Abstract
The siliceous skeletal elements of the sponges, the spicules, represent one of the very few examples from where the molecule toolkit required for the formation of an extracellular mineral-based skeleton, has been elucidated. The distinguished feature of the inorganic matrix, the bio-silica, is its enzymatic synthesis mediated by silicatein. Ortho-silicate undergoes in the presence of silicatein a polycondensation reaction and forms bio-silica under release of reaction water. The protein silicatein aggregates non-covalently to larger filaments, a process that is stabilized by the silicatein-associated protein, silintaphin-1. These structured clusters form the axial filament that is located in the center of the spicules, the axial canal. Surprisingly it has now been found that the initial axial orientation, in which the spicules grow, is guided by cell processes through evagination. The approximately two µm wide cell extensions release silicatein that forms the first organic axial filament, which then synthesizes the inner core of the siliceous spicule rods. In parallel, the radial growth of the spicules is controlled by a telescopic arrangement of organic layers, into which bio-silica and ortho-silicate are deposited. Hence, the formation of a mature siliceous spicule is completed by a centrifugal accretion of bio-silica mediated by the silicatein in the axial filament, and a centripetal bio-silica deposition catalyzed by the extra-spicular silicatein. Finally this contribution highlights that for the ultimate determination of the spicule shapes, their species-specific morphologies, bio-silica hardens during a process which removes reaction water. The data presented can also provide new blueprints for the fabrication of novel biomaterials for biomedical applications.
Keywords: siliceous spicules, silicatein, evagination, bio-silica, aquaporin, axial/radial growth
Introduction
Sponges [phylum Porifera; including the siliceous sponges Demospongiae and Hexactinellida and the calcareous sponges, the Calcarea] are the oldest and the only multicellular animal taxon still existing today. They evolved first from the common ancestor of all metazoan phyla [Urmetazoa]reviewed in,1 a finding that has now been widely confirmed after painful discussions.2,3 This elucidation bases on molecular biological data obtained from those proteins that are provided with structural and functional properties.4 However, this positioning of the sponges also implies that all animals which comprise a mineralized skeleton, have a common organization of their major reinforcing mineralized skeletal systems.5 The common principle of both, the silica- (sponge spicules) and the calcium-based skeletal structures (vertebrate bone or molluscan shell) is the composition of an inorganic architectural material that displays unusual combinations of mechanical properties (e.g., as strength, stiffness, and toughness).6 The formation of the sponge silica structures, the spicules, and the vertebrate bone skeletons are ruled by permanent anabolic and catabolic re-modeling processes.
The formation of the spicules in siliceous sponges involves an exceptional process, the enzymatic polycondensation of amorphous silica.7 This inorganic polymer, formed of siloxane bonds under simultaneous release of reaction water, is mediated by the anabolic enzyme silicatein.8,9 The reverse, catabolic reaction, the hydrolysis, is catalyzed by the silicase.10 Because this silica material is metabolically formed by silicatein, it has been termed bio-silica.7 Silicatein is located in the central axial canal of the spicules, where it forms multimers.11,12 The stabilization of these aggregates is controlled by silintaphin-1, a non-enzymatically acting structural protein.13,14 Recently, a second silicatein-associated protein has been described, silintaphin-2, a protein that binds Ca2+ cations that are required in the extra-spicular space for the formation of the organic cylinder around the growing spicule. After having been described as the major proteinaceous component of the axial filament silicatein has later been identified also in the extra-spicular space.15,16 As outlined below, the silicateins present in the intra-spicular axial filament and the extra-spicular space [the mesohyl/the extra-cellular bulky matrix] allow and control spicule growth. The major cell biological studies contributing to our understanding of the spicule formation have been performed with the demosponge Suberites domuncula.16
Cell-based formation of spicules
Until recently it was not possible to define cell types in sponges in a strict manner.17 Now molecular markers have been worked out that allow also the distinction of some differentiation steps of stem cells to the sclerocyte lineage, those cells that are involved in the formation of spicules; they are positive for silicatein.18 Within those cells the spicules are initially formed, either within the cell nucleus or within specific organelles, the silicasomes (Fig. 1A).16,19,20 This observation has first been seen in S. domuncula and has subsequently been confirmed for the homosclerophorid Corticium candelabrum.21 It is conceivable that not the complete spicules, most of them have sizes larger than 100 µm, can be formed intracellularly. The spicules are after having reached sizes around 7 µm are extruded from the sclerocytes into the extracellular space, where the final synthesis and the shaping of the spicules is performed.
