Abstract
In African mormyrid fishes, evolutionary change in a sensory region of the brain established an ability to detect subtle variation in electric communication signals. In one lineage, this newfound perceptual ability triggered a dramatic increase in the rates of signal evolution and species diversification. This particular neural innovation is just one in a series of nested evolutionary novelties that characterize the sensory and motor systems of mormyrids, the most speciose group of extant osteoglossomorph fishes. Here we discuss the behavioral significance of these neural innovations, relate them to differences in extant species diversity, and outline possible scenarios by which some of these traits may have fueled diversification. We propose that sensory and motor capabilities limit the extent to which signals evolve and, by extension, the role of communication behavior in the process of speciation. By expanding these capabilities, neural innovations increase the potential for signal evolution and species diversification.
Keywords: brain evolution, speciation, key innovation, Mormyridae, electrosensory, electromotor, electric organ discharge (EOD)
Neural innovations in mormyroid electric fishes
Communication often plays a key role in animal speciation. In African weakly electric fishes (superfamily Mormyroidea), an innovative electric communication system has arisen from a number of component evolutionary novelties.1,2 Recent investigation of the influence of these novel traits on mormyroid species radiation sheds new light on the interplay among animal communication, nervous system evolution, and speciation.1
According to Pigliucci,3 “evolutionary novelties are new traits or behaviors, or novel combinations of previously existing traits or behaviors, arising during the evolution of a lineage, and that perform a new function within the ecology of that lineage.” To evolutionary developmental biologists, ‘novelty’ represents a discontinuity in homology,4 yet evo-devo also recognizes that novelties arise from pre-existing traits, often building upon deeper homologies.5 The related evolutionary concept of ‘key innovation’ emphasizes the effect that new traits can have on the tempo and breadth of species radiation. According to its most common usage, a key innovation is a trait that allows a lineage to invade underutilized ecological or phenotypic space, facilitating an increase in species diversification rate.6-8 Clearly, many component traits of electrocommunication in mormyroid fishes are novelties. In addition, some of these traits have recently been found to fit the definition of key innovation,1,2,9 as they are associated with dramatic increases in rates of species diversification and phenotypic (signal) divergence10 (Fig. 1).
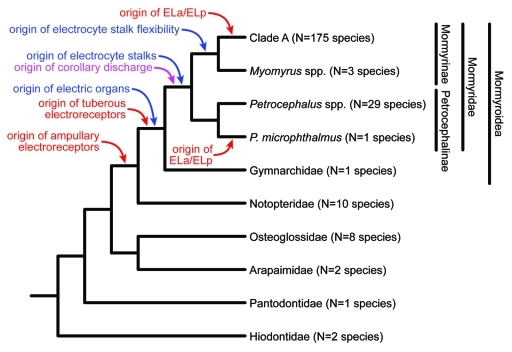
Figure 1.
