Abstract
Advances in genomics have revealed that many genes implicated in the nervous systems of bilaterians were already present in the last common ancestor (LCA) of animals, and some even before that.1-5 This new information coincides with a growing reinterpretation of cnidarian nervous systems which holds that they are ‘fundamentally conventional’ with regards to bilaterian nervous systems,6 and do not represent ancient forms. Since in general adult forms are expected to be the most derived features of organisms, the study of non-bilaterian larval forms may be a better way to investigate potential plesiomorphies. We recently showed that voltage-gated sodium channel (Nav) genes, which make action potentials in nerves and muscles, were present in the LCA of animals and choanoflagellates, the closest unicellular relatives to animals.2 This addendum will attempt to put this finding within the context of the new views of nervous system evolution.
The New View
Studying the evolution of the first nervous systems is fraught with difficulties. The behaviors that rely on nervous function, and upon which selection acts, may be achieved by an unknown manifold of morphological and physiological means. Conversely, there appears to be widespread convergence or parallelism of nervous system traits, making homology difficult to establish.7,8 The avalanche of genomic data is beginning to shed new light on these old problems, however, just as it has done for developmental studies.9 Specifically, comparative genomic data tells us the minimal genomic content of the LCA of living taxa. It has become clear that many of the genes which are expressed in synapses,1,5 nervous system development,10 and the electrical properties of nervous systems2,11 were present in the LCA of animals or before.
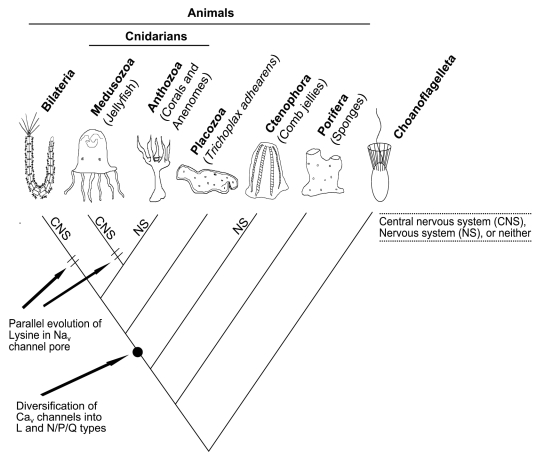
The more traditional biophysical and cell biological studies tended to focus on adult forms, and most of the work was done in medusozoan cnidarians, the true jellyfish. This was for practical reasons: amongst non-bilaterians, only these animals, with a notable exception,12 have large enough nerves for in-depth electrophysiological work. Anthozoans and ctenophores tend to have fine, diffuse nerve-nets (Fig. 1). The growing consensus from these studies, as well as developmental and behavioral studies13-16 is that medusozoan cnidarians have nervous systems which approximate bilaterian brains in their complexity. Medusozoans can navigate with visual cues,16 have fast escape responses mediated by giant axons,17 and have localized ganglia integrated with complex sense organs like the cubozoan eyes,14,15 which resemble the eyes of vertebrates and cephalopods. In other words, medusozoans may be said to have radially symmetrical central nervous systems (CNS) which are convergent with the bilaterally symmetrical CNS in bilateria.13,14 G.O. Mackie called this the “fundamental conventionality of hydromedusan neurophysiology.”6 Anthozoans do not share these features, so medusozoan CNSs may be no more “basal”, and no more indicative of the plesiomorphic condition than ours are (see Fig. 1 for phylogeny).
Figure 1 .
A provisional phylogeny based on new evidence from gene content analyses28-30 and the phylogeny of voltage-gated ion channel genes.2 The figure shows the types of nervous systems present at the tips of the branches, and places where an important amino acid replacement and gene-family expansion are supposed to have occurred.
