Abstract
To reveal the neural and behavioral dynamics of social interaction, single-person studies are increasingly complemented by research designs that simultaneously assess two or more interacting individuals. In this article, we review studies on neural mechanisms and markers of social interactions that use multi-person functional magnetic resonance imaging and electrophysiological recordings. We propose a terminology for investigating social interaction dynamics, show how forward models of action regulation may serve as a framework for investigating interpersonal action coordination and discuss different methodological approaches to studying functional brain connectivity.
Keywords: social interaction, social cognition, action coordination, EEG and fMRI hyperscanning, phase synchronization, functional brain connectivity
“Neuroscientists do agree: humans and their brains and minds are shaped, and normally function, in continuous interaction with other people” (Hari and Kujala,1 p. 454)
Coordinated social interaction is an essential and ubiquitous part of human life. Aligning one’s gaze with a conversation partner, carrying home the groceries with the help of a friend, doing the dishes with a roommate or helping someone getting dressed – all of these common actions pose coordinative demands.2-4 The skilled performances of sports teams, ensemble dancers, or musicians also come to mind. Nevertheless, the real-time neural dynamics of interpersonally coordinated behavior have remained largely unexplored, presumably reflecting difficulties in studying the complexities of social interaction in tightly controlled experimental settings. There is a need to create and refine experimental paradigms that probe the mechanisms of social interaction at behavioral and neural levels of analysis.
Terminology
In the following, we refer to the field of social cognition as the mechanisms that allows us to understand others.5 Social cognition includes mentalizing or theory of mind, that is, the ability to represent other people’s mental states,6 as well as the know-how needed for interaction and the formation of social relations.7 In contrast, social interaction is more narrowly defined as turn-taking among active, autonomous agents who follow social rules and control their action and reactions according to their perceptions.8 Finally, joint action is seen as any form of coordinated action bringing about change in the environment,9 which might also take place in the structured setting of a game or a particular setting that imposes constraints on roles, the sequence of turn-taking and the modality of communication. Coordination is generally defined as the “non-accidental correlation between the behaviors of two or more systems that are in sustained coupling, or have been coupled in the past, or have been coupled to another, common, system.”10 In the context of joint actions, coordination results in interpersonal action coordination. In contrast, coordination elicited by an external event (e.g., a simultaneous orienting response to a strange sound7) or without a particular aim (e.g., the synchronization of speech and movements between persons in a conversation7) does not qualify as interpersonal action coordination.
Extending the Forward Model of Action Regulation to Interpersonal Action Coordination
Interpersonal action coordination requires the perception, representation, and anticipation of one’s own and the partner’s actions. These requirements can be integrated in a forward model. This notion of a forward model, which was initially introduced for individual action control by Wolpert and colleagues,11 can be traced back to the far more complex physiological theory of functional systems proposed by Petr K. Anokhin.11,12 In a forward model, any intended action is stored together with a variety of sensory inputs and appropriate motor commands, which in turn are associated with the corresponding efference copy signals to predict the sensory effects of the intended action. When the action is performed, its actual sensory consequences and the predictions are compared. The result of this comparison can then be used to determine the source of the sensory event,13 assuming that agents are better able to predict the sensory consequences of their own actions than the sensory consequences of external events.
The interpersonal extension of the forward model postulates that in coordinated actions, both interaction partners do not only have a forward model of their own actions, but do also predict their partner’s actions and the according effects and compare the actual outcome to this prediction. Especially interactive couples that are highly skilled in their coordinative task, e.g., dancers and musicians, may also maintain an additional suprapersonal action representation. This emulation of two models in one joint forward model is likely to engage the mirror neuron system.5,14-16
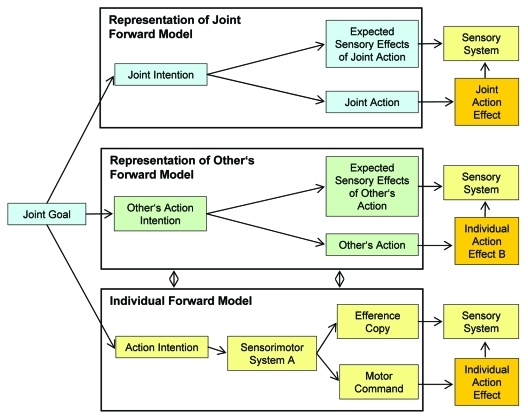
The model in Figure 1 shows the three layers of forward models of one person engaged in an interactive task. It also posits a joint goal, from which a concrete joint intention is derived. This in turn determines the individual intentions and accordingly the actions. It remains to be clarified, how joint intentions are formed and how individual intentions relates to them,17 but still it appears important to tentatively include them into the model.
Figure 1.
