Abstract
Western Pacific amyotrophic lateral sclerosis (ALS) and parkinsonism-dementia complex (PDC), a prototypical neurodegenerative disease (tauopathy) affecting distinct genetic groups with common exposure to neurotoxic chemicals in cycad seed, has many features of Parkinson's and Alzheimer's diseases (AD), including early olfactory dysfunction. Guam ALS-PDC incidence correlates with cycad flour content of cycasin and its aglycone methylazoxymethanol (MAM), which produces persistent DNA damage (O6-methylguanine) in the brains of mice lacking O6-methylguanine methyltransferase (Mgmt-/-). We described in Mgmt-/-mice up to 7 days post-MAM treatment that brain DNA damage was linked to brain gene expression changes found in human neurological disease, cancer, and skin and hair development. This addendum reports 6 months post-MAM treatment- related brain transcriptional changes as well as elevated mitogen activated protein kinases and increased caspase-3 activity, both of which are involved in tau aggregation and neurofibrillary tangle formation typical of ALS-PDC and AD, plus transcriptional changes in olfactory receptors. Does cycasin act as a "slow (geno)toxin" in ALS-PDC?
Keywords: amyotrophic lateral sclerosis, Parkinson disease, Alzheimer disease, methylazoxymethanol, tauopathy
We showed that methylazoxymethanol (MAM), the genotoxic metabolite of the cycad plant carcinogen cycasin (MAM-β-D-glucoside), induced in young adult mice lacking O6-methylguanine (O6-mG) methyltransferase (Mgmt−/−)—the enzyme that repairs O6-mG DNA lesions—a O6-mG-linked brain transcriptional response associated with human neurological disease.1 This supports an etiologic role for the azoxyglycoside cycasin in the genesis of a disappearing degenerative brain disease (amyotrophic lateral sclerosis and parkinsonism-dementia complex, ALS-PDC) found among the genetically distinct island populations of Guam and Rota (Chamorros), Honshu (Japanese) and New Guinea (Papuan New Guinean), which used cycad seed as medicine applied orally (Kii Peninsula, Honshu) or topically (West Papua), or for both topical medicine and food (Guam and Rota). In all three disease foci, periods of years or decades intervene between exposure to cycad seed and the development of ALS-PDC, suggesting the operation of a “slow toxin” able to trigger a progressive neuronal disease reminiscent of look-a-like disorders of old age (e.g., Alzheimer disease, AD) elsewhere in the world.2 Both AD and ALS-PDC have neurofibrillary tangles containing the microtubule tau protein in a hyperphosphorylated state,3-5 which has been linked to both activation of serine-threonine kinases (Erk-1/2, p38, c-Jun NH2-terminal kinase) in the mitogen-activated protein kinase (MAPK) pathway and to phosphorylation of the C-terminal fragment of amyloid precursor protein (APP).6 More recent studies suggest the activation of non-apoptotic caspases may be one of the earliest events that triggers tau aggregation and the accumulation of neurofibrillary tangles in tauopathies.7-9 Here, we supplement data on the short-term actions (up to 7 d) of MAM on brain gene expression in O6-mG-deficient mice (MAMearly) with preliminary findings on caspase activity and the transcription and protein expression of brain cell signaling proteins 6 mo post-treatment (MAMlate).
Seven 11-week-old male Mgmt−/− mice were treated with a single intraperitoneal dose of MAM (20 mg/kg body weight, n = 4) or a comparable volume of vehicle consisting of 0.5% acetic acid in saline (n = 3). Animals were housed singly, fed rodent chow ad libitum for 6 mo, during which all animals grew and maintained apparent health, and decapitated by guillotine.