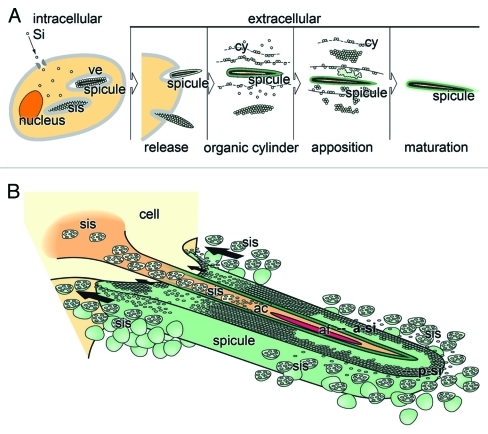
Figure 1.
Schematic outline of the spicule formation in S. domuncula. (A) Intracellularly, spicules are formed in vesicles (ve). The ortho-silicate (Si) is taken up by the cells and chanaled into those vesicles, also termed silicasomes (sis). The spicules are subsequently released into the extracellular space, where it grows by formation of organic lamellae/cylinder (cy) that are concentrically located around the growing spicule. After the appositional layering of silica lamellae the mature spicule is formed after fusion of the lamellae to a solid rod. (B) Axial growth process of the spicule via evagination of one cell protrusion and bioinorganic deposition of bio-silica. The cell (sclerocyte) extends into the axial canal and releases silicasome (sis). In the axial canal (ac) the silicasomes (sis) deposit the central, axial bio-silica lamellae (a-si) during the polycondensation reaction that is driven by silicatein and it substrate ortho-silicate. From the extra-spicular space silicasomes that are secreted from those cells that surround the growing spicule, release ortho-silicate and silicatein that deposit bio-silica onto the surface under formation of the peripheral bio-silica rod (p-si). Finally the two siliceous cylinders fuse together and form one solid rod. The arrows mark the direction of the retroaction between the cell and the spicule resulting in a translocation of the growing spicule, away from the synthesizing cell.
Silica is actively taken up by a Na+/HCO3-[Si(OH)4] cotransporter.22 In the first steps silicatein is synthesized as a pro-enzyme (signal peptide-propeptide-mature enzyme: 36.3 kDa) and processed via the 34.7 kDa form (propeptide-mature enzyme) to the 23 kDa mature enzyme. Very likely during the transport through the endoplasmic reticulum and the Golgi complex, silicatein undergoes phosphorylation and is transported into vesicles where it forms rods, the axial filaments (Fig. 1A). After assembly to filaments the first layer(s) of silica is (are) formed. After having reached a size of about seven µm the immature spicules are extruded from the sclerocytes.16 There, the bio-silica deposition occurs in two directions; first, driven by the silicateins in the axial filament in axial direction (Fig. 1B), and second, in radial, perpendicular direction which results in the thickening of the spicules (Fig. 2).
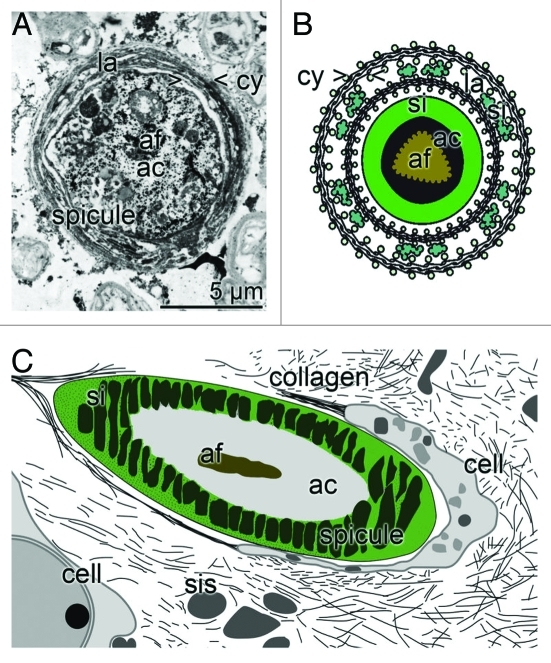
Figure 2.