Tentative forward model for action coordination. In coordinative actions, the forward model of each of the interaction partners is assumed to be 3-fold. The elements of the forward model concerning one’s self are depicted in yellow here, green color indicates the representation of the other person (which could also be more persons in an interacting group) and the suprapersonal representation of the joint action is marked in blue. Actual, observable action effects are shown in orange. On layer 1 (Individual Forward Model), one’s own action intention, as derived from the joint goal, is represented. The sensorimotor system thereupon builds a motor command and an according efference copy. The action effect caused by the execution of the motor command is compared with the efference copy in the sensory system. The result of this comparison (sensory congruency or discrepancy) influences the following actions such that the joint goal is approached and reached in the end. On a second layer (Representation of Other’s Forward Model), the other’s action intention and the corresponding implementation with its assumed sensory consequences are represented. With regard to the interaction partner, one’s own sensory system thus compares the expected and the actual action effects. Especially when the involved individuals are highly skilled in their interactive tasks, an additional third layer (Representation of Joint Forward Model) is assumed, in which the joint intention is represented and the joint action and its effects are predicted detached from the individual contribution. Again, a comparison of prediction and outcome takes place in the individual sensory system, thereby determining the progress of coordinated action. The various representational layers of the actors are intertwined by sensorimotor feedback loops.
All the behavioral activities indicated in the forward model induce activation and co-activation of specific brain regions. So far, these activation patterns have been assessed and described, for the most part, for one brain in the context of one-person action.18-21 It seems reasonable to assume that joint action activates similar or overlapping brain areas as individual action. At the same time, additional brain regions may become activated as well. Currently, little is known about the brain areas that are involved and the neural mechanisms that implement interpersonally coordinated behavior. Relevant neuroimaging and neuroelectrical studies of interacting brains are reviewed below. Given the pronounced differences in spatial and temporal resolution between EEG (electroencephalography) and MEG (magnetoencephalography), on the one hand, and neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), one the other, EEG and MEG are more appropriate for investigating the temporal dynamics of interpersonal action coordination, whereas neuroimaging techniques are more appropriate for localizing activation and co-activation patterns in the context of joint action.
Functional Imaging of Interacting Brains
A recently introduced twin-volume head coil allows for the simultaneous recording of two interacting brains using one scanner.22 This may enable face-to-face interaction to be investigated with fMRI methods in the future. Until now, however, this technique has not found its way into research practice. Hence, the physical configuration of MRI scanners does not permit investigating face-to-face interactions. To circumvent this limitation, researchers use avatars, project interaction partners by video, or ask the research participants to imagine a social interaction.
Interacting with virtual others
Using fMRI, Schilbach et al.23 found that research participants’ medial prefrontal cortex (mPFC) showed activity when they were merely observing the facial expressions exchanged between avatars, as well as when they themselves were personally involved, that is, when an avatar directed its gaze at the subject. They also found that the dorsal mPFC (dmPFC) appears to differentially support the detection of self-relevance, presumably because an intersubjective context was established to evaluate communicative signals. The ventral part of mPFC (vmPFC) was particularly active when facial expressions were being processed. When social relevance of facial expression and self-involvement occurred together, the left parahippocampal region and right superior temporal regions were activated in addition to the vmPFC. In line with these findings, another fMRI study of participants taking part in a virtual ball tossing game with two avatars showed overlapping mPFC activation when subjects were actively playing from a first-person perspective.24 This suggests that the mPFC makes a critical contribution to the neural basis of the social self.
Cooperating and competing with others
In a computerized monetary “trust and reciprocity” game, fMRI recordings of cooperative subjects, who risked to miss out in order to enable greater winnings for both partners or accepted a smaller win in favor of their partner, showed a greater activation in the middle frontal gyrus and the frontal pole than recordings of non-cooperators.25 The authors link these findings to a prefrontal “active convergence zone,” which combines joint attention to reciprocal gains with inhibition of immediate gratification. While in non-cooperative subjects there was no difference in prefrontal cortex activation between human (i.e., a person sitting at a computer in another room) and computer counterpart conditions, cooperators showed higher levels of prefrontal involvement when playing with another person as opposed to a computer. Likewise, a positron emission tomography (PET) study of subjects playing a computerized version of “stone, paper, scissors” established higher levels of differential bilateral activation in the anterior paracingulate cortex when participants thought of their opponent as a rational agent, that is, when they believed they were playing against the researcher despite the fact that the opponent was, in effect, a computer randomly displaying the different gestures.26 An additional confirmation of this result was provided by a study using a non-interactive comic comprehension task: the anterior paracingulate cortex was not involved in understanding other people’s intentions in general, but only in situations linked to understanding the intentions of socially interacting people.27
Decety and his colleagues28 had their subjects play a computerized cooperation/ competition game and used pre-recorded introduction videos to make them believe that they would be playing against two different opponents, the first being cooperative and second competitive, despite the fact that they were actually playing against the same researcher the whole time. The subjects were able to either help or hinder their opponent in creating a particular pattern of tokens by placing their own tokens so as to either help create the pattern or bring it into disarray respectively. When compared with an independent play condition, both cooperation and competition were associated with increased activation of a frontoparietal network, presumably to support executive functions, and increased activation of the insula, presumably to enhance the sense of agency, action attribution and autonomic arousal. The orbitofrontal cortex was selectively involved in cooperation. According to the authors, this may reflect behavioral choices and the motivational control of goal-directed behavior. In association with competitive behavior, the inferior parietal and medial prefrontal cortices were differentially activated. This can be explained by the role of the inferior parietal cortex in distinguishing between self-generated actions and those performed by others and the involvement of the medial prefrontal cortex in the process of mentalizing, which is relevant both for cooperation and competition.28