Consensus cladogram of osteoglossomorph fishes.11-13 The evolutionary origins of sensory novelties (red), motor novelties (blue), and sensorimotor novelties (violet) are based on most parsimonious reconstructions of trait distribution across extant lineages. Estimated total numbers of extant species in each lineage (N) are shown, based on a combination of valid species counts,10 and estimated numbers of undescribed species.1,14-18 Note that ampullary electroreceptors were subsequently lost in one group nested within the Notopteridae.11
Ampullary electroreceptors serve in the passive sensing of bioelectric fields, thereby mediating prey detection, and sometimes predator avoidance and mate localization.19 Although they appear to be an ancestral vertebrate trait, ampullary electroreceptors were lost during the evolution of teleost fishes, only to be ‘rediscovered’ in the lineage leading to both mormyroids and their sister taxon, the Notopteridae.11 Among osteoglossomorphs, only mormyroid fishes possess electric organs that actively generate electric organ discharges (EODs) as well as the tuberous electroreceptors that detect them, together mediating electrocommunication20 and active electrolocation21 (orientation and navigation based on detecting distortions in the self-generated electric field). The electric organs of mormyroids are composed of excitable cells called electrocytes that are derived from skeletal muscle but exhibit a number of novel modifications including the loss of contractile ability.22 The evolution of electrocyte stalks in the family Mormyridae,22 and later, developmental flexibility in stalk morphology in the subfamily Mormyrinae,12 established an enhanced capacity for generating a variety of EOD waveforms.1 Further, a corollary discharge pathway that relays the timing of EOD output to electrosensory processing regions allows mormyrids to separately process information related to electrocommunication and active electrolocation, and to adaptively filter this information based on changing conditions.23 Finally, in two separate mormyrid lineages, one within the subfamily Mormyrinae and another within the subfamily Petrocephalinae, an electrosensory region of the midbrain called the exterolateral nucleus (EL) became enlarged and subdivided into separate anterior and posterior divisions (ELa/ELp).1 This neuroanatomical change resulted in a newfound ability to detect subtle variation in EOD waveforms.1 Only ‘clade A’ within the subfamily Mormyrinae possesses each of these neural innovations: there are more than 160 described, valid, extant species within this recently-evolved clade,10 plus at least 15 additional undescribed, valid species;1,14-17 by contrast, there are just 57 extant species among all other osteoglossomorph lineages combined10 (Fig. 1).
Exploitation of electric signal space
The evolution of electric organs and tuberous electroreceptors established a fairly private channel of communication, relatively free from constraints imposed by environmental effects, predators, and competing signalers.9,24,25 In mormyrids, the resulting exploitation of signal space took place in the form of two distinct aspects of electric signals, the waveform of the pulse-type EOD itself and interpulse intervals (IPIs) between EODs24,26 (Fig. 2). Within clade A, the fine temporal structure of the EOD waveform plays a critical role in species recognition and mate choice,27-31 and this is associated with relatively high rates of EOD waveform divergence.1,9 By comparison, non-clade A species, all of which lack the unique combination of electrocyte stalk flexibility and an enlarged ELa/ELp (Fig. 1), have experienced much lower rates of EOD waveform evolution1 (Fig. 2), suggesting that these species have not exploited temporal features of the EOD waveform for communication.
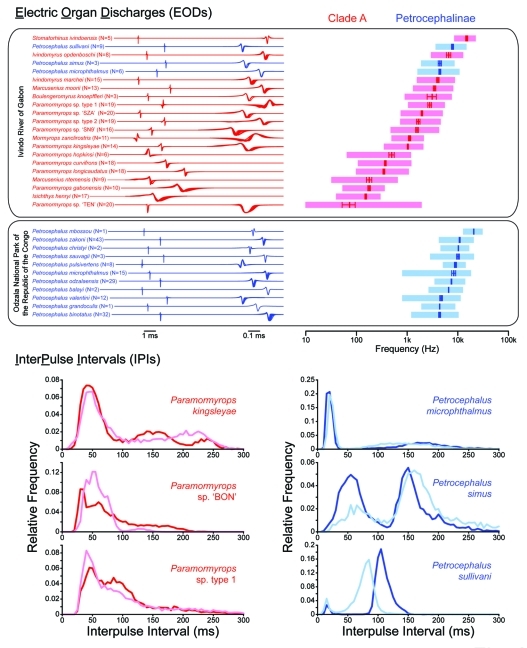
Figure 2.