In bilaterians and some medusozoans, Nav channels are the powerhouse behind the electrical pulses called action potentials (APs) in nerve and muscle. They encode neural signals by creating finely tuned waveforms that travel down nerves and induce cellular signaling at the synapse by activating voltage-gated calcium channels (Cav), from which they are thought to have evolved. Because of calcium’s role in intra-cellular signaling pathways, the Cav channel is the archetypical tool for transducing electrical signals carried by sodium into cellular signals, such as translational regulation, but this very same property makes them less fit to carry the main current across spaces where such signaling is not needed.18 Thus, Nav channels are specialized for APs and Cav channels, which can be divided into low and high-voltage activated groups, are specialized for sensitivity to APs. This division of labor underlies the finely tuned neural code that is present across bilaterians, almost without exception, and is present in some medusozoans.6,17
Mutagenesis experiments show that a lysine in the pore of Nav channels is critical for selectivity for sodium over calcium in bilaterians.19,20 In our paper, we showed that two medusozoans that have such a “conventional” nervous system have evolved a lysine residue in the pore of their Nav channel convergently with bilaterians, which have lysines as well but in a different domain of the protein.2 The convergence to lysine is coincident with the convergent CNSs of these two groups. Anthozoans and ctenophores do not have this lysine, and APs in these animals are less dependent on sodium.17,21
The question in this case, as in others, is what the genes did before they played a role in nerves. In this case, what did channels without a lysine in the pore do (and still do in the wide range of species that have them)? Such problems are made even trickier by the fact that there is still no consensus on the phylogeny of non-bilaterian animals.
Animal phylogeny
Almost every possible phylogenetic arrangement of non-bilaterian groups has been proposed,22-26 and some researchers have even suggested that the problem is insoluble.27 A provisional phylogeny of the lineages discussed here is represented in Figure 1. Sponges and placozoans are unique among animals in that they do not have nervous systems at any point in their life history.
A recent re-analysis of studies that had come to mutually exclusive conclusions,25 together with evidence from other studies,4,28 has solidified the placement of two lineages: Sponges appear to be the earliest branching lineage and cnidarians seem to be a sister group to bilaterians. The phylogenetic positions of ctenophores and placozoans are not fully resolved.
Our phylogeny of sodium channels agrees with this placement of sponges and cnidarians, but supports the non-traditional placement of the placozoan lineage between the ctenophore and cnidarian lineages (Fig. 1), rather than placing ctenophores with cnidarians, whom they physically resemble.25 Our study agrees with evidence from gene content analyses that found that ctenophores have a complement of homeodomain, Wnt, and nuclear receptor genes that are more similar to placozoans and sponges than to cnidaria.28-30 The complement of homeodomain genes in particular strongly suggests a placement of ctenophores between sponges and placozoans.28 We found a similar pattern in Cav channels: placozoans, cnidarians and bilaterians had all three major types of Cav channels while ctenophores only had one (Fig. 1).2
Our study admittedly suffers from low taxon sampling, which can adversely affect probabilistic phylogenetic inference,25,31 and gene presence/absence matrices are rarely used for phylogenetic inference because they rely on the non-probabilistic assumption that genes are unlikely to be lost. But if a pattern of presence/absence is present across many groups of genes, as it appears to be in ctenophores, it represents a genomic trend that informs phylogeny, especially when probabilistic methods have poor resolution.
In our phylogeny the ctenophore Cav channel grouped with N/P/Q channels, suggesting a loss of other Cav genes in this taxon rather than the presence of an ancestral channel. But this placement had very low support (bootstrap = 61) and general BLAST searches with this sequence return L-type channels, so it is possible that the channel stemmed from an ancestral channel before the separation of the N/P/Q and L type groups. In addition, ctenophore Cav and Nav channels always branched before placozoan channels with moderately high support (boostrap = 85 and 94), so we feel the representation in Figure 1 is the most likely.
Perhaps the main surprise in finding ctenophores diverging from other animals before placozoans is that ctenophores have a nervous system and musculature, while placozoans are the simplest animals morphologically and have neither. But this may not be so surprising if we consider two things: First, placozoans may be secondarily simplified, as has been previously suggested;32 Second, living adult forms may say very little about ancestral forms, as we have already seen with medusozoan cnidarians. Ctenophores are known to be capable of dissogony, where the larval stage is sexually mature; maybe the ancestor of ctenophores and placozoans more resembled a ctenophore larva than a ctenophore adult.33
Larvae
A biphasic life history, characterized by a pelagic larval stage and a benthic adult stage, is a widespread phenomenon in animals, and it may have been present in the LCA of animals.34 The importance of studying the pelagic larval forms is becoming recognized in the fields of morphological33,35,36 and nervous system evolution.37 It has even been suggested that some of the main animal lineages are derived from larval stages of earlier lineages.33,35
A biphasic life style may have been present in some form even before the LCA of animals. Some choanoflagellates have an analogous life history where cells adhere to bacterial mats, form a silaceous, cage-like “lorica,” and then bud off naked juveniles who swim away to find their own spot to settle or adhere directly to the parent’s lorica, forming small colonies.38 Choanoflagellates may therefore be a good comparison system to study pathways involved in settlement. Finally, pelagic larvae suffer massive mortality,39 and the survival of benthic adult forms depends on the larvae settling in a good spot. This suggests that most of the selection pressure since the LCA of animals to develop sensory-motor abilities may have fallen on the larval stages.