Two studies performed by Montague et al.29,30 are particular noteworthy because two individuals were scanned simultaneously in separate scanners while completing a deception task29 and respectively an economic exchange task (a paradigm referred to as hyperscanning).30 The deception task involved one of the subjects taking on the role of the “receiver” to decide whether the other subject, the “sender,” was being honest or not. The sender was shown either a red or a green screen and then sent on one of the two colors to the receiver, who then had to decide whether that color was the same color the sender had seen (honesty) or the other color (deception). The functional data sets of the two subjects were then combined in a so-called “hyperbrain” and analyzed with independent component analysis (ICA) to separate individual activity modes from interacting ones. Remarkably, Montague and colleagues were also able to measure the frequency-wise degree of common power between the two signals in the form of cross-spectral coherence, thereby quantifying their functional coupling at the base frequency of the task (0.04 Hz) with a cluster of activity in the supplementary motor area.29
Similarly, King-Casas et al. used a setup in which each of two participants was lying in a different fMRI scanner (one in Pasadena, CA, the other in Houston, TX) while playing multiple rounds of an economic exchange task via an online transmission device. The “investor” was supposed to give any portion of the 20 dollars he received to the “trustee.” The trustee’s share then tripled, and he could decide how much to give back to the investor. Intrabrain correlations between the anterior cingulated cortex (ACC) and the caudate of the “trustee” as well as interbrain correlations of the trustee’s ACC and caudate with the middle cingulated cortex (MCC) of the “investor” were discovered. While the strongest correlation between MCC and an “intention to trust” signal occurred after the investor’s decision in the first rounds of the game, it came about 14 sec earlier, i.e., before the investor’s decision, in the late rounds, possibly because the “trustee” built a model of the investor’s response over the course of the game.30
With wearable near-infrared spectroscopy (NIRS) devices, Funane et al. could have their participating pairs sitting face-to-face at one table.31 In a cooperative task, each partner had to silently count 10s before pressing a button. This reaction was supposed to be performed simultaneously by both partners and each one of them got feedback after each trial indicating whether he was too late or too early. The authors found a significantly smaller interval between the button-press times in trials where there was a high positive covariance of prefrontal activation values of both participants during the counting. This relationship was not found when no feedback in timing was given. Therefore, the Funane and colleagues31 conclude that the synchronized activation might not only be caused by both participants integrating working-memory exigencies (counting) with attention allocation (right timing of the button press), but might also indicate mentalizing activity, that could have been induced by making the participants think of each other through the feedback.
Imitating another agent
Decetey and colleagues32 took PET scans during reciprocal imitation of hand movements between the subject and a researcher whose gestures were visually transmitted into the scanner by means of a video-mirror setup. Left inferior parietal regions were involved in imitating the researcher’s movements, while the right counterpart of this region was differentially activated when the research imitated the subject’s movements. This finding would imply that this region plays a potentially essential role in agency. In a similar set-up, Guionnet et al.33 added a condition of free imitation, in which participants could decide whether they wanted to imitate the experimenter’s hand movements or not, while the experimenter followed a protocol of alternately making predefined gestures and imitating the participant’s hand movements. Apart from areas having been associated with imitation before (left pars opercularis, motor areas, inferior frontal gyrus, left inferior parietal lobe and insula), they found the same pattern of activation for free imitation and being imitated (regardless of condition), namely in dorsolateral prefrontal cortex (dlPFC), dorsal anterior cingulate cortex (dACC) and pre-supplementary motor area (pre-SMA). The authors implicated this with the anticipation and monitoring of the partner’s behavior. A pattern of dACC and insula activation with simultaneous deactivation of the default mode network in periods in which subjects were imitated by the experimenter either freely or on instruction, was interpreted as a correlate of the integration of sensory, visceral, autonomic, and hedonic information, possibly enabling an agent to make behavioral decisions in the interaction with others.
Functional interbrain connectivity during gestural and verbal communication
Two groups recently studied between-brain effects in interpersonal communication with real persons acting as senders and receivers, respectively. Schippers et al.34 introduced the technique of between-brain Granger causality mapping (bbGCM), which is used to measure the influence of a selected region in one brain on all the voxels of another brain by statistically comparing the Granger causalities in both directions. Granger causality posits that one signal causes another in the Granger sense if knowledge of the past of the first signal reduces the variance of the prediction error of the second signal as opposed to when only the past of the second signal is known.35,36 Using this statistical technique, which is based on strong assumptions (for a critical examination see ref.37), the activity in the putative mirror neuron system (pMNS) of a “gesturer,” who was scanned while gesturing a given word into a camera as if playing charades, was found to predict the brain activity of the respective “guesser,” who was scanned while trying to guess the word from the recording, more so than vice versa. This directed influence was significantly reduced when the “guesser” did not actually guess, but rather just watched the video without having to do anything. This, according to the authors, indicates that the moment-to-moment activity in the guesser’s pMNS mirrors the very recent past of the gesturer’s pMNS activity. Lower Granger causality scores for post-hoc surrogate pairs supported this interpretation. Note, however, that the factual gesturer-guesser pairs were also romantic partners in real life, which may have created a-priori differences between the original and the randomly combined surrogate pairs.