The right half of the MAMlate brain was employed for gene expression analysis using mouse oligo microarrays (~21,000 features) by Agilent (Santa Clara, CA). As in the previously published MAMearly study,1 brain cellular networks putatively perturbed in MAMlate Mgmt−/− mice were identified by integrating the transcripts with their gene products and overlaying these with known molecular interactions using Ingenuity™ Pathway Analysis (IPA, Redwood City, CA). The three top-ranked IPA Biofunction Diseases and Disorders included: inflammatory response, cancer and genetic disorders. We identified 355 genes that were differentially expressed between the brains of MAM- and vehicle-treated Mgmt−/− mice. IPA analysis retained 85% of these genes (302 of the 355) in the construction of 176 networks. The three top-scoring IPA networks contained 23 (#1), 25 (#2) and 21 (#3) focus molecules, including hubs for Akt, transforming growth factor β (Tgfβ) and histone h3 (#1), calcineurin and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB complex) (#2) and Erk1/2, MAPK and collagen(s) (#3). The most significant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were determined with DAVID (the Database for Annotation, Visualization and Integrated Discovery) bioinformatics software. Based on 291 genes, DAVID identified six pathways (n = 4 or more genes per pathway) common to MAMearly and MAMlate and three unique to MAMlate (Table 1): the latter included genes coding for olfactory receptors that were both upregulated (n = 25) and downregulated (n = 3). Parkinson disease was one of 23 additional KEGG pathways (n = 2 or 3 genes per pathway). Brain KEGG pathways common to both MAMlate and MAMearly animals included: Pathways in Cancer, MAPK, Focal Adhesion Pathway, Neuroactive Receptor Interaction Pathway, Steroid Hormone Biosynthesis and the Calcium Signaling Pathway (Table 1). The MAPK signaling pathway involved 3 upregulated genes (activin A receptor, type 1B (Acvr1b) a member of the transforming-growth-factor-β family linked to skin epithelial cell proliferation and hair development,10 calcium channel voltage dependent α 2/delta subunit 4; serine/threonine kinase-3) (CACNA2D3), a neuroblastoma marker gene,11 and 1 downregulated gene raffinose permease (RafB), a MAPK protein pathway gene.12
Table 1. KEGG pathways from DAVID based on 407/443 (DAVID recognized /submitted) Affymetrix probe ids (MAMearly) or 291/355 GENBANK accession numbers (MAMlate). Both sets restricted to pathways containing at least four probes/genes.
| KEGG Pathway | MAMearly | MAMlate |
|---|---|---|
| Pathways in cancer |
13 |
4 |
| Insulin signaling pathway |
9 |
|
| Wnt signaling pathway |
10 |
|
| Purine metabolism |
9 |
|
| MAPK signaling pathway |
7 |
4 |
| Prostate cancer |
8 |
|
| Acute myeloid leukemia |
5 |
|
| Chronic myeloid leukemia |
5 |
|
| Neurotrophin signaling pathway |
6 |
|
| Huntington’s disease |
5 |
|
| Focal adhesion |
6 |
4 |
| Neuroactive ligand-receptor interaction |
5 |
4 |
| Nucleotide excision repair |
4 |
|
| Steroid hormone biosynthesis |
4 |
4 |
| Endometrial cancer |
5 |
|
| Glioma |
4 |
|
| Long-term potentiation |
4 |
|
| Long-term depression |
5 |
|
| Small cell lung cancer |
4 |
|
| Colorectal cancer |
5 |
|
| Apoptosis |
4 |
|
| ErbB signaling pathway |
5 |
|
| Melanogenesis |
6 |
|
| Axon guidance |
5 |
|
| Calcium signaling pathway |
4 |
4 |
| Endocytosis |
4 |
|
| Regulation of actin cytoskeleton |
5 |
|
| Chemokine signaling pathway |
5 |
4 |
| Basal cell carcinoma |
4 |
|
| Alzheimer disease |
4 |
|
| Olfactory transduction |
|
28 |
| ECM-receptor interaction |
|
5 |
| Cytokine-cytokine receptor interaction | 5 |
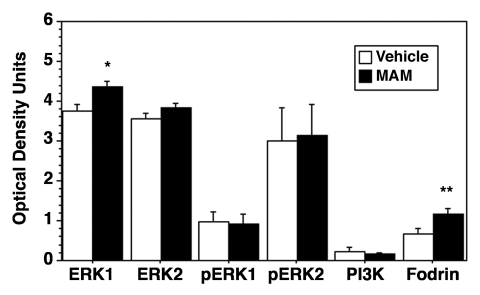
The left half of each brain was analyzed by protein gel blotting for components of the MAPK (perturbed in both MAMlate and MAMearly) and phosphatidylinositol 3-kinase/Akt (PI3K/Akt) signaling pathways and activity of caspase-3 (the APP cleavage protein linked to AD).8,9 In MAMlate, significant increases were found in Erk-1 (p < 0.03) and fodrin cleavage (p < 0.01) (Fig. 1). Brain fodrin cleavage, which was increased in MAMlate animals, indicates the activation of caspase-3, an enzyme with an important role in cleaving tau.14 MAMlate transcriptional changes in extracellular-matrix-receptor interaction (4 genes upregulated, 1 downregulated gene) and cytokine-cytokine receptor interaction (3 genes upregulated, 2 downregulated genes) suggest brain inflammatory response modulation, which is consistent with early changes in tau-related neurodegeneration.15
Figure 1.
Effect of MAMlate on components of the MAPK signaling pathway and caspase activity. The left half brains of vehicle- (n = 3) and MAM- (n = 4) treated Mgmt−/− mice were flash frozen in liquid N2, the frozen tissue subjected to ultrasonication in gel electrophoresis buffer to avoid loss of protein modifications or lysis, and the homogenate heat denatured at 95°C for 5 min. An aliquot of the brain tissue homogenate (50 μg) was resolved on a 10% polyacrylamide gel, transferred to PVDF membranes, the blocked membranes probed with monoclonal antibodies to ERK (ERK1, ERK2), phosphorylated ERK (pERK1, pERK2), PI3K (p110γ) (Santa Cruz Biotechnology, Inc.) and α-fodrin (Chemicon) and the bands visualized by chemiluminescence detection. α-Fodrin cleavage was determined using the 120 kDA band. Membranes were scanned on a Microtek flatbed scanner and each band quantified using Molecular Analyst software (BioRad, Inc) with background subtraction as described previously.13 Values are the mean ± standard error. Significantly different from vehicle (*p < 0.03 or ** p < 0.01 by ANOVA).