Evolution of sodium selectivity
The evolution of complex structures requires that those structures at least be advantageous enough to offset the additional cost of maintenance that they incur. Similarly, proteins are under selective constraint at every point in their evolution.40 With this in mind, the preceding discussion raises two questions: How did the nervous system arise in a stepwise fashion in pelagic larvae? How did sodium selectivity arise in a stepwise fashion from calcium selectivity? I will claim that these are answerable in a similar (and speculative) way.
Jekely37 suggests an answer to the first question. Nerves may have arisen as coordinators of cilia beating in larval phototaxis. Jekely’s elegant argument combines comparative evidence with a theory that invokes a plausible selection regime. The idea is as follows. Many eukaryotic species have photactic behavior mediated by pigment spots and ciliary motion. Volvox colonies and sponge larvae, both of which display phototaxis but don’t have nerves, allocate many cells, each with pigment and a cilium, to one sensory/motor modality.41,42 Larvae from the annelid worm Playnereis, who have nerves, can navigate with just two directionally oriented pigment cells and a few multi-ciliated cells. Nerves first arose as sensory cells that coordinated many separate ciliated cells via paracrine signals. They then formed longer processes which integrated and amplified the signal, allowing the allocation of fewer sensors to one sensory-motor pathway. Thus it is nerve’s role as a signal amplifier that made it so beneficial. The amplification of signals allowed a more complex battery of sensors to evolve while keeping the total number of cells constant, essentially replacing numerous sensors with wiring and integration.
Perhaps selection for sodium selectivity involved a similar dynamic. Cav channels evolved in eukaryotic protists to coordinate calcium signaling machinery. All-or-nothing action potentials carried by calcium are common in eukaryotes, both single and multicellular, and serve to deliver calcium in precise bursts. Cav channels mediate flagellar motion in chlorophyte algae, fertilization signals in multicellular brown algae, bioluminescence in diatoms, avoidance response in Paramecium, and probably many other functions yet to be discovered.43 Choanoflagellates have a complement of calcium signaling genes rivaling that of animals.11 Perhaps sodium selectivity arose in a choanoflagellate-like ancestor to boost the voltage of calcium signals without introducing more calcium. Such a role would be analogous to the signal amplification role of nerves.
Just as nerve’s role in signal amplification may have allowed diversification of sensors, the specialization of Nav channels for impulse conduction may have facilitated the diversification of calcium channels (the T, L, and N/P/Q-type groups), which our paper suggests happened in the common ancestor of placozoans, cnidarians, and bilaterians. The LCA of modern Nav and Cav channels was probably a Cav that produced APs. Its role in both AP production and calcium signaling precluded its becoming specialized for either, but a gene-duplication may have freed it from such constraint. This is the “escape from adaptive conflict” model of novel protein function.44,45 The final step in sodium selectivity may have come with the introduction of lysines in the pores of Nav channels. Perhaps this final step allowed the parallel development of complex nervous systems in medusozoans and bilaterians.