Stephens, Silbert, and Hasson38 scanned a native English speaker and a native Russian speaker while telling an unrehearsed real-life story in their respective languages. Twelve other English-speaking subjects were subsequently scanned while listening to recordings of these stories. To check for spatial and temporal couplings between the speaker’s and the listener’s neural activity, Stephens and his colleagues then used a general linear model, which involved temporally shifted voxel time series of one brain being linearly summed up. This connectivity study found couplings in those brain areas involved in linguistic, semantic and social processing when the story was told in English rather than Russian, which, within the context of different control conditions, can be attributed to successful communication having taken place. The speaker-listener coupling in early auditory areas was aligned to speech utterances (synchronized alignment). In addition, directed influences from the speaker’s brain to the listener’s brain and vice versa were found as well. While the speaker’s brain activity preceded the listener’s activity in posterior areas, including the right temporal-parietal junction (TPJ) and the precuneus, the listener’s brain activity preceded the speaker’s brain activity in striatum and anterior frontal areas, including the mPFC and dorsolateral prefrontal cortex (dlPFC). The extent of cortical areas where the listeners‘ activity preceded the speaker’s was strongly correlated with story comprehension, thereby highlighting the importance of prediction activity for successful communication.
Interestingly, when applying a similar unbiased voxel-by-voxel synchronization analysis to the brain activity of individuals watching the same video one after the other, Hasson et al.39 noted a tendency for individual brains to “tick collectively.” Voxel-by-voxel synchronization between individuals was found not only in primary and secondary visual and auditory areas, but also in association cortices, suggesting that the synchronization between brains reflecting not the similarity of sensory streams between the two individuals, but also between-person similarities in association processes activated during natural vision.
Neuroelectrical Recording of Interacting Brains
Following the above summary of findings obtained by using (more or less) interactive paradigms regarding the brain regions involved in different aspects of social interaction, such as agency, mentalizing, imitation, and cooperation vs. competition, we now turn to studies that have investigated the real-time dynamics of face-to-face action coordination by making use of the high temporal resolution and greater design flexibility attainable by electroencephalography (EEG).
EEG components of interpersonal action coordination
Sebanz et al.40 examined P300, an event-related potential reflecting response inhibition, in a go/no-go task that subjects performed either alone or together with a partner. More pronounced P300 was found in the joint condition, which might suggest an increase in response inhibition and can therefore be interpreted as evidence of the subject having formed a representation of the other’s action. This result is consistent with the claim that shared representations are a cornerstone of social cognition. For instance, the result may point representations of joint actions and the formation of shared intentions, as suggested by the model shown in Figure 1.
Tognoli et al.41 applied high-resolution spectral analysis to EEG data simultaneously obtained from pairs of subjects asked to make synchronous self-paced finger movements. In this way, they found a depression in occipital α and rolandic mu rhythms during movement interaction independently of whether the behavior was coordinated or not. In addition, they were able to determine two lateralized centro-parietal spectral peaks in the range between 9.2 and 11.5 Hz, which could be used to distinguish between effective and ineffective coordination, and were defined as phi1 and phi2 rhythms respectively. While a power increase in the phi2 rhythm was observed during synchronized behavior, the phi1 rhythm was enhanced during non-synchronized behavior. The authors argue that phi2 reflects the enhancement of the mirror neuron system and an inhibition of intrinsic premotor activity, whereas Phi1 reflects an inhibition of the mirror neuron system and an enhancement of intrinsic premotor activity.
Neural synchronization in interpersonal action coordination
Several recent studies on action coordination have focused on neural synchrony. The guiding hypothesis of this line of research is that synchronous brain oscillations support interpersonally coordinated behavior and social interaction through reciprocal sensory and motor feedbacks. This hypothesis builds upon theorizing and empirical observations about the functional properties of brain oscillations. Specifically, brain oscillations are: (a) are fast enough to allow information to be exchanged with the necessary speed and precision needed for interpersonal action coordination;42 (b) bind spatially distributed but functionally related neural information;43 and (c) support both perception and motor function.44-46 It follows that coherent activity between brains should be observed when interacting individuals adjust their activity patterns in the pursuit of a joint action whose successful execution requires synchronization of behavioral patterns.47-49