The MAMlatetranscriptional profile was dominated by the presence of 28 (of a total of ~1300) genes involved in olfactory transduction,16 including chloride channel activated 6, which suggest the presence of a MAM-induced change in olfaction status. While caution is merited when comparing rodent and human data, olfactory dysfunction is among the first signs of neurodegenerative disease.17 Marked olfactory deficits, first reported in Guam PDC, are also similarly present in Chamorro patients with ALS, pure parkinsonism, and pure dementia, and in some controls with possible sub-clinical ALS-PDC.18 Olfactory deficits are also among the first signs of Alzheimer disease and idiopathic Parkinson disease.19,20 The inability to distinguish the nature of olfactory dysfunction among Guam PDC, AD21 and ALS patients18 suggests a common neurologic substrate and underlines the close relationship between ALS-PDC and the more familiar neurodegenerative disorders seen in the West.
Acknowledgments
This work was funded by the National Institute of Environmental Health Sciences grant ES11384.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17603
References
- 1.Kisby GE, Fry RC, Lasarev MR, Bammler TK, Beyer RP, Churchwell M, et al. The cycad genotoxin MAM modulates brain cellular pathways Involved in neurodegenerative disease and cancer in a DNA damage-linked manner. PLoS ONE. 2011;6:e20911. doi: 10.1371/journal.pone.0020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer PS, Kisby GE, Ludolph AC. Slow toxins, biologic markers, and long-latency neurodegenerative disease in the western Pacific region. Neurology. 1991;41(Suppl 2):62–6. doi: 10.1212/wnl.41.5_suppl_2.62. [DOI] [PubMed] [Google Scholar]
- 3.Mawal-Dewan M, Schmidt ML, Balin B, Perl DP, Lee VM, Trojanowski JQ. Identification of phosphorylation sites in PHF-Tau from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J Neuropathol Exp Neurol. 1996;55:1051–9. [PubMed] [Google Scholar]
- 4.Itoh N, Ishiguoro K, Arai H, Kokubo Y, Sasaki R, Narita Y, et al. Biochemical and ultrastructural study of neurofibrillary tangles in amyotrophic lateral sclerosis/parkinsonism-dementia complex in the Kii peninsula of Japan. J Neuropathol Exp Neurol. 2003;62:791–8. doi: 10.1093/jnen/62.7.791. [DOI] [PubMed] [Google Scholar]
- 5.Winton MJ, Joyce S, Zhukareva V, Practico D, Perl DP, Galasko D, et al. Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathol. 2006;111:401–12. doi: 10.1007/s00401-006-0053-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010; 1802: 396-405. [DOI] [PubMed]
- 7.Avila J. Alzheimer’s disease: caspases first. Nat Rev Neurol. 2010;6:587–8. doi: 10.1038/nrneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 8.D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 9.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer’s disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–12. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 10.Qiu W, Li X, Tang H, Huang AS, Panteleyev AA, Owens DM, et al. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J Invest Dermatol. 2011;131:1067–76. doi: 10.1038/jid.2010.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorell K, Bergman A, Carén H, Nilsson S, Kogner P, Martinsson T, et al. Verification of genes differentially expressed in neuroblastoma tumours: a study of potential tumour suppressor genes. BMC Med Genomics. 2009;2:53. doi: 10.1186/1755-8794-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang F, Sun T, Trostinskaia A, Wygle D, Puscheck E, et al. Entire mitogen activated protein kinase (MAPK) pathway is present in preimplantation mouse embryos. Dev Dyn. 2004;231:72–87. doi: 10.1002/dvdy.20114. [DOI] [PubMed] [Google Scholar]
- 13.Kisby GE, Standley M, Park T, Olivas A, Fei S, Jacob T, et al. Proteomic analysis of the genotoxicant methylazoxymethanol (MAM)-induced changes in the developing cerebellum. J Proteome Res. 2006;5:2656–65. doi: 10.1021/pr060126g. [DOI] [PubMed] [Google Scholar]
- 14.Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–54. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 15.McGeer EG, McGeer PL. Brain inflammation in Alzheimer diseases and the therapeutic implications. Curr Pharm Des. 1999;5:821–36. [PubMed] [Google Scholar]
- 16.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nature Neurosc 2002; 124-33. [DOI] [PubMed] [Google