Abstract
The hippocampus plays a central role in learning and memory. Although synaptic delivery of AMPA-type glutamate receptors (AMPARs) contributes to experience-dependent synaptic strengthening, its role in hippocampus-dependent learning remains elusive. In a recent study,we found that the inhibitory avoidance (IA) task, a hippocampus-dependent contextual fear learning paradigm, drives GluR1-containing AMPARs into CA3-CA1 synapses of the dorsal hippocampus.We expressed mutated membrane-proxymal region (14 amino acids) of the GluR1-cytoplasmic tail (serines mutated to phospho-mimicking aspartates:MPR-DD) in the dorsal hippocampus to block the synaptic delivery of endogenous AMPARs. Learning-driven synaptic AMPARs delivery in CA1 neurons was prevented by the expression of MPR-DD. Bilateral expression of MPR-DD in CA1 region of the dorsal hippocampusattenuated IA learning, indicating that synaptic GluR1 trafficking in the hippocampus is required for encoding contextual fear memories. Furthermore, fraction of CA1 neurons with synaptic strengthening positively correlated with the performance in the IA fear memory task. Thus, the robustness of a contextual fear memory may depend on thenumber of hipppocampal neurons that are involved in the encoding of a memory trace.
Long-term potentiation (LTP) has been suggested to play a crucial role in learning and memory.1,2 Glutermatergic excitatory synaptic transmission is a major neuronal system utilized in cognitive functions such as learning. AMPARsare ionotropic glutamate receptors and form heterotetramers, comprised of a combination of four subunits (GluR1-GluR4). Synaptic incorporation of GluR1-containing AMPARs contributes to the synaptic potentiation during LTP and experience-dependent neuronal plasticity.3-10
Contextual fear learning leads to synaptic strengthening in CA1 region of the dorsal hippocampus.11 GluR1knock out mice exhibits impaired hippocampus-dependent memory.12 Furthermore, fear conditioning recruits GluR1-containing AMPARs into spines of the hippocampus.13 These studies, however, did not address ifsynaptic strengthening through AMPARsdelivery into synapses participates in and is required for hippocampus-dependent learning.
In our recent study, we used hippocampus dependent inhibitory avoidance (IA) task, a contextual fear learning paradigm. In this paradigm, rats are allowed to cross from an illuminated box to a dark box where an electric foot shock is delivered. Thus, rats learn to avoid the dark box and stay in a light box, which they naturally would not prefer. Acquisition of contextual memories can thereby be evaluated as the tendency to avoid the dark box (Fig.). We reported 1) IA learning drives GluR1 into synapses formed from CA3 to CA1 of the dorsal hippocampus, 2) blockade of synaptic GluR1 delivery with MPR-DD in the hippocampal CA1 region attenuates IA learning, 3) the fraction of CA1 neurons with synaptic strengthening positively correlated with the performance in the IA task.14 These results showed that synaptic GluR1 delivery at CA3-CA1 synapses is required for IA learning and robustness of fear memory depends on the number of neurons with synaptic strengthening.
We observed that IA strengthened CA3-CA1 synapses by the synaptic trafficking of GluR1-containing AMPA-Rs in large fraction of CA1 neurons (~90% of neurons).14 If synaptic circuits are strengthened over the majority of CA1 neurons during IA learning, how can a fear memory retain its contextual specificity? A previous study showed that approximately 30% of recording electrodes exhibited LTP-like enhancement of fEPSP in IA-trained rats in vivo, indicating that limited fraction of circuits enhanced transmission.11 One possibility is that contextual learning also enhances inhibitory circuits and selectively veils some of potentiated glutamatergic circuits. It will be interesting if IA also enhances inhibitory circuits.
Figure 1.
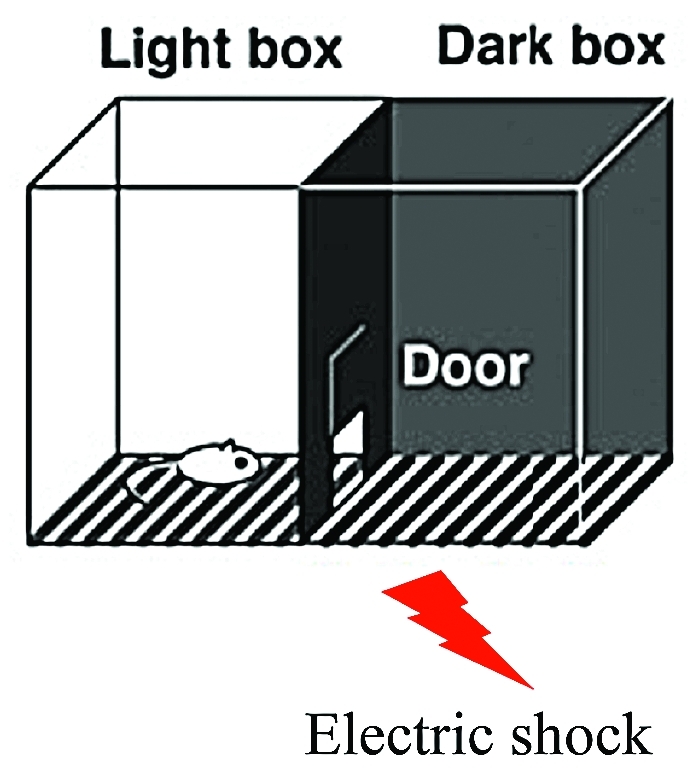
Inhibitory Avoidance task. Rats are allowed to cross from an light box to a dark box where an electric foot shock is delivered. Thus, rats learn to avoid the dark box and stay in a light box. Acquisition of contextual memories can thereby be evaluated as the tendency to avoid the dark box.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17505
References
- 1.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–8. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- 5.Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–70. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–8. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 7.Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, et al. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–92. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 9.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–79. doi: 10.1016/S0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 10.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 12.Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–73. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–7. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1104558108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