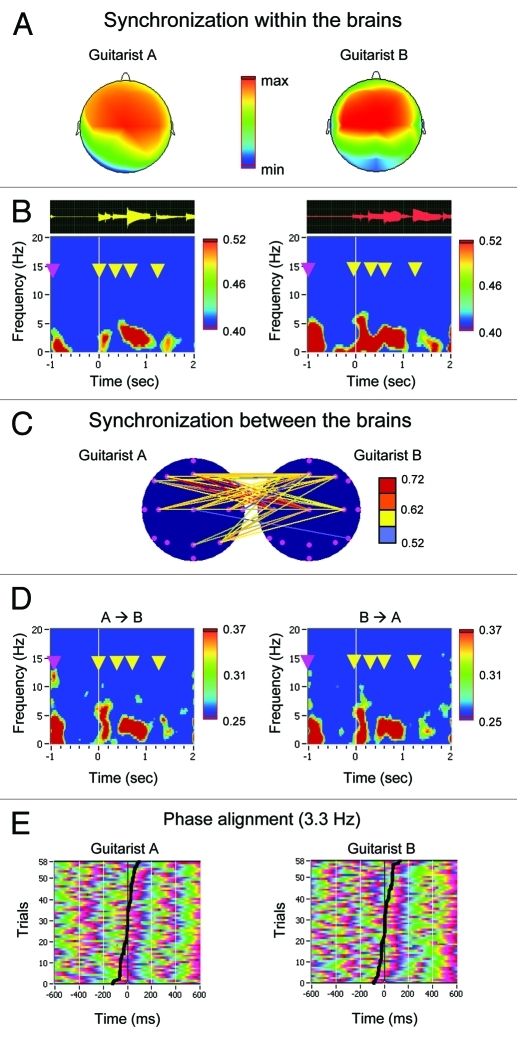
Lindenberger and his colleagues50 collected simultaneous EEG data from each of the brains of eight different pairs of guitarists playing a short melody together. The authors analyzed two measures of intra- and interbrain synchronization: The Phase Locking Index (PLI), which refers to the phase invariance at single electrodes of one brain across trials in the time-frequency domain, and Interbrain Phase Coherence (IPC), which refers to the invariance of phase difference between two electrodes of two different brains across trials. The authors found a significant increase in phase synchronization within and between the brains during periods of preparatory metronome tempo setting and at the onset of coordinated play. Interpersonally coordinated actions were thus preceded and accompanied by within- and between-brain oscillatory couplings. These couplings were observed in the delta and theta frequency ranges and were most prominent at frontal and central electrodes. In addition, the intrabrain phase alignment was strongly related to the behavioral play-onset synchrony between the two guitarists of a pair. Figure 2 summarizes some of the results obtained in this study during the onset of synchronized playing of a guitar duet. With regard to the forward model presented in Figure 1, it shows individual and joint effects interpreted as indicating synchronization within and between the brains.
Figure 2.
Example results of phase locking analyses applied to EEG data obtained from a guitar duet. (A) Topological distribution of PLI (Phase Locking Index) in the pair of guitarists (A and B) at the low theta frequency (3.3 Hz) 800 ms after guitarist A has begun to play. Fronto-central maximum of PLI is shown in both guitarists. (B) Guitar traces recorded through the microphones and time-frequency diagram of the average PLI for guitarists A and B separately. PLI was averaged across six fronto-central electrodes (F3, Fz, F4, C3, Cz, and C4). Only significant PLI values (p < 0.01) are shown. Time zero is time locked to the onset of play for the leading guitarist (A). The leading guitarist’s finger gesture to start playing together is indicated with a red arrow. The yellow arrows refer to the single guitar strokes as recorded. High phase synchronization in both guitarists took place not only at the onset of play but also at the time point of the gesture serving as a starting signal and at the individual guitar strokes. (C) Interbrain synchronization between the two guitarists measured by IPC (Interbrain Phase Coherence) at the low theta frequency (3.3 Hz) 800 ms after the onset of play. Synchrony between electrode pairs of the two guitarists is indicated by colored lines, corresponding to a significant interbrain synchronization. Only IPC values higher than 0.51 are highlighted. The interbrain coupling is highest between fronto-central electrode locations of the two guitarists. (D) Time-frequency diagram of the average IPC averaged across six electrode pairs. On the left diagram (A - > B), the selected electrode pairs represent phase coherence between one electrode of guitarist A (Cz) to the six fronto-central electrodes of guitarist B (F3, Fz, F4, C3, Cz, and C4). On the right diagram (B - > A), the selected electrode pairs represent phase coherence between one electrode of guitarist B to the six fronto-central electrodes of guitarist A. Only significant IPC-values (p < 0.01) are highlighted. (E) Phase alignment of phase angles at the frequency of 3.3 Hz across trials in guitarists A and B. Trials were sorted by behavioral onset asynchrony between the players, which is depicted by the black curve. Asynchrony is defined as the time difference (in ms) between play onsets of the two guitarists across 58 trials (see ref.51 for details).
In a second study, Dumas et al.51 investigated interbrain phase synchronization by means of simultaneous EEG recordings of interacting dyads. They applied the Phase Locking Value (PLV), which measures the adjustment of cortical phase between different cortical sites in a given time window. In synchronous episodes of a gestural imitation task, i.e., when the gestures of the two subjects started and ended simultaneously, a distributed network of neural synchronization was found between certain regions of the model’s and the imitator’s brain across the α-mu (right centro-parietal regions in both partners), β (central and right parieto-occipital regions resp.) and gamma band (centro-parietal and parieto-occipital regions, respectively). This finding can be interpreted as an indicator of complex cognitive processes that involve cortical areas not in close proximity to one another or flexible and efficient communication between these areas. Synchronization in the α band most robustly discriminated between synchrony vs. non-synchrony, which can be seen as hinting at how the mirror neuron system functions. The absence of significant differences between episodes with and without similar morphology and direction of movement is taken as evidence that synchrony does not only represent the execution and perception of similar movements. The fact that right parietal cortex apparently played an important role may suggest that it functioned as a “when pathway” linked to temporal estimation and anticipation as well as turn-taking in endogenous oscillators.