Demosponge siliceous spicule formation; appositional lamellar growth. (A) During the radial growth of the spicules an organic cylinder (> < cy) is formed into which both silicatein and bio-silica is deposited. In the center an axial filament (af) is located that is harbored by the axial canal (ac). Cross section that had been contrasted with immunogold labeled anti-silicatein antibodies; TEM. (B) Appositional telescopic arrangement of radial, organic cylinders (cy) which become increasingly filled with bio-silica (si), formed form ortho-silicate and the polymerizing enzyme silicatein. In the center is the axial filament (af) located in the axial canal (ac). (C) Schematic section through sponge tissue showing the cut though spicule and the surrounding silica envelope (si). In the center the axial filament (af) and the axial canal (ac) is located. Around the spicule the sclerocytes are arranged, that release the silicasomes (sis) and also collagen.
Evagination of cells controls bio-silica formation and maturation during axial spicule growth
Invagination and also evagination processes are not rare in metazoans. A well established classical system for a cell-layer invagination process is the amphibian gastrulation,23 a cell migration process during which the cells from the vegetal pole migrate into the blastocoel and form the endoderm.24 Evagination processes occur on tissue level, e.g., in Hydra bud evagination when the cells in the epithelial tissue change their cleavage direction and, in turn, their movement resulting in the formation of evaginating centers,25 or on the cellular level, as during dendritic evaginations in the nerve system.26
Somehow surprising was the recent finding that it is cell protrusions that direct the axial growth of the spicules along the axial filament.27 Before this discovery it had been proposed that the organization of the axial filament, based on the stoichiometric aggregation of the silicateins (pentamer) with one single silintaphin-1 molecule, is the key process of the initial axial growth of the spicule.12 The application of the primmorph system, a three-dimensional cell/tissue culture, allowed for the first time a study under controlled laboratory conditions. Previously, by using intact animals, such an evagination process had not been seen, since the growth of the spicules in animals is too fast.19 Studying the freshwater sponge Ephydatia muelleri it had been shown that a spicule of an average length of 200–350 µm and a thickness of 15 µm is completely formed during one day. Now, using this primmorph system and analyzing sections through primmorphs by TEM cellular protrusions could be identified in the axial direction of the growing spicule within the axial canal (Fig. 1B).27 At the terminus of one cell process within one axial canal of a growing spicule the axial filament is formed/elongated, filling the space between the cell surface and the inner surface of the axial canal. Toward the closed, distal, terminus of the axial canal (diameter of 1–2 µm) the axial filament condenses and appears as a 0.5–1 µm solid cord.
It is assumed that the initial, intracellularly formed primordial spicules are extruded from the cells via evagination (Fig. 1B), a process which is presumably driven by hydro-mechanical forces. These forces are the result of differences in the resistance forces of the cell membrane and the forces that originate from the intracellular composition of the (macro)molecules and the osmotic pressure. And finally, not to forget, also the enzymatic bio-silica polycondensation causes tension forces, which result from the processes of bio-silica formation. Those interactions exert a retroaction between the cells and the spicule with the consequence that the cells and/or the spicules are shoved in opposite directions. Consequently, this cellular mechanism allows bio-silica deposition and – in parallel – a directed migration of the spicule-forming cells away from the growing spicule, leaving behind the spicule growing in axial orientation.