Electric signal components in two lineages of mormyrids, clade A within the subfamily Mormyrinae (red) and the non-clade A subfamily Petrocephalinae (blue). The electric organ discharge (EOD) is a stereotyped, all-or-none pulse of electricity with a species-typical waveform (top left) and power spectrum (top right). EOD waveforms were recorded from individuals collected in two different locales.1 Multiple waveforms from different individuals of the same species are superimposed, amplitude-normalized, plotted head-positive up, and aligned to the head-positive peak (except for Paramormyrops sp ‘TEN’, for which waveforms are aligned to the head-negative peak). The left and right columns show waveforms at two different timescales (1 ms and 0.1 ms scale bars, respectively). The longest waveforms are shown only in the left column. Spectral contents of the same EODs are shown as the mean peak power frequency (± s.e.m.), with the shaded area corresponding to the mean bandwidth of the power spectra, 3 dB below the peak power. The interpulse interval (IPI) is variable (bottom). IPI histograms with a bin size of 5 ms were obtained from 10 min recordings of freshly-caught specimens from southeastern Gabon. Shown are two individuals of each of three species within the genus Paramormyrops and two individuals of each of three species within the genus Petrocephalus.
This raises an obvious question: what do mormyrid species outside of clade A use for species recognition and mate choice? Within the electrosensory domain, the frequency content of EODs (i.e., power spectra) could potentially be used for species recognition,32,33 although there is considerable overlap among the power spectra of sympatric non-clade A petrocephaline species (Fig. 2). Another possibility is that species outside of clade A could use IPIs for species recognition and mate choice, similar to the role of pulse repetition rates in acoustic communication for orthopteran insects34 and anuran amphibians.35 A comparison of IPI distributions from several freshly-caught field specimens provides preliminary support for this hypothesis: the IPIs generated by different species of the clade A genus Paramormyrops show considerable overlap, whereas the IPIs generated by different species of the non-clade A genus Petrocephalus are much more distinct (Fig. 2). Species in clade A generate a variety of IPI patterns to communicate contextual social information.20,24,26 If non-clade A mormyrids do indeed use IPIs for species recognition and mate choice, then this would limit the degree to which they could vary IPIs to communicate additional information. Thus, by establishing a new signal dimension to code for species identity, the ability to both generate and detect variation in the fine temporal structure of EOD waveforms in clade A would have expanded the total information carrying capacity of electrocommunication.
Evolutionary scenarios and mechanisms of selection
Neural innovations can only be part of the mechanism responsible for higher rates of signal divergence and species diversification in clade A. One or more agents of selection, and/or other influences on divergence, must have been coupled with these innovations to cause the increased tempo of evolution observed in this lineage. One possible agent is sexual selection by female mate choice, which can be a potent driver of speciation because it acts directly on signals involved in pre-mating reproductive isolation.36,37 Several patterns of phenotypic variation in clade A suggest that divergent sexual selection between geographically isolated populations may have contributed to the radiation of this group. First, both species recognition based on EODs27,28,30,31 and intraspecific sexual selection on EODs via female choice29 have been demonstrated for clade A mormyrids, although studies of within-population sexual selection on EODs are needed in additional species. Demonstrating a continuum (among populations or species) between directional sexual selection and stabilizing selection for species recognition provides evidence that sexual selection has contributed to speciation.38-40 Among mormyrids outside clade A there is no evidence of either species recognition or sexual selection targeting variable EOD features. Second, many clade A species exhibit a hallmark of sexual selection: strong sexual dimorphism of a courtship trait (here, the EOD) that is preferred by females and/or affects the outcome of male-male contests.17,20,24 Sexual dimorphisms of EODs are absent or weak outside clade A.17 Importantly, we find EOD sex differences in the most basal group within clade A (Mormyrops; Figure 3) – a speciose lineage with high interspecific EOD diversity that is the sister group of all other clade A species.10,41 Third, based largely on clade A mormyrids, EODs have diverged much more rapidly than traits that are directly linked to ecology9 (i.e., ecomorphology and trophic resource use). Unless key axes of ecological divergence have gone undetected, this suggests that direct sexual selection on EODs may have acted as the earliest driver of divergence in many mormyrid species. However, no data have yet illuminated the precise evolutionary mechanisms42 by which sexual selection presumably arose and persists in clade A.