The studies by Lindenberger et al.50 and Dumas et al.51 did not attempt to ascertain directed effects between the two interaction partners. More recently, the examination of directed couplings within and between interacting brains has emerged as another way of approaching simultaneously recorded brain data of interacting subjects. With regard to within-brain analyses, this may lead to insights into how intrabrain networks function in supporting social cognition and interaction. Regarding interbrain analyses, the consideration of directed functional connections might not only reveal in how far the brains of interaction partners merge into a joint network. It also allows for tracing the direction of effects as a function of different interactional roles as it has already been done in the the fMRI studies by Schippers and Stephens and their respective colleagues.34,38
To determine directed influences, Fabio Babiloni, Laura Astolfi and colleagues have made use of multiple-person simultaneous high resolution EEG recordings (again referred to as hyperscanning29) of pairs of participants playing the Prisoner’s Dilemma52 and measured the Partial Directed Coherence (PDC), a full multivariate spectral measure based on the concept of Granger causality. They found that the mPFC was consistently activated in all behavioral situations of the Prisoner’s Dilemma (cooperation, defection, tit-for-tat) and that the ACC played a differential role in the case of defection: It was only in this condition that a high number of reliable within-brain connections emanating from the ACC was found. The authors attribute this to the generation of an accurate guess about the opponent’s behavior in a defect situation. Moreover, a higher level of global integration among the cortical areas in the α band was found for this condition compared with the others.
Using the same recording and analysis techniques, Babiloni and his colleagues investigated groups of four subjects in a second study.53 This time, the subjects played a card game involving two groups of two players, whereby the first player of each group started the game, with the respective second players then having to exploit the situation set by the first players in such a way that the chance of winning the respective round was increased. Defining activity in terms of in- and out-degree, that is, the total in- and outflow of functional links from the respective area to all the others areas in the same subject, Babiloni and colleagues found a concentration of activity in the prefrontal right hemisphere of the first players’ brains across all frequencies, as well as in sensorimotor areas in the β band. The latter is interpreted as representing the intention of putting the card on the desk. In the second players’ brains, activity was found to be more restricted to the right prefrontal cortex. This difference can be seen as representing different strategies: ACC and cingulated motor area (CMA) involvement in the first players might suggest theory of mind activity, while the correlated activity in the right PFC and parietal areas of the second players’ brains can be seen as representing the recall of pictorial material before playing the second card.
In another study, Astolfi et al.54 again used the card game design and the PDC technique to calculate interbrain connectivity. Across the seven groups analyzed, only players that belonged to the same duo showed significant functional connectivity between signals from different cortical areas. According to Granger causality analyses, ACC activity in the second players was shown to depend on the signal of different cortical areas of the respective first player, while prefrontal activity (BA8) in both brain hemispheres in the first players was found to influence the ACC and parietal regions of the corresponding second player.
Conclusions and Outlook
In this review, we presented a range of different neuroscientific designs and methods that have been used to investigate the brain basis of social interaction. We discussed the results of imaging studies that examined single subjects interacting either with computers, virtual or real counterparts to reveal the brain regions involved in the social-cognitive functions of agency,28,32 cooperation and competition,25,28,30 the intentional stance,26 self-relevance and the interpretation of facial expressions.23,24 Based on investigations using simultaneous neuroelectrical recordings of two subjects, we reviewed the EEG components associated with the formation of shared action representations40 and movement coordination,41 and also reported evidence indicating that brain oscillations synchronize within and between the brains when people engage in different forms of action coordination.50,51 Furthermore, we presented findings on directed functional intra- and interbrain coupling provided by EEG hyperscanning during games of cooperation and competition52,53 and by sequential fMRI scanning of communicative partners respectively.34,38 As all of these aspects are important facets of social interaction, the neural correlates found in association with them will make a crucial contribution to the general understanding of the neural basis of social interaction accordingly - certainly as a function of the degree of “interactivity” of the respective designs, in part at least.
The majority of the studies reviewed here showed the crucial role of the prefrontal cortex in social interaction and particularly in interbrain coupling. In addition, several regions of parietal cortex, which together with prefrontal cortices form the frontoparietal network, are involved in interbrain interactions. Thus, synchronization found between the brains is not only due to a similar sensory stream, but can also be attributed to association processes activated during social interaction.
The data reviewed in this article support the general idea that specific brain regions are activated and synchronized in two or more individuals during joint action. However, the present review also yields the impression that the behaviors and neural mechanisms that support joint action, be it on the basis of the forward model shown in Figure 1 or on the basis of some other model, are not yet well understood. Specifically, the neural mechanisms supporting emulation of others and joint action with others in real time remain unclear. In the following, we suggest three dimensions that seem critical for improving our understanding of the neural basis of joint action.
First, a major challenge in studying interpersonal action coordination is to reconcile the dynamics of the phenomenon with the requirements of experimental control. Real-life social interactions are spontaneous, reciprocal, and multimodal, and thereby pose great challenges to experimental design and the ability to draw causal inferences. Future research needs to strike a balance between primarily exploratory studies of relatively unconstrained joint actions, such as in conversation (as it has been studied by Rotondo and Boker regarding behavioral synchronization55) or musical improvisation, and more constrained settings that test the relative importance of specific input modalities or brain areas for well-defined aspects of joint action outcomes.