Radial growth of the spicules by appositional layering of bio-silica
The bio-silica shell of the spicule is synthesized from two directions. First, the inner layer around the axial filament forms the axial canal. This precedes the secondary the thickening process of the spicule. As described already in 2005/2006 the radial growth of the spicule proceeds by formation of organic cylinders that are telescopically arranged (Fig. 2A and B).16,28 The extracellularly existing silicatein molecules constitute together with galectin concentric cylinders within which bio-silica is deposited during the enzyme function of silicatein and the condensation of ortho-silicate. Immunogold electron microscopic analyses revealed that the silicatein molecules are lined up along strings, which are organized in parallel to the surfaces of the spicules. In the presence of Ca2+ silicatein associates with galectin and allows the appositional growth of the spicules. Evidence has been presented that the targeted delivery of Ca2+ to the region of organic cylinder formation is mediated by the cation-binding silintaphin-2.29 Since also the surface of a new siliceous spicule is covered with silicatein, the appositional growth/thickening of a spicule proceeds apparently from two directions [centrifugal and centripetal]. During the process of extra-spicular appositional bio-silica deposition, which forms a new lamella on a pre-formed and a pre-existing completed bio-silica surface, the spicule grows in radial, centrifugal direction. Finally, in demosponges the individual lamellae fuse together to a solid rod.30
Extracellular phase (shaping) of the spicules
The morphology of sponge spicules is strictly species-specifically determined. This implies that the synthesis is genetically determined. The elucidation of the gene sequences and the identification of the array of proteins, controlling the species-specific sizes, shapes and also special locations of the extracellularly formed skeletal structures is a complex task. At present the major proteins involved in the enzymatic formation of the silica structures, silicatein,14 silintaphin-1 and silintaphin-2, are well defined and functionally characterized.13,29 The subsequent task is to identify the soluble or structural molecules that direct the organic cylinders to form the intricate spicules. Again, data gathered with the S. domuncula model strongly suggested that the galectin-containing strings are organized by collagen fibers to net-like structures (Fig. 2C).28 It is very likely that collagen, which is released by specialized cells the collencytes, provides the organized platform for the morphogenesis of the spicules. Recently, it was shown that bio-silica undergoes after its enzymatic formation a process of hardening/aging via removal of reaction water through aquaporin pores.31 During this process of syneresis new siloxane bonds are formed that give the bio-silica product a tougher mechanical quality.
Outlook
The elucidation of the different phases of spiculogenesis and of the molecules involved allows now a wide application of silicatein in the field of nano-biotechnology. In sponges silica deposition is a biologically controlled process. The key enzyme is silicatein which is non-covalently linked to galectin. The organized process of biosilica deposition is very likely guided by an organic matrix, collagen. This principle has been utilized e.g., by the group of Tremel to synthesize biosilica on a gold surface (matrix) from monomeric precursors.32-34 The matrix used had been activated by cysteamine to allow binding to a reactive polymer; in turn this polymer is able to chemisorb the nitrilotriacetic acid ligand. Finally, this architecture facilitated the binding of histidine-tagged silicatein. Surprisingly, silicatein immobilized on this matrix has the capacity to catalyze bio-titania and bio-zirconia from the monomeric metal oxide precursors. This is a further striking example to show that nature can be used as a biological blueprint for nanobiotechnological applications. In addition, this technology introduces a new concept, the synthesis of inorganic polymers by an organic molecule (silicatein). Just In opposite, in 1828 Wöhler succeeded with his epochal experiments on the synthesis of urea, an organic compound, from inorganic basic materials [“die künstliche Erzeugung eines organischen, und zwar animalischen, Stoffes aus unorganischen Stoffen”].35
Acknowledgments
This work was supported by grants from the European Commission (PROJECT N. 266033244967 “SPECIAL”; X.H. W.), the Bundesministerium für Bildung und Forschung Germany (project “Center of Excellence BIOTECmarin”; W.E.G. M.), the International Human Frontier Science Program (W.E.G. M.), the International S and T Cooperation Program of China (Grant No. 2008DFA00980; X.H. W.) and the Public Welfare Project of Ministry of Land and Resources of the People’s Republic of China (Grant No. 201011005–06; X.H. W.). W.E.G. M. is holder of an ERC Individual Advanced Grant (no 268476 BIOSILICA).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17090
References
