Abstract
Swarming is a group motility behavior exhibited by bacteria that coordinate to spread over surfaces. Swarms of the bacterium Pseudomonas aeruginosa often develop tendril patterns and tendril development requires production of the surfactant rhamnolipid. We recently showed that harder surfaces limit induction of quorum sensing genes including those responsible for rhamnolipid synthesis, but it is not yet clear why similar populations of cells should behave differently on hard surfaces compared with soft (agar) surfaces. Here we explore the population dynamics during P. aeruginosa swarming. We find that the population of P. aeruginosa does not immediately increase as the swarm expands. We also detail three stages of population development during swarming.
Keywords: rhamnolipid, motility, bacterial growth
Swarming is a type of surface motility exhibited by Pseudomonas aeruginosa and other bacteria where groups of organisms coordinate to expand as an entire population over new surfaces.1,2 Robust swarming is dependent upon growth of a minimum population that is induced to produce rhamnolipid via the rhl quorum sensing system of P. aeruginosa.3-6 We recently showed that P. aeruginosa surface growth and quorum sensing vary with changes to the surface environment.7 P. aeruginosa quorum sensing and rhamnolipid production are impaired when growing on harder semi-solid agar surfaces compared with growth on soft surfaces that yields robust rhamnolipid production and swarming. It is not yet understood why quorum sensing genes near the advancing swarm edge were less induced on hard agar; even addition of excess acyl homoserine lactone did not improve swarming. We have further investigated the differentiation between populations of cells growing on these two types of surfaces. Here we show that swarm zone expansion is not synchronous with population growth under conditions of optimal swarming.
Given the increased surface coverage that occurs for tendril-forming swarms, we theorized that this spreading should provide a growth advantage. Swarming was investigated on both soft and hard agar plates containing FAB-glucose.7 Plate assays were inoculated with 1 µL culture containing 2.0 ± 0.1 × 107 cfu (pregrown planktonically on FAB-glucose) and incubated at 30°C for 39 h. At designated intervals, swarms were harvested using sterile spatulas and collected in 10 mL sterile buffer. These samples were serially diluted and 10 µL of each dilution was spotted on LB plates (in triplicate) for five replicates (i.e., n = 15) and incubated at 28°C for ~24 h.
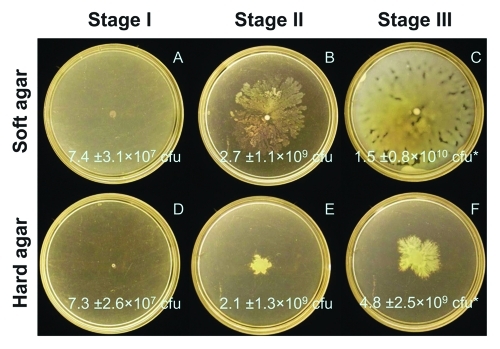
We observed that swarming expansion was not synonymous with increases in bacterial cell population. Swarms that develop on soft vs. hard agar can be differentiated into three distinct stages of behavior (Fig. 1). During Stage I (approximately the first 20 h of our experiment) there are no differences between soft and hard agar swarms. During Stage II (hours 20–30) we see expansion of soft agar swarms (Fig. 1B) but no expansion of hard agar swarms (Fig. 1E). Curiously, the total bacterial population is not different on soft and hard agar at Stage II—both swarm types have increased nearly two orders of magnitude from Stage I. During Stage III (greater than 30 h) we see a continued expansion of swarm area upon soft agar and we now observe a clear advantage in the population growth on soft agar. While hard agar swarm populations have doubled since Stage II, soft agar swarms have increased nearly 10-fold (c.f., Figure 1C and 1F).
Figure 1.
P. aeruginosa swarming over time on (A-C) soft (0.4%) and (D-F) hard (0.6%) agar FAB-glucose plates. Plate counts of colony forming units (cfu) are given for n = 12–15 measurements for an initial inocula of 2.0 ± 0.1 × 107 cfu. (A,D) Stage I (13 h) represents no differentiation between agar types; (B,E) Stage II (26 h) represents an observed difference in swarm pattern between agar types with no difference in population; and (C,F) Stage III (39 h) represents the period when both swarm pattern and overall population are distinct between agar types. *Population different by Student T-test (p = 0.0015).