Second, there is a need for studies that assess the target behavior as well as the behavioral cues exchanged between the interaction partners in real time, and relate these measures to neural synchronization within and between brains. Interbrain synchronization during interpersonal action coordination clearly depends on multimodal perceptual cues (e.g., gestures, facial expressions, movements), but the relation between these cues and interbrain synchronization is rarely assessed or analyzed. Autonomous physiological responses and their role in social interaction have recently been investigated by Müller and Lindenberger56 using the example of a small choir. Their results suggest that the oscillatory coupling of cardiac and respiratory patterns provide a physiological basis for interpersonal action coordination.
Third, statistical methods used to infer causality in the context of between-brain relations need to be critically examined and further developed. Granger Causality, which has been applied in several investigations of directed functional brain connectivity reviewed here, was originally developed in the social sciences and econometrics.35 The lack of a biologically based generative model is a major limitation when applying this method in neuroscience because it may lead to the estimation of spurious causal connections due to differences in the hemodynamic lags of brain regions.37,57 In addition, Granger Causality has been criticized for disregarding contemporaneous relations between neural regions, thereby rendering biased estimates.58,59 Dynamic causal modeling (DCM),60 Unified Structural Equation Modeling (uSEM),61 and machine learning approaches such as Dynamic Bayesian Network (DBN) Inference62 may provide potentially more powerful and valid statistical tools than Granger Causality, and should be used more frequently and comparatively to examine synchronization of neural, physiological, and behavioral signals in the course of joint action. Informed by the work of Kitzbichler et al.,63 Müller and Lindenberger56 recently derived a new frequency-resolved, single-trial measure of directed phase coupling. Their Integrative Coupling Index (ICI) combines different quantifications of in-phase synchronization between physiological time-series such that an asymmetric estimate of the direction of phase coupling is obtained.
In concert, ecologically valid yet well-controlled experimental paradigms data, dense and simultaneous assessments of neural, other physiological and behavioral responses, and the use of adequate statistical methods may help to unravel the mechanisms that permit and regulate interpersonal action coordination.
Acknowledgments
This work was supported by the Max Planck Society. Johanna Sänger is supported by the International Max Planck Research School “The Life Course: Evolutionary and Ontogenetic Dynamics” (LIFE, www.imprs-life.mpg.de; participating institutions: MPI for Human Development, Humboldt-University Berlin, Free University Berlin, University of Michigan, University of Virginia, and the University of Zurich).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17934
References
- 1.Hari R, Kujala MV. Brain basis of human social interaction: from concepts to brain imaging. Physiol Rev. 2009;89:453–79. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- 2.Allport FH. Social psychology. Boston: Houghton Mifflin, 1924. [Google Scholar]
- 3.Argyle M, Cook M. Gaze and mutual gaze. Cambridge: Cambridge University Press, 1976. [Google Scholar]
- 4.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/S0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher S. Two problems of intersubjectivity. J Conscious Stud. 2009;16:289–308. [Google Scholar]
- 6.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jaegher H, Di Paolo E, Gallagher S. Can social interaction constitute social cognition? Trends Cogn Sci. 2010;14:441–7. doi: 10.1016/j.tics.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Nagao K, Takeuchi A. Social interaction: multimodal conversation with social agents. Proceedings of the 12th national conference on Artificial Intelligence (AAAI-94). Seattle, Washington: The MIT Press, 1994:22-8. [Google Scholar]
- 9.Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends Cogn Sci. 2006;10:70–6. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.De Jaegher H, Di Paolo E. Participatory sense-making: an enactive approach to social cognition. Phenom Cogn Sci. 2007;6:485–507. [Google Scholar]
- 11.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 12.Anokhin PK. Beiträge zur allgemeinen Theorie des funktionellen Systems. Jena: Gustav Fischer Verlag, 1978. [Google Scholar]
- 13.Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–79. doi: 10.1016/S0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 14.Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nat Rev Neurosci. 2001;2:561–7. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- 15.Pacherie E, Dokic J. From mirror neurons to joint actions. Cogn Syst Res. 2006;7:101–12. doi: 10.1016/j.cogsys.2005.11.012. [DOI] [Google Scholar]
- 16.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–70. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 17.Knoblich G, Sebanz N. Evolving intentions for social interaction: from entrainment to joint action. Philos Trans R Soc Lond B Biol Sci. 2008;363:2021–31. doi: 10.1098/rstb.2008.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105:16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nat Rev Neurosci. 2007;8:547–58. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 22.Lee RF, Weiming D, Dix W. A twin-volume head coil for fMRI to study two interacting brains in one scanner. Neural Engineering, 2009 NER '09 4th International IEEE/EMBS Conference, 2009:167-70. [Google Scholar]