- 1.Müller WEG. How was the metazoan threshold crossed? The hypothetical Urmetazoa. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:433–60. doi: 10.1016/S1095-6433(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–6. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schierwater B, Eitel M, Jakob W, Osigus J, Hadrys H, Dellaporta L, et al. Concatenated analysis sheds light on early metazoan evolution and fuels a modern “Urmetazoon” hypothesis. PLoS Biol. 2009;7:e20. doi: 10.1371/journal.pbio.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller WEG. Molecular phylogeny of metazoa [Animals] (1995) Monophyletic origin. Naturwiss. 1995;82:321–9. doi: 10.1007/BF01131528. [DOI] [PubMed] [Google Scholar]
- 5.Müller WEG. Spatial and temporal expression patterns in animals. In: Meyers RA, ed. Encyclopedia of Molecular Cell Biology and Molecular Medicine. Weinheim, WILEY-VCH Press, Vol.13, 2005; pp. 269-309. [Google Scholar]
- 6.Mayer G. Rigid biological systems as models for synthetic composites. Science. 2005;310:1144–7. doi: 10.1126/science.1116994. [DOI] [PubMed] [Google Scholar]
- 7.Morse DE. Silicon biotechnology: harnessing biological silica production to construct new materials. Trends Biotechnol. 1999;17:230–2. doi: 10.1016/S0167-7799(99)01309-8. [DOI] [Google Scholar]
- 8.Cha JN, Shimizu K, Zhou Y, Christianssen SC, Chmelka BF, Stucky GD, et al. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc Natl Acad Sci USA. 1999;96:361–5. doi: 10.1073/pnas.96.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krasko A, Batel R, Schröder HC, Müller IM, Müller WEG. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur J Biochem. 2000;267:4878–87. doi: 10.1046/j.1432-1327.2000.01547.x. [DOI] [PubMed] [Google Scholar]
- 10.Schröder HC, Krasko A, Le Pennec G, Adell T, Wiens M, Hassanein H, et al. Silicase, an enzyme which degrades biogenous amorphous silica: contribution to the metabolism of silica deposition in the demosponge Suberites domuncula. Prog Mol Subcell Biol. 2003;33:249–68. doi: 10.1007/978-3-642-55486-5_10. [DOI] [PubMed] [Google Scholar]
- 11.Murr MM, Morse DE. Fractal intermediates in the self-assembly of silicatein filaments. Proc Natl Acad Sci USA. 2005;102:11657–62. doi: 10.1073/pnas.0503968102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller WEG, Boreiko A, Schloßmacher U, Wang XH, Tahir MN, Tremel W, et al. Fractal-related assembly of the axial filament in the demosponge Suberites domuncula: relevance to biomineralization and the formation of biogenic silica. Biomaterials. 2007;28:4501–11. doi: 10.1016/j.biomaterials.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Wiens M, Wang XH, Schröder HC, Kolb U, Schloßmacher U, Ushijima H, et al. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials. 2010;31:7716–25. doi: 10.1016/j.biomaterials.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Schlossmacher U, Wiens M, Schröder HC, Wang XH, Jochum KP, Müller WEG. Silintaphin-1: interaction with silicatein during structure guiding biosilica formation. FEBS J. 2011;278:1145–55. doi: 10.1111/j.1742-4658.2011.08040.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu K, Cha J, Stucky GD, Morse DE. Silicatein alpha: cathepsin L-like protein in sponge biosilica. Proc Natl Acad Sci USA. 1998;95:6234–8. doi: 10.1073/pnas.95.11.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller WEG, Rothenberger M, Boreiko A, Tremel W, Reiber A, Schröder HC. Formation of siliceous spicules in the marine demosponge Suberites domuncula. Cell Tissue Res. 2005;321:285–97. doi: 10.1007/s00441-005-1141-5. [DOI] [PubMed] [Google Scholar]
- 17.Müller WEG. The stem cell concept in sponges (Porifera): metazoan traits. Semin Cell Dev Biol. 2006;17:481–91. doi: 10.1016/j.semcdb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Müller WEG, Wiens M, Müller IM, Schröder HC. The chemokine networks in sponges: potential roles in morphogenesis, immunity and stem cell formation. Prog Mol Subcell Biol. 2004;34:103–43. doi: 10.1007/978-3-642-18670-7_5. [DOI] [PubMed] [Google Scholar]
- 19.Imsiecke G, Steffen R, Custodio M, Borojevic R, Müller WEG. Formation of spicules by sclerocytes from the freshwater sponge Ephydatia muelleri in short-term cultures in vitro. In Vitro Cell Dev Biol Anim. 1995;31:528–35. doi: 10.1007/BF02634030. [DOI] [PubMed] [Google Scholar]
- 20.Schröder HC, Natalio F, Shukoor I, Tremel W, Schloßmacher U, Wang XH, et al. Apposition of silica lamellae during growth of spicules in the demosponge Suberites domuncula: biological/biochemical studies and chemical/biomimetical confirmation. J Struct Biol. 2007;159:325–34. doi: 10.1016/j.jsb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado M, Riesgo A. Intra-epithelial spicules in a homosclerophorid sponge. Cell Tissue Res. 2007;328:639–50. doi: 10.1007/s00441-007-0385-7. [DOI] [PubMed] [Google Scholar]
- 22.Schröder HC, Perović-Ottstadt S, Rothenberger M, Wiens M, Schwertner H, Batel R, et al. Silica transport in the demosponge Suberites domuncula: fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem J. 2004;381:665–73. doi: 10.1042/BJ20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeckel, E. Die Gastraea-Theorie, die phylogenetische Classification des Thierreichs und die Homologie der Keimblätter. Jen Zts Naturwiss 1874; 8:1-55 + 1 pl. (I).