Acknowledgments
I would like to thank Harold H. Zakon and David M. Hillis for support and for helpful comments on the manuscript and Ammon Thompson for comments as well. I’d also like to acknowledge Andy Baxevanis and Joseph Ryan in the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health, for providing sequences from the Mnemiopsis genome and for helpful discussion on the animal phylogeny. This work was funded by National Institute of Health grant R01GM084879.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17069
References
- 1.Alié A, Manuel M. The backbone of the post-synaptic density originated in a unicellular ancestor of choanoflagellates and metazoans. BMC Evol Biol. 2010;10:34. doi: 10.1186/1471-2148-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebeskind BJ, Hillis DM, Zakon HH. Evolution of sodium channels predates the origin of nervous systems in animals. Proc Natl Acad Sci USA. 2011;108:9154–9. doi: 10.1073/pnas.1106363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards GS, Simionato E, Perron M, Adamska M, Vervoort M, Degnan BM. Sponge genes provide new insight into the evolutionary origin of the neurogenic circuit. Curr Biol. 2008;18:1156–61. doi: 10.1016/j.cub.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–6. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, et al. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE. 2007;2:e506. doi: 10.1371/journal.pone.0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacKie GO. The elementary nervous system revisited. Am Zool. 1990;30:907–20. [Google Scholar]
- 7.Nishikawa KC. Evolutionary convergence in nervous systems: insights from comparative phylogenetic studies. Brain Behav Evol. 2002;59:240–9. doi: 10.1159/000063561. [DOI] [PubMed] [Google Scholar]
- 8.Holland ND. Early central nervous system evolution: an era of skin brains? Nat Rev Neurosci. 2003;4:617–27. doi: 10.1038/nrn1175. [DOI] [PubMed] [Google Scholar]
- 9.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–8. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs DK, Nakanishi N, Yuan D, Camara A, Nichols SA, Hartenstein V. Evolution of sensory structures in basal metazoa. Integr Comp Biol. 2007;47:712–23. doi: 10.1093/icb/icm094. [DOI] [PubMed] [Google Scholar]
- 11.Cai X. Unicellular Ca2+ signaling “toolkit” at the origin of metazoa. Mol Biol Evol. 2008;25:1357–61. doi: 10.1093/molbev/msn077. [DOI] [PubMed] [Google Scholar]
- 12.Mackie GO, Mills CE, Singla CL. Giant axons and escape swimming in Euplokamis dunlapae (Ctenophora: Cydippida) Biol Bull. 1992;182:248–56. doi: 10.2307/1542118. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe H, Fujisawa T, Holstein TW. Cnidarians and the evolutionary origin of the nervous system. Dev Growth Differ. 2009;51:167–83. doi: 10.1111/j.1440-169X.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 14.Satterlie RA. Do jellyfish have central nervous systems? J Exp Biol. 2011;214:1215–23. doi: 10.1242/jeb.043687. [DOI] [PubMed] [Google Scholar]
- 15.Garm A, Ekström P, Boudes M, Nilsson D-E. Rhopalia are integrated parts of the central nervous system in box jellyfish. Cell Tissue Res. 2006;325:333–43. doi: 10.1007/s00441-005-0134-8. [DOI] [PubMed] [Google Scholar]
- 16.Garm A, Oskarsson M, Nilsson D-E. Box jellyfish use terrestrial visual cues for navigation. Curr Biol. 2011;21:798–803. doi: 10.1016/j.cub.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Anderson PAV. Evolution of the First Nervous Systems. Springer: 1990. [Google Scholar]
- 18.Hille B. Ion Channels of Excitable Membranes. Sinauer Associates: 2001. [Google Scholar]
- 19.Schlief T, Schönherr R, Imoto K, Heinemann SH. Pore properties of rat brain II sodium channels mutated in the selectivity filter domain. Eur Biophys J. 1996;25:75–91. doi: 10.1007/s002490050020. [DOI] [PubMed] [Google Scholar]
- 20.Lipkind GM, Fozzard HA. Voltage-gated Na channel selectivity: the role of the conserved domain III lysine residue. J Gen Physiol. 2008;131:523–9. doi: 10.1085/jgp.200809991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman MA, Anderson PAV. Voltage-activated ionic currents in myoepithelial cells Isolated from the sea anemone Calliactis tricolor. J Exp Biol. 1991;161:333–46. [Google Scholar]
- 22.Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 23.Schierwater B, Eitel M, Jakob W, Osigus HJ, Hadrys H, Dellaporta SL, et al. Concatenated analysis sheds light on early metazoan evolution and fuels a modern “Urmetazoon” hypothesis. PLoS Biol. 2009;7:e20. doi: 10.1371/journal.pbio.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippe H, Derelle R, Lopez P, Pick K, Borchiellini C, Boury-Esnault N, et al. Phylogenomics revives traditional views on deep animal relationships. Curr Biol. 2009;19:706–12. doi: 10.1016/j.cub.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Philippe H, Brinkmann H, Lavrov DV, Littlewood DT, Manuel M, Wörheide G, et al. Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol. 2011;9:e1000602. doi: 10.1371/journal.pbio.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddall ME. Unringing a bell: metazoan phylogenomics and the partition bootstrap. Cladistics. 2010;26:444–52. doi: 10.1111/j.1096-0031.2009.00295.x. [DOI] [PubMed] [Google Scholar]