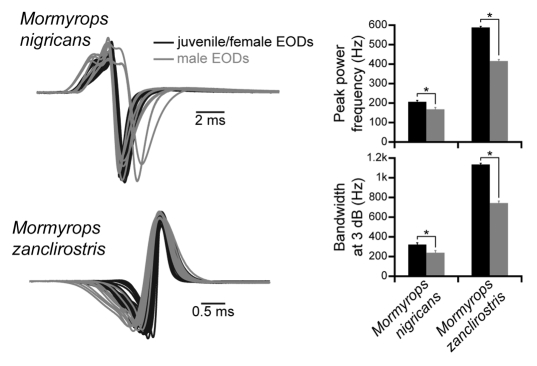
Figure 3.
Sex differences in EOD waveform for two species within the basal clade A genus Mormyrops. Multiple waveforms from different individuals of the same species are superimposed, amplitude-normalized, plotted head-positive up, and aligned to the head-positive peak (M. nigricans: n = 13 juveniles/females, n = 8 males; M. zanclirostris: n = 24 juveniles/females, n = 10 males). All recordings were made in Gabon. In both species, adult males (shown in gray) generally have longer EODs than adult females and juveniles (shown in black). This is reflected in significantly lower peak power frequencies and smaller bandwidths in males of both species (bar plots show the mean ± s.e.m.; *p < 0.01 based on two-sample t-tests).
Mechanisms underlying large species radiations are likely to be multifaceted, and sexual selection has certainly operated in concert with other factors during the radiation of clade A. For example, divergent sexual selection has a much greater scope for driving speciation when it is coupled with at least some spatial isolation among populations.43,44 Drift can also cause divergence among isolated populations, particularly when they are small. In riverine systems in which the radiation of clade A species has been most extensive, opportunities for geographic isolation exist among headwater tributaries and across main channel discontinuities such as waterfalls or the low velocity runs that isolate patches of riffles.16,45 It is possible that ecologically-based divergent natural selection may be the primary cause of mormyrid species divergence in some cases. Under this scenario, reinforcement46 and reproductive character displacement47 can subsequently enhance EOD differences among evolving species. When a single trait simultaneously experiences divergent natural selection and governs assortative mating due to pleiotropic coupling, it is referred to as a ‘magic trait’.48 Such traits can greatly facilitate speciation because, in these cases, selection does not have to overcome recombination between a key ecological trait and one or more mating trait(s).49,50 A magic trait hypothesis was recently proposed for EODs in clade A mormyrids because these signals function both in mate choice and active electrolocation.51 While this hypothesis certainly merits consideration due to the dual function of EODs, demonstrating that EODs act as magic traits will require studies showing that EOD variation among species is environmentally optimized and/or that interspecific divergence in these signals relates systematically to resource or habitat use patterns. It will also need to account for the dramatic sexual signal dimorphisms observed in many clade A species,17,20,24 which do not presently appear to underlie intraspecific resource use polymorphisms.9 Regardless of the selection pressures and other evolutionary influences that have been most important in speciation, we propose that greater opportunity for EOD divergence and an enhanced capacity for these signals to communicate information on nascent gene pool membership would have facilitated diversification under any of the evolutionary scenarios discussed above. Upon secondary contact following slight ecological divergence in allopatry by natural selection or drift, for example, a communication channel with higher information carrying capacity may prolong an initial period of weak adaptive isolation until further ecological divergence evolves and stabilizes long-term species coexistence.9,52
Neural innovations and the evolution of signals and species
Diverse communication signals and novel neural structures are found in a number of speciose lineages such as orthopterans,34,53 anurans,35,54 bats55,56 and songbirds,57,58 suggesting that neural innovation may prove to be a general mechanism for triggering rapid diversification. Indeed, increased anuran diversification has been linked to anatomical change in the inner ear59 (but see60), and modeling studies suggest that high rates of songbird diversification may relate to song learning,61,62 mediated by a specific neural circuit.63
The evolution of signal diversity depends on both the ability of senders to exploit new regions of signal space and the ability of receivers to detect the resulting signal variation.1 Therefore, to the extent that communication fuels speciation,64 the sensory and motor capabilities of animals can limit the rate of species diversification. However, neural novelties are not sufficient to trigger rapid diversification; the right form of selection and/or evolutionary scenario is necessary for these novelties to act as key innovations that drive signal divergence and species diversification. An illustrative example are the hominins (modern humans and closely-related extinct lineages), which have not exhibited particularly extensive species radiation,65 but are nevertheless characterized by dramatically enlarged brains conferring unique perceptual and motor abilities,66,67 as well as a permanently descended larynx essential for phonetic articulation.68 We propose that rapid diversification triggered by neural innovation is most likely to occur when an innovation is directly related to the generation or detection of signals used specifically for mate choice and species recognition, as opposed to signals used more broadly in other social contexts. In the latter case, neural innovations related to communication can increase information transmission within a species, as in the evolution of human language.68 Here, the exploitation of signal space is realized by the expansion of a single species’ range of communication behavior, rather than by different species each occupying distinct regions within an expanded signal space.
