Abstract
The essence of neuronal function is to generate outputs in response to synaptic potentials. Synaptic integration at a synapse determines neuronal outputs in the CNS. In a recent study, we describe that excitatory and inhibitory transmitter-gated channels physically crosstalk each other at the cellular and molecular level. Increased membrane expression of ATP P2X4 receptors by using an interference peptide competing with the intracellular endocytosis motif enhances neuronal excitability, which is further enhanced by reciprocal interaction between post-synaptic ATP- and GABA-gated channels. Molecular interaction is supported by experiments of co-immunoprecipitation and mutagenesis of P2X4 subunit. Two amino acids in the intracellular carboxyl tail of P2X4 subunit appears to be responsible for this crosstalk. Our recent study provides molecular and electrophysiological evidence for physical interaction between excitatory and inhibitory receptors that appears to be crucial in determining synaptic strength at central synapses.
Keywords: Synapse, ligand-gated channels, P2X, GABA-A receptors, plasticity, crosstalk, trafficking, ATP, GABA, interaction
Fast synaptic transmission between neurons is achieved through the release of one or more neurotransmitters from the same presynaptic terminal, resulting in the activation of different classes of ligand-gated ion channels co-localized at the same post-synaptic site.1-4 Although it has been long believed that each receptor type acts independently of the other, recent studies have revealed that ATP-gated channels crosstalk with cys loop receptors in recombinant expression and cell culture preparations.5-12 Of particular interest is that an excitatory neurotransmitter, ATP is always considered a co-transmitter and is released either with an inhibitory neurotransmitter, GABA,4,13-15 or with an excitatory neurotransmitter, glutamate in the CNS.16,17 Therefore, the process of synaptic integration at mixed synapses is potentially complicated by the presence of ATP P2X receptors.
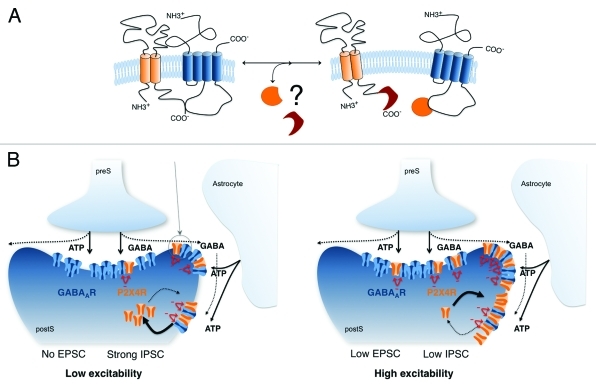
Our recent study clearly demonstrates the physical and functional interactions between excitatory ATP P2X4 and inhibitory GABAA receptors at the cellular and molecular levels.18 Their interactions appear to be critical in regulating synaptic strength at the synaptic level and, as a result, neuronal excitability (Fig. 1). A series of experiments, including co-immunoprecipitation, peptide-based pull downs, mutagenesis and overexpression of peptides in heterogeneous system provides converging evidence for a physical interaction between a specific intracellular motif (Tyr 374, Val 375) within the C-terminal tail of P2X4 subunits and GABAA β subunits. In addition, our prior studies show that the main intracellular loop of mainly GABAA/c β or ρ subunits are involved in the coupling with P2X2 or P2X3 receptors.7,8,12 Furthermore, a recent FRET study confirms the close proximity of the C-terminal tail of P2X2 subunits and intracellular loop of GABAA subunits.19 It is thus believed that the C-terminal tail of P2X subunits interacts directly with the main intracellular loop of subunits of cys loop receptor family, including GABAA, nicotinic or 5-HT3 receptors. Importantly, the interaction motif identified in P2X4 subunit is different from that identified in P2X3. These identified motifs are absent in P2X2 subunit. Likewise, the interacting regions of cys loop receptors, including GABA, nicotinic, and 5-HT3 subunits show no primary sequence homology. It thus appears that the interaction between P2X and cys loop receptors is subunit-specific. In addition to the direct interaction between two receptors, undefined interacting proteins and/or regulatory factors may trigger or alter this negative interaction since these receptors appear to act independently in some neuronal populations20 (Fig. 1).
Figure 1.