- 27.Rokas A. Animal evolution and the molecular signature of radiations compressed in time. Science. 2005;310:1933–8. doi: 10.1126/science.1116759. [DOI] [PubMed] [Google Scholar]
- 28.Ryan JF, Pang K, NISC Comparative Sequencing Program. Mullikin JC, Martindale MQ, Baxevanis AD, et al. The homeodomain complement of the ctenophore Mnemiopsis leidyi suggests that Ctenophora and Porifera diverged prior to the ParaHoxozoa. EvoDevo. 2010;1:9. doi: 10.1186/2041-9139-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang K, Ryan JF, Mullikin JC, Baxevanis AD, Martindale MQ. Genomic insights into Wnt signaling in an early diverging metazoan, the ctenophore Mnemiopsis leidyi. EvoDevo. 2010;1:10. doi: 10.1186/2041-9139-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitzel AM, Pang K, Ryan JF, Mullikin JC, Martindale MQ, Baxevanis AD, et al. Nuclear receptors from the ctenophore Mnemiopsis leidyi lack a zinc-finger DNA-binding domain: lineage-specific loss or ancestral condition in the emergence of the nuclear receptor superfamily? . EvoDevo. 2011;2:3. doi: 10.1186/2041-9139-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedtke SM, Townsend TM, Hillis DM. Resolution of phylogenetic conflict in large data sets by increased taxon sampling. Syst Biol. 2006;55:522–9. doi: 10.1080/10635150600697358. [DOI] [PubMed] [Google Scholar]
- 32.Pearse VB, Voigt O. Field biology of placozoans (Trichoplax): distribution, diversity, biotic interactions. Integr Comp Biol. 2007;47:677–92. doi: 10.1093/icb/icm015. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen C. Six major steps in animal evolution: are we derived sponge larvae? Evol Dev. 2008;10:241–57. doi: 10.1111/j.1525-142X.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 34.Degnan SM, Degnan BM. The origin of the pelagobenthic metazoan life cycle: what’s sex got to do with it? Integr Comp Biol. 2006;46:683–90. doi: 10.1093/icb/icl028. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado M. Choanoflagellates, choanocytes, and animal multicellularity. Invertebr Biol. 2004;123:1–22. doi: 10.1111/j.1744-7410.2004.tb00138.x. [DOI] [Google Scholar]
- 36.Degnan SM, Degnan BM. The initiation of metamorphosis as an ancient polyphenic trait and its role in metazoan life-cycle evolution. Philos Trans R Soc Lond B Biol Sci. 2010;365:641–51. doi: 10.1098/rstb.2009.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jékely G. Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc Biol Sci. 2011;278:914–22. doi: 10.1098/rspb.2010.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leadbeater BSC. Developmental and ultrastructural observations on two stalked marine Choanoflagellates, Acanthoecopsis spiculifera Norris and Acanthoeca spectabilis Ellis. Proc R Soc Lond B Biol Sci. 1979;204:57–66. doi: 10.1098/rspb.1979.0012. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado M, Riesgo A. Reproductive output in a Mediterranean population of the homosclerophorid Corticium candelabrum (Porifera, Demospongiae), with notes on the ultrastructure and behavior of the larva. Mar Ecol (Berl) 2008;29:298–316. doi: 10.1111/j.1439-0485.2008.00244.x. [DOI] [Google Scholar]
- 40.Smith JM. Natural selection and the concept of a protein space. Nature. 1970;225:563–4. doi: 10.1038/225563a0. [DOI] [PubMed] [Google Scholar]
- 41.Maldonado M, Durfort M, McCarthy DA, Young CM. The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Mar Biol. 2003;143:427–41. doi: 10.1007/s00227-003-1100-1. [DOI] [Google Scholar]
- 42.Ueki N, Matsunaga S, Inouye I, Hallmann A. How 5000 independent rowers coordinate their strokes in order to row into the sunlight: phototaxis in the multicellular green alga Volvox. BMC Biol. 2010;8:103. doi: 10.1186/1741-7007-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verret F, Wheeler G, Taylor AR, Farnham G, Brownlee C. Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol. 2010;187:23–43. doi: 10.1111/j.1469-8137.2010.03271.x. [DOI] [PubMed] [Google Scholar]
- 44.Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–24. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- 45.Storz JF. Genome evolution: Gene duplication and the resolution of adaptive conflict. Heredity. 2009;102:99–100. doi: 10.1038/hdy.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]