- 24.Spemann H, Mangold H. Ueber Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Arch Mikr Anat Entw Mech. 1924;100:599–638. [Google Scholar]
- 25.Dübel S. Cell differentiation in the head of Hydra. Differentiation. 1989;41:99–109. doi: 10.1111/j.1432-0436.1989.tb00737.x. [DOI] [Google Scholar]
- 26.Eckenhoff MF, Pysh JJ. Double-walled coated vesicle formation: evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. J Neurocytol. 1979;8:623–38. doi: 10.1007/BF01208513. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Wiens M, Schröder HC, Schloßmacher U, Pisignano D, Jochum KP, et al. Evagination of cells controls bio-silica formation and maturation during spicule formation in sponges. PLoS ONE. 2011;6:e20523. doi: 10.1371/journal.pone.0020523. [doi:10.1371/journal.pone.0020523] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder HC, Boreiko A, Korzhev M, Tahir MN, Tremel W, Eckert C, et al. Co-Expression and functional interaction of silicatein with galectin: matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J Biol Chem. 2006;281:12001–9. doi: 10.1074/jbc.M512677200. [DOI] [PubMed] [Google Scholar]
- 29.Wiens M, Schröder HC, Wang XH, Link T, Steindorf D, Müller WEG. Isolation of the silicatein-α interactor silintaphin-2 by a novel solid-phase pull-down assay. Biochemistry. 2011;50:1981–90. doi: 10.1021/bi101429x. [DOI] [PubMed] [Google Scholar]
- 30.Wang XH, Wiens M, Schröder HC, Hu S, Mugnaioli E, Kolb U, et al. Morphology of sponge spicules: silicatein a structural protein for bio-silica formation. Advanced Biomaterials. Adv Eng Mater. 2010;12:B422–37. doi: 10.1002/adem.200980042. [DOI] [Google Scholar]
- 31.Müller WEG, Wang XH, Wiens M, Schloßmacher U, Jochum KP, Schröder HC. Hardening of bio-silica in sponge spicules involves an aging process after its enzymatic polycondensation: evidence for an aquaporin-mediated water absorption. Biochim Biophys Acta [General Subjects] 2011; 1810:713–726 [DOI] [PubMed] [Google Scholar]
- 32.Tahir MN, Théato P, Müller WEG, Schröder HC, Janshoff A, Zhang J, et al. Monitoring the formation of biosilica catalysed by histidin-tagged silicatein. ChemComm. 2004;24:2848–9. doi: 10.1039/b410283e. [DOI] [PubMed] [Google Scholar]
- 33.Tahir MN, Théato P, Müller WEG, Schröder HC, Boreiko A, Faiß S, et al. Formation of layered titania and zirconia catalysed by surface-bound silicatein. Chem Commun (Camb) 2005;44:5533–5. doi: 10.1039/b510113a. [DOI] [PubMed] [Google Scholar]
- 34.Tahir MN, Natalio F, Therese HA, Yella A, Metz N, Shah MR, et al. Enzyme-mediated deposition of a TiO2 coating onto biofunctionalized WS2 chalcogenide nanotubes. Adv Funct Mater. 2009;19:285–91. doi: 10.1002/adfm.200800841. [DOI] [Google Scholar]
- 35.Wöhler F. Ueber künstliche Bildung des Harnstoffs. Annalen der Physik und Chemie. 1828;12:253–6. [Google Scholar]