Interaction between P2X4 and GABAA receptors at a central synapse. (A) Schematic diagrams to describe the interaction between P2X4 and GABAA subunits. Unknown proteins and/or factors may regulate or initiate physical coupling between the C-terminal tail of P2X and the second intracellular loop of GABAA subunits. If this is the case, the cross-inhibition would be regulated by these factors. (B) Surface trafficking of P2X4 receptors and subsequent interaction with GABAA receptors at synapses and/or peri/extra synaptic sites downregulate inhibitory synaptic inputs. Additionally, this interaction may alter trafficking of other receptors, including GABAA receptors.
P2X4-GABAA receptor interaction results in an instantaneous and reciprocal current inhibition in recombinant expression system. In other words the amplitude of the currents evoked by concomitant application of ATP and GABA is significantly smaller than the predicted sum of the responses to separate application of ATP and GABA. Although P2X4 and GABAA receptors form separate channels in recombinant expression system, the co-activation of both receptors results in non-additive responses due to reciprocal inhibition of both channel types. It should be emphasized that this current occlusion is abrogated by mutation of Tyr374 and/or Val375 as well as by intracellular administration of a peptide corresponding to amino acid 372–377 region of P2X4 receptor (YV6). This peptide appears to bind to GABA β subunits, which in turn occludes the binding of the P2X4 receptors.
Among P2X receptors, P2X4-containing receptors constitutively cycle into and out of the membrane in a dynamin-dependent mechanism. As a result, P2X4 receptors appear to be predominantly retained in intracellular compartments.21 Blockade of P2X4 receptor internalization with a site-specific peptide increases surface expression of P2X4 subunits and the mean amplitude of ATP responses. Furthermore, increased surface expression of P2X4 receptors induces a decrease in the frequency and the amplitude of GABAergic post-synaptic currents. This depression of GABAergic currents is abolished following intracellular administration of YV6 peptide that disrupts interaction between P2X4 and GABAA receptors. We think that the physical interaction between P2X4 and GABAA receptors at the synaptic level plays an essential role in regulating inhibitory synaptic strength.
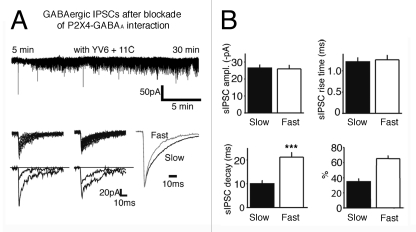
Since spontaneous P2X receptor-mediated postsynaptic currents are scarce following blockade of P2X4 receptor internalization, P2X4 receptors appear to be located mainly at peri/extrasynaptic areas in the CNS. If it is the case, P2X4 receptors may play a role in the modulation of synaptic transmission. For instance, P2X4 would preferentially interact with neighboring synaptic GABAA receptors at GABAergic synapses in the VMH. Indeed, in-depth analysis of individual sIPSCs following blockade of the interaction reveals two distinct GABAergic synaptic currents (Figs. 2A and B): 34.8 ± 4% of sIPSCs have a fast decay time course of 10 ± 1.3 ms (n = 11 neurons) and 65.2 ± 4% of sIPSCs have a slow decay phase of 21.2 ± 1.9 ms (n = 11 neurons). As the composition of GABAA subunits determines the subcellular localization as well as biophysical properties, including decay time phase of GABAA receptors,22-24 we may speculate that GABAA receptors having a slow decay phase are physically coupled with P2X4 receptors. In fact, the desensitization of αβ- or αβδ-containing GABAA receptors is slower than that of αβγ-containing GABAA receptors.25 These αβγ -containing receptors appear to be located at synaptic sites, whereas δ subunit-containing receptors or αβ-containing receptors without γ subunits are found at extrasynaptic sites.26 Furthermore, native α2- or α3 subunit-containing GABAA receptors decay more slowly than α1-expressing GABAA receptors.27 It is thus possible that P2X4 receptors would interact with synaptic GABAA receptors containing α2 or α3, β and γ subunits. This is, somewhat, consistent with prior studies showing that extrasynaptic GABAA receptors do contain neither α2 nor α3.28-30 Given that the interaction between P2X and GABA receptors influences receptor trafficking,7,19 the expression of P2X4 receptors on the membrane would be critical in altering targeting of GABAA receptors in favor of a peri/extra synaptic location.
Figure 2.