Acknowledgments
Supported by NSF IOS-0818390.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17483
References
- 1.Carlson BA, Hasan SM, Hollmann M, Miller DB, Harmon LJ, Arnegard ME. Brain evolution triggers increased diversification of electric fishes. Science. 2011;332:583–6. doi: 10.1126/science.1201524. [DOI] [PubMed] [Google Scholar]
- 2.Arnegard ME, Zwickl DJ, Lu Y, Zakon HH. Old gene duplication facilitates origin and diversification of an innovative communication system—twice. Proc Natl Acad Sci USA. 2010;107:22172–7. doi: 10.1073/pnas.1011803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pigliucci M. What, if anything, is an evolutionary novelty? Philos Sci. 2008;75:887–98. doi: 10.1086/594532. [DOI] [Google Scholar]
- 4.Wagner GP, Lynch VJ. Evolutionary novelties. Curr Biol. 2010;20:R48–52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–23. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 6.Simpson GG. The Major Features of Evolution. New York: Columbia University Press, 1953. [Google Scholar]
- 7.Hunter JP. Key innovations and the ecology of macroevolution. Trends Ecol Evol. 1998;13:31–6. doi: 10.1016/S0169-5347(97)01273-1. [DOI] [PubMed] [Google Scholar]
- 8.Schluter D. The Ecology of Adaptive Radiation. New York: Oxford University Press, 2000. [Google Scholar]
- 9.Arnegard ME, McIntyre PB, Harmon LJ, Zelditch ML, Crampton WGR, Davis JK, et al. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am Nat. 2010;176:335–56. doi: 10.1086/655221. [DOI] [PubMed] [Google Scholar]
- 10.Eschmeyer WN, Fricke R. Catalog of Fishes. San Francisco: California Academy of Sciences, 2011. [Google Scholar]
- 11.Lavoué S, Sullivan JP. Simultaneous analysis of five molecular markers provides a well-supported phylogenetic hypothesis for the living bony-tongue fishes (Osteoglossomorpha: Teleostei) Mol Phylogenet Evol. 2004;33:171–85. doi: 10.1016/j.ympev.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JP, Lavoué S, Hopkins CD. Molecular systematics of the African electric fishes (Mormyroidea: Teleostei) and a model for the evolution of their electric organs. J Exp Biol. 2000;203:665–83. doi: 10.1242/jeb.203.4.665. [DOI] [PubMed] [Google Scholar]
- 13.Lavoué S, Miya M, Arnegard ME, McIntyre PB, Mamonekene V, Nishida M. Remarkable morphological stasis in an extant vertebrate despite tens of millions of years of divergence. Proc Biol Sci. 2011;278:1003–8. doi: 10.1098/rspb.2010.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan JP, Lavoué S, Hopkins CD. Discovery and phylogenetic analysis of a riverine species flock of African electric fishes (Mormyridae: Teleostei) Evolution. 2002;56:597–616. doi: 10.1111/j.0014-3820.2002.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 15.Feulner PGD, Kirschbaum F, Tiedemann R. Adaptive radiation in the Congo River: An ecological speciation scenario for African weakly electric fish (Teleostei; Mormyridae; Campylomormyrus) J Physiol Paris. 2008;102:340–6. doi: 10.1016/j.jphysparis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Arnegard ME, Bogdanowicz SM, Hopkins CD. Multiple cases of striking genetic similarity between alternate electric fish signal morphs in symaptry. Evolution. 2005;59:324–43. [PubMed] [Google Scholar]