- 23.Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, et al. Being with virtual others: neural correlates of social interaction. Neuropsychologia. 2006;44:718–30. doi: 10.1016/j.neuropsychologia.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 24.David N, Bewernick BH, Cohen MX, Newen A, Lux S, Fink GR, et al. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. J Cogn Neurosci. 2006;18:898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- 25.McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci USA. 2001;98:11832–5. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16:814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- 27.Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. J Cogn Neurosci. 2004;16:1854–63. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- 28.Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: a fMRI investigation. Neuroimage. 2004;23:744–51. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montague PR, Berns GS, Cohen JD, McClure SM, Pagnoni G, Dhamala M, et al. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 2002;16:1159–64. doi: 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- 30.King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 31.Funane T, Kiguchi M, Atsumori H, Sato H, Kubota K, Koizumi H. Synchronous activity of two people's prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. J Biomed Opt. 2011;16:077011. doi: 10.1117/1.3602853. [DOI] [PubMed] [Google Scholar]
- 32.Decety J, Chaminade T, Grezes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15:265–72. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- 33.Guionnet S, Nadel J, Bertasi E, Sperduti M, Delaveau P, Fossati P. Reciprocal imitation: toward a neural basis of social interaction. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr177. In press. [DOI] [PubMed] [Google Scholar]
- 34.Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci USA. 2010;107:9388–93. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–38. doi: 10.2307/1912791. [DOI] [Google Scholar]
- 36.Gourévitch B, Bouquin-Jeannes RL, Faucon G. Linear and nonlinear causality between signals: methods, examples and neurophysiological applications. Biol Cybern. 2006;95:349–69. doi: 10.1007/s00422-006-0098-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, et al. Network modelling methods for FMRI. Neuroimage. 2011;54:875–91. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 38.Stephens GJ, Silbert LJ, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci USA. 2010;107:14425–30. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–40. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- 40.Sebanz N, Knoblich G, Prinz W, Wascher E. Twin peaks: an ERP study of action planning and control in co-acting individuals. J Cogn Neurosci. 2006;18:859–70. doi: 10.1162/jocn.2006.18.5.859. [DOI] [PubMed] [Google Scholar]
- 41.Tognoli E, Lagarde J, DeGuzman GC, Kelso JA. The phi complex as a neuromarker of human social coordination. Proc Natl Acad Sci USA. 2007;104:8190–5. doi: 10.1073/pnas.0611453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roelfsema PR, Engel AK, Konig P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–61. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- 43.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 44.Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA. 1993;90:4470–4. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makeig S, Jung TP. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Brain Res Cogn Brain Res. 1996;4:15–25. doi: 10.1016/0926-6410(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 46.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–45. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–64. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–60. doi: 10.1016/S0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 50.Lindenberger U, Li SC, Gruber W, Muller V. Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 2009;10:22. doi: 10.1186/1471-2202-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. Inter-brain synchronization during social interaction. PLoS ONE. 2010;5:e12166. doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babiloni F, Astolfi L, Cincotti F, Mattia D, Tocci A, Tarantino A, et al. Cortical activity and connectivity of human brain during the prisoner's dilemma: an EEG hyperscanning study. Conference Proceedings: 29th Annual International Conference of the IEEE. Engineer Med Biol Soc 2007; 2007:4953-6. [DOI] [PubMed] [Google Scholar]
- 53.Babiloni F, Cincotti F, Mattia D, De Vico Fallani F, Tocci A, Bianchi L, et al. High resolution EEG hyperscanning during a card game. Conference Proceedings: 29th Annual International Conference of the IEEE. Engineer Med Biol Soc 2007; 2007:4957-60. [DOI] [PubMed] [Google Scholar]
- 54.Astolfi L, Toppi J, De Vico Fallani F, Vecchiato G, Salinari S, Mattia D, et al. Neuroelectrical hyperscanning measures simultaneous brain activity in humans. Brain Topogr. 2010;23:243–56. doi: 10.1007/s10548-010-0147-9. [DOI] [PubMed] [Google Scholar]
- 55.Rotondo J, Boker SM. Behavioral synchronization in human conversational interaction. Mirror neurons and the evolution of brain and language. In: Stamenov M G, V, ed. Mirror neurons and the evolution of brain and language, Advances in Consciousness Research. Amsterdam: John Benjamins Publishing Company, 2002:151-62. [Google Scholar]
- 56.Müller V, Lindenberger U. Cardiac and respiratory patterns synchronize between persons during choir singing. PLoS ONE. 2011 doi: 10.1371/journal.pone.0024893. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gates KM, Molenaar PC, Hillary FG, Ram N, Rovine MJ. Automatic search for fMRI connectivity mapping: an alternative to Granger causality testing using formal equivalences among SEM path modeling, VAR, and unified SEM. Neuroimage. 2010;50:1118–25. doi: 10.1016/j.neuroimage.2009.12.117. [DOI] [PubMed] [Google Scholar]
- 59.Gates KM, Molenaar PC, Hillary FG, Slobounov S. Extended unified SEM approach for modeling event-related fMRI data. Neuroimage. 2011;54:1151–8. doi: 10.1016/j.neuroimage.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 60.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Zhu W, Chang L, Bentler PM, Ernst T. Unified structural equation modeling approach for the analysis of multisubject, multivariate functional MRI data. Hum Brain Mapp. 2007;28:85–93. doi: 10.1002/hbm.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajapakse JC, Zhou J. Learning effective brain connectivity with dynamic Bayesian networks. Neuroimage. 2007;37:749–60. doi: 10.1016/j.neuroimage.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Kitzbichler MG, Smith ML, Christensen SR, Bullmore E. Broadband criticality of human brain network synchronization. PLOS Comput Biol. 2009;5:e1000314. doi: 10.1371/journal.pcbi.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]