Disruption of the interaction between P2X4 and GABAA receptors increases GABAergic synaptic transmission and reveal two populations of sIPSCs. (A) Recording samples of the baseline GABAergic synaptic activity in the presence of both 11C and YV6 in the patch pipette. Analysis of individual sIPSCs recorded in the presence of 11C and YV6 revealed that there were two distinct GABAA receptor-mediated sIPSCs (fast vs. slow components). ~two third of sIPSCs had a slow decay phase. Bottom panel: Superimposition of traces of sIPSCs (left, fast sIPSCs; middle, slow sIPSCs; right, normalized traces of average amplitude of sIPSCs to shows the difference in the decay time course of sIPSCs). HP = -70 mV. (B) Summary of the basic characteristics of slow and fast sIPSCs recorded from 11 different SF-1 GFP-positive neurons (***, p < 0.0001).
In summary, the observed crosstalk between excitatory and inhibitory ligand-gated channels appears to be a novel form of short-term synaptic plasticity at central synapses. P2X4 subunit-mediated fine tuning of GABAergic transmission would contribute to the regulation of synaptic strength, thereby regulating neuronal outputs in neural circuits fundamental to feeding behavior in particular and likely in brain.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17291
References
- 1.Zander JF, Munster-Wandowski A, Brunk I, Pahner I, Gomez-Lira G, Heinemann U, et al. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–45. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–87. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, et al. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–52. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–5. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- 5.Barajas-López C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J Physiol. 1998;513:671–83. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boué-Grabot E, Barajas-Lopez C, Chakfe Y, Blais D, Belanger D, Emerit MB, et al. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci. 2003;23:1246–53. doi: 10.1523/JNEUROSCI.23-04-01246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boué-Grabot E, Emerit MB, Toulme E, Seguela P, Garret M. Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J Biol Chem. 2004;279:6967–75. doi: 10.1074/jbc.M307772200. [DOI] [PubMed] [Google Scholar]
- 8.Boué-Grabot E, Toulme E, Emerit MB, Garret M. Subunit-specific coupling between gamma-aminobutyric acid type A and P2X2 receptor channels. J Biol Chem. 2004;279:52517–25. doi: 10.1074/jbc.M410223200. [DOI] [PubMed] [Google Scholar]
- 9.Karanjia R, Garcia-Hernandez LM, Miranda-Morales M, Somani N, Espinosa-Luna R, Montano LM, et al. Cross-inhibitory interactions between GABAA and P2X channels in myenteric neurones. Eur J Neurosci. 2006;23:3259–68. doi: 10.1111/j.1460-9568.2006.04861.x. [DOI] [PubMed] [Google Scholar]
- 10.Khakh BS, Humphrey PP, Henderson G. ATP-gated cation channels (P2X purinoceptors) in trigeminal mesencephalic nucleus neurons of the rat. J Physiol. 1997;498:709–15. doi: 10.1113/jphysiol.1997.sp021895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–10. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- 12.Toulmé E, Blais D, Leger C, Landry M, Garret M, Seguela P, et al. An intracellular motif of P2X(3) receptors is required for functional cross-talk with GABA(A) receptors in nociceptive DRG neurons. J Neurochem. 2007;102:1357–68. doi: 10.1111/j.1471-4159.2007.04640.x. [DOI] [PubMed] [Google Scholar]
- 13.Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–30. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo YH, Role LW. Cholinergic modulation of purinergic and GABAergic co-transmission at in vitro hypothalamic synapses. J Neurophysiol. 2002;88:2501–8. doi: 10.1152/jn.00352.2002. [DOI] [PubMed] [Google Scholar]
- 15.Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci. 2002;22:4794–804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–23. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio ME, Soto F. Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–53. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Cross-talk between P2X4 and {gamma}-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrivastava AN, Triller A, Sieghart W, Sarto-Jackson I. Regulation of GABA(A) receptor dynamics by interaction with purinergic P2X(2) receptors. J Biol Chem. 2011;286:14455–68. doi: 10.1074/jbc.M110.165282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khakh BS, Fisher JA, Nashmi R, Bowser DN, Lester HA. An angstrom scale interaction between plasma membrane ATP-gated P2X2 and alpha4beta2 nicotinic channels measured with fluorescence resonance energy transfer and total internal reflection fluorescence microscopy. J Neurosci. 2005;25:6911–20. doi: 10.1523/JNEUROSCI.0561-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–24. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol. 2004;92:1718–27. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- 23.Koksma JJ, Fritschy JM, Mack V, Van Kesteren RE, Brussaard AB. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol Cell Neurosci. 2005;28:128–40. doi: 10.1016/j.mcn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi MT, Macdonald RL. Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol. 2002;544:3–18. doi: 10.1113/jphysiol.2002.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–78. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, et al. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–14. doi: 10.1016/S0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 28.Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, et al. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, et al. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–87. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]