- 17.Lavoué S, Arnegard ME, Sullivan JP, Hopkins CD. Petrocephalus of Odzala offer insights into evolutionary patterns of signal diversification in the Mormyridae, a family of weakly electrogenic fishes from Africa. J Physiol Paris. 2008;102:322–39. doi: 10.1016/j.jphysparis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Lavoué S, Sullivan JP, Arnegard ME. African weakly electric fishes of the genus Petrocephalus (Osteoglossomorpha: Mormyridae) of Odzala National Park, Republic of the Congo (Lékoli River, Congo River basin) with description of five new species. Zootaxa. 2010;2600:1–52. [Google Scholar]
- 19.Wilkens LA, Hofmann MH. Behavior of animals with passive, low-frequency electrosensory systems. In: Electroreception. Bullock TH, Hopkins CD, Popper AN, Fay RR (eds). New York: Springer, 2005:229-63. [Google Scholar]
- 20.Carlson BA. A neuroethology of electrocommunication: Senders, receivers, and everything in between. In: Communication in Fishes. Ladich F, Collin SP, Moller P, Kapoor BG (eds). Enfield, NH: Science Publishers, 2006:805-48. [Google Scholar]
- 21.von der Emde G. Active electrolocation of objects in weakly electric fish. J Exp Biol. 1999;202:1205–15. doi: 10.1242/jeb.202.10.1205. [DOI] [PubMed] [Google Scholar]
- 22.Bass AH. Electric organs revisited: Evolution of a vertebrate communication and orientation organ. In: Electroreception. Bullock TH, Heiligenberg W (eds). New York: John Wiley and Sons, 1986:13-70. [Google Scholar]
- 23.Bell CC. Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol. 1989;146:229–53. doi: 10.1242/jeb.146.1.229. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins CD. Behavior of Mormyridae. In: Electroreception. Bullock TH, Heiligenberg W (eds). New York: John Wiley and Sons, 1986:527-76. [Google Scholar]
- 25.Hopkins CD. Design features for electric communication. J Exp Biol. 1999;202:1217–28. doi: 10.1242/jeb.202.10.1217. [DOI] [PubMed] [Google Scholar]
- 26.Carlson BA. Electric signaling behavior and the mechanisms of electric organ discharge production in mormyrid fish. J Physiol Paris. 2002;96:405–19. doi: 10.1016/S0928-4257(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins CD, Bass AH. Temporal coding of species recognition signals in an electric fish. Science. 1981;212:85–7. doi: 10.1126/science.7209524. [DOI] [PubMed] [Google Scholar]
- 28.Arnegard ME, Jackson BS, Hopkins CD. Time-domain signal divergence and discrimination without receptor modification in sympatric morphs of electric fishes. J Exp Biol. 2006;209:2182–98. doi: 10.1242/jeb.02239. [DOI] [PubMed] [Google Scholar]
- 29.Machnik P, Kramer B. Female choice by electric pulse duration: Attractiveness of the males' communication signal assessed by female bulldog fish, Marcusenius pongolensis (Mormyridae, Teleostei) J Exp Biol. 2008;211:1969–77. doi: 10.1242/jeb.016949. [DOI] [PubMed] [Google Scholar]
- 30.Feulner PGD, Plath M, Engelmann J, Kirschbaum F, Tiedemann R. Electrifying love: Electric fish use species-specific discharge for mate recognition. Biol Lett. 2009;5:225–8. doi: 10.1098/rsbl.2008.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowski B, Baier B, Kramer B. Differentiation in electrical pulse waveforms in a pair of sibling Dwarf Stonebashers, Pollimyrus castelnaui and P. marianne: Possible mechanisms and functions (Mormyridae, Teleostei) Behaviour. 2008;145:115–35. doi: 10.1163/156853908782687223. [DOI] [Google Scholar]
- 32.Lavoué S, Hopkins CD, Toham AK. The Petrocephalus (Pisces, Osteoglossomorpha, Mormyridae) of Gabon, Central Africa, with the description of a new species. Zoosystema. 2004;26:511–35. [Google Scholar]
- 33.Moritz T, Engelmann J, Linsenmair KE, von der Emde G. The electric organ discharges of the Petrocephalus species (Teleostei: Mormyridae) of the Upper Volta System. J Fish Biol. 2009;74:54–76. doi: 10.1111/j.1095-8649.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- 34.Hedwig B. Pulses, patterns and paths: Neurobiology of acoustic behaviour in crickets. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:677–89. doi: 10.1007/s00359-006-0115-8. [DOI] [PubMed] [Google Scholar]
- 35.Kelley DB. Vocal communication in frogs. Curr Opin Neurobiol. 2004;14:751–7. doi: 10.1016/j.conb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Ptacek MB. The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav Processes. 2000;51:111–34. doi: 10.1016/S0376-6357(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie MG. Sexual selection and speciation. Annu Rev Ecol Evol Syst. 2007;38:79–102. doi: 10.1146/annurev.ecolsys.38.091206.095733. [DOI] [Google Scholar]
- 38.Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47:647–57. doi: 10.2307/2410076. [DOI] [PubMed] [Google Scholar]
- 39.Boake CRB, DeAngelis MP, Andreadis DK. Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. Proc Natl Acad Sci USA. 1997;94:12442–5. doi: 10.1073/pnas.94.23.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal GG, Ryan MJ. Conflicting preferences within females: Sexual selection versus species recognition. Biol Lett. 2011;7:525–7. doi: 10.1098/rsbl.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins CD, Lavoué S, Sullivan JP. Mormyridae. In: The Fresh and Brackish Water Fishes of Lower Guinea, West-Central Africa. Stiassny MLJ, Teugels GG, Hopkins CD (eds). Paris: IRD Éditions, 2007:219-334. [Google Scholar]
- 42.Kokko H, Jennions MD, Brooks R. Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst. 2006;37:43–66. doi: 10.1146/annurev.ecolsys.37.091305.110259. [DOI] [Google Scholar]
- 43.Lande R. Rapid origin of sexual isolation and character divergence in a cline. Evolution. 1982;36:213–23. doi: 10.2307/2408039. [DOI] [PubMed] [Google Scholar]
- 44.Arnegard ME, Kondrashov AS. Sympatric speciation by sexual selection alone is unlikely. Evolution. 2004;58:222–37. [PubMed] [Google Scholar]
- 45.Gallant JR, Arnegard ME, Sullivan JP, Carlson BA, Hopkins CD. Signal variation and its morphological correlates in Paramormyrops kingsleyae provide insight into the evolution of electrogenic signal diversity in mormyrid electric fish. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:799–817. doi: 10.1007/s00359-011-0643-8. [DOI] [PubMed] [Google Scholar]
- 46.Servedio MR, Noor MAF. The role of reinforcement in speciation: Theory and data. Annu Rev Ecol Evol Syst. 2003;34:339–64. doi: 10.1146/annurev.ecolsys.34.011802.132412. [DOI] [Google Scholar]
- 47.Crampton WGR, Lovejoy NR, Waddell JC. Reproductive character displacement and signal ontogeny in a sympatric assemblage of electric fish. Evolution. 2011;65:1650–66. doi: 10.1111/j.1558-5646.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 48.Gavrilets S. Fitness Landscapes and the Origin of Species. Princeton, New Jersey, 2004. [Google Scholar]