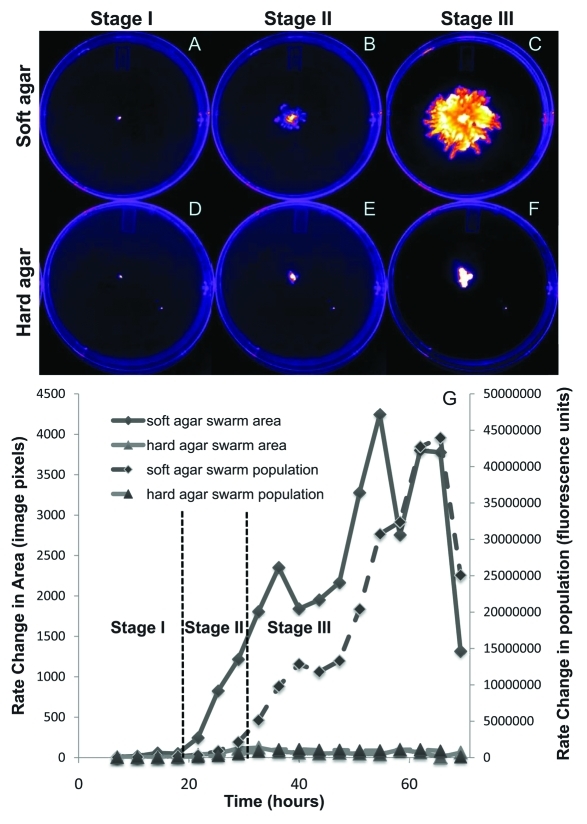
We again examined population development during swarming on soft vs. hard agar by monitoring fluorescence of GFP-labeled cells over time using a Carestream Multispectral FX image station (Woodbridge, CT). Swarm assay plates were inoculated and incubated in the image station in the dark. Because of the location of the instrument, these plates were incubated at room temperature, thus the overall growth is slightly less than the experiments shown above but the trends are the same. This non-destructive technique allowed for more frequent measurements of the same plate assay.10 Excitation and emission wavelengths for GFP and Nile Red were 480/535 nm and 540/600 nm, respectively. Images were further processed using ImageJ.8 Representative fluorescent images from this experiment are shown in Figure 2.
Figure 2.
Fluorescent imaging analysis of P. aeruginosa swarming over time on (A-C) soft (0.45%) and (D-F) hard (0.6%) agar. Representative fluorescent images from (A,D) Stage I (~0–20 h), (B,E) Stage II (20–30 h), and (C,F) Stage III (> 30 h). Fluorescence intensity from all images is pseudo-colored to display highest cell density in white. (G) Rate of change (i.e., first derivative) in the swarm area and population with time.
Results from this fluorescent monitoring experiment are similar to those presented in Figure 1, with swarm development separated into distinct stages. Figure 2G shows the changes of swarm area (determined by the sum of fluorescent pixels above background) and total bacterial population (determined by total fluorescence intensity above background) over time. During Stage I, there is no difference between these rates on soft and hard agar. During Stage II, the swarm area increases sharply upon soft agar, yet the population does not increase significantly. Lastly during Stage III, the growth rate increases on soft agar, and a growth advantage for swarming is apparent. This result is in strong agreement with those of Xavier et al.,9 who showed a significant growth advantage by bacteria swarming on soft agar over non-swarming bacteria on hard agar or a rhamnolipid-deficient mutant. Stages I, II, and III are readily distinguished by plotting the change in area and fluorescence intensity per unit time (i.e., the first derivative of the area and total fluorescence with time).
The findings we classify as Stage II, where no growth advantage is apparent between these swarm conditions were not expected. We were initially surprised that Stage II lasted for such a long duration, namely hours in our experiments. Close inspection of the cell counts, however, suggests that bacterial growth during Stages II and III of swarming is comparatively slow to the transition between Stage I to Stage II. Thus, as soft agar swarm populations produce ample rhamnolipid to aid swarming, swarm spreading becomes rapid compared with cell growth.
It is still unclear why quorum sensing and rhamnolipid production is more inhibited on hard agar despite similar populations of cells. This result, however, suggests that bacterial growth is not inhibited under poor swarming conditions. It will be useful to probe these populations further to understand the physiology of bacteria growing on optimal swarming conditions. Previous study suggests these cells are sensing a better nutrient environment and are more induced for quorum sensing.7,9
Acknowledgments
This work was supported by the Indiana Clinical and Translational Science Institute (NIH # UL1RR025761) to J.D.S and the University of Notre Dame College of Science (Summer Undergraduate Research Fellowship) to M.J.S.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17109
References
- 1.Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nat Rev Microbiol. 2008;6:466–76. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 2.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–44. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caiazza NC, Shanks RM, O'Toole GA. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol. 2005;187:7351–61. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nozawa T, Tanikawa T, Hasegawa H, Takahashi C, Ando Y, Matsushita M, et al. Rhamnolipid-dependent spreading growth of Pseudomonas aeruginosa on a high-agar medium: marked enhancement under CO2-rich anaerobic conditions. Microbiol Immunol. 2007;51:703–12. doi: 10.1111/j.1348-0421.2007.tb03959.x. [DOI] [PubMed] [Google Scholar]
- 5.Déziel E, Lépine F, Milot S, Villemur R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology. 2003;149:2005–13. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- 6.Köhler T, Curty LK, Barja F, van Delden C, Pechére JC. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–6. doi: 10.1128/JB.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamatkar NG, Shrout JD. Surface hardness impairment of quorum sensing and swarming for Pseudomonas aeruginosa. PLoS ONE. 2011;6:e20888. doi: 10.1371/journal.pone.0020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasband WS. ImageJ. U.S. National Institutes of Health, Bethesda, MD., 1997-2011.
- 9.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol. 2011;79:166–79. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris JD, Hewitt JL, Wolfe LG, Kamatkar NG, Chapman SM, Diener JM, et al. Shrout imaging and analysis of pseudomonas aeruginosa swarming and rhamnolipid production. Appl Environ Microbiol. 2011;77:8310–7. doi: 10.1128/AEM.06644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]