori-motor learning and its relevance for task specialization in bumble bees. Behav Ecol Sociobiol. 1997;41:385–98. doi: 10.1007/s002650050400. [DOI] [Google Scholar]
- 21.Wells H, Rathore RRS. Foraging ecology of the Asian hive bee, Apis cerana indica, within artificial flower patches. J Apic Res. 1994;33:219–30. [Google Scholar]
- 22.von Frisch K. The dance language and orientation of bees. Cambridge, Massachusetts: Harvard University Press, 1967. [Google Scholar]
- 23.Beekman M, Ratnieks FLW. Long-range foraging by the honey-bee, Apis mellifera L. Funct Ecol. 2000;14:490–6. doi: 10.1046/j.1365-2435.2000.00443.x. [DOI] [Google Scholar]
- 24.Chapman RE, Wang J, Bourke AFG. Genetic analysis of spatial foraging patterns and resource sharing in bumble bee pollinators. Mol Ecol. 2003;12:2801–8. doi: 10.1046/j.1365-294X.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson CE, Orozco BS, Hill PSM, Wells H. Honeybee (Apis mellifera ligustica) response to differences in handling time, rewards and flower colours. Ethology. 2006;112:937–46. doi: 10.1111/j.1439-0310.2006.01245.x. [DOI] [Google Scholar]
- 26.Grüter C, Moore H, Firmin N, Helanterä H, Ratnieks FLW. Flower constancy in honey bee foragers (Apis mellifera) depends on ecologically realistic rewards. J Exp Biol. 2011;214:1397–402. doi: 10.1242/jeb.050583. [DOI] [PubMed] [Google Scholar]
- 27.Banschbach VS. Colour association influences honey bee choice between sucrose concentrations. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1994;175:107–14. doi: 10.1007/BF00217441. [DOI] [Google Scholar]
- 28.Waddington KD, Gottlieb N. Actual vs. perceived profitability: a study of floral choice of honey bees. J Insect Behav. 1990;3:429–41. doi: 10.1007/BF01052010. [DOI] [Google Scholar]
- 29.Chittka L. Does bee color vision predate the evolution of flower color? Naturwissenschaften. 1996;83:136–8. doi: 10.1007/BF01142181. [DOI] [Google Scholar]
- 30.Seeley TD. The wisdom of the hive: The social physiology of honey bee colonies. Cambridge, Massachusetts: Harward University Press, 1995. [Google Scholar]
- 31.Grüter C, Farina WM. The honeybee waggle dance: can we follow the steps? Trends Ecol Evol. 2009;24:242–7. doi: 10.1016/j.tree.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Farina WM, Núñez JA. Trophallaxis in the honeybee, Apis mellifera (L) as related to the profitability of food sources. Anim Behav. 1991;42:389–94. doi: 10.1016/S0003-3472(05)80037-5. [DOI] [Google Scholar]
- 33.Farina WM, Grüter C, Diaz PC. Social learning of floral odours within the honeybee hive. Proc Biol Sci. 2005;272:1923–8. doi: 10.1098/rspb.2005.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grüter C, Acosta LE, Farina WM. Propagation of olfactory information within the honeybee hive. Behav Ecol Sociobiol. 2006;60:707–15. doi: 10.1007/s00265-006-0214-0. [DOI] [Google Scholar]
- 35.Farina WM, Grüter C. Trophallaxis - A mechanism of information transfer. In: Jarau S, Hrncir M, eds. Food exploitation by social insects: Ecological, behavioral, and theoretical approaches. Boca Raton, Florida: CRC Press, 2009:173-187. [Google Scholar]
- 36.Grüter C, Leadbeater E, Ratnieks FLW. Social learning: the importance of copying others. Curr Biol. 2010;20:R683–5. doi: 10.1016/j.cub.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 37.Cakmak I, Sanderson C, Blocker TD, Pham LL, Checotah S, Norman AA, et al. Different solutions by bees to a foraging problem. Anim Behav. 2009;77:1273–80. doi: 10.1016/j.anbehav.2009.01.032. [DOI] [Google Scholar]
- 38.Loo SK, Bitterman ME. Learning in honeybees (Apis mellifera) as a function of sucrose concentration. J Comp Psychol. 1992;106:29–36. doi: 10.1037/0735-7036.106.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Slaa EJ, Tack AJM, Sommeijer MJ. The effect of intrinsic and extrinsic factors on flower constancy in stingless bees. Apidologie (Celle) 2003;34:457–68. doi: 10.1051/apido:2003046. [DOI] [Google Scholar]
- 40.Greggers U, Menzel R. Memory dynamics and foraging strategies of honeybees. Behav Ecol Sociobiol. 1993;32:17–29. doi: 10.1007/BF00172219. [DOI] [Google Scholar]
- 41.Chittka L, Gumbert A, Kunze J. Foraging dynamics of bumble bees: correlates of movements within and between plant species. Behav Ecol. 1997;8:239–49. doi: 10.1093/beheco/8.3.239. [DOI] [Google Scholar]
- 42.Kulahci IG, Dornhaus A, Papaj DR. Multimodal signals enhance decision making in foraging bumble-bees. Proc Biol Sci. 2008;275:797–802. doi: 10.1098/rspb.2007.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marden JH, Waddington KD. Floral choices by honey bees in relation to the relative distances to flowers. Physiol Entomol. 1981;6:431–5. doi: 10.1111/j.1365-3032.1981.tb00658.x. [DOI] [Google Scholar]
- 44.Kunin WE. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology. 1993;74:2145–60. doi: 10.2307/1940859. [DOI] [Google Scholar]