- 49.Felsenstein J. Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution. 1981;35:124–38. doi: 10.2307/2407946. [DOI] [PubMed] [Google Scholar]
- 50.Servedio MR, van Doorn GS, Kopp M, Frame AM, Nosil P. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol Evol. 2011;26:389–97. doi: 10.1016/j.tree.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Feulner PGD, Plath M, Engelmann J, Kirschbaum F, Tiedemann R. Magic trait electric organ discharge (EOD) Commun Integr Biol. 2009;2:329–31. doi: 10.4161/cib.2.4.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rundell RJ, Price TD. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol Evol. 2009;24:394–9. doi: 10.1016/j.tree.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Mendelson TC, Shaw KC. Sexual behavior: Rapid speciation in an arthropod. Nature. 2005;433:375–6. doi: 10.1038/433375a. [DOI] [PubMed] [Google Scholar]
- 54.Wiens JJ. Global patterns of diversification and species richness in amphibians. Am Nat. 2007;170:S86–106. doi: 10.1086/519396. [DOI] [PubMed] [Google Scholar]
- 55.Jones G, Teeling EC. The evolution of echolocation in bats. Trends Ecol Evol. 2006;21:149–56. doi: 10.1016/j.tree.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Jones KE, Purvis A, MacLarnon A, Bininda-Emonds OR, Simmons NB. A phylogenetic supertree of the bats (Mammalia: Chiroptera) Biol Rev Camb Philos Soc. 2002;77:223–59. doi: 10.1017/S1464793101005899. [DOI] [PubMed] [Google Scholar]
- 57.Slabbekoorn H, Smith TB. Bird song, ecology, and speciation. Philos Trans R Soc Lond B Biol Sci. 2002;357:493–503. doi: 10.1098/rstb.2001.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konishi M. Birdsong: from behavior to neuron. Annu Rev Neurosci. 1985;8:125–70. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- 59.Ryan MJ. Neuroanatomy influences speciation rates among anurans. Proc Natl Acad Sci USA. 1986;83:1379–82. doi: 10.1073/pnas.83.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richards CL. Has the evolution of complexity in the amphibian papilla influenced anuran speciation rates? J Evol Biol. 2006;19:1222–30. doi: 10.1111/j.1420-9101.2006.01079.x. [DOI] [PubMed] [Google Scholar]
- 61.Ellers J, Slabbekoorn H. Song divergence and male dispersal among bird populations: a spatially explicit model testing the role of vocal learning. Anim Behav. 2003;65:671–81. doi: 10.1006/anbe.2003.2081. [DOI] [Google Scholar]
- 62.Lachlan RF, Servedio MR. Song learning accelerates allopatric speciation. Evolution. 2004;58:2049–63. doi: 10.1111/j.0014-3820.2004.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 63.Mooney R. Neurobiology of song learning. Curr Opin Neurobiol. 2009;19:654–60. doi: 10.1016/j.conb.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoskin CJ, Higgie M. Speciation via species interactions: the divergence of mating traits within species. Ecol Lett. 2010;13:409–20. doi: 10.1111/j.1461-0248.2010.01448.x. [DOI] [PubMed] [Google Scholar]
- 65.Wood B, Richmond BG. Human evolution: taxonomy and paleobiology. J Anat. 2000;197:19–60. doi: 10.1046/j.1469-7580.2000.19710019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–7. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–31. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- 68.Fitch WT. The evolution of language: a comparative review. Biol Philos. 2005;20:193–203. doi: 10.1007/s10539-005-5597-1. [DOI] [Google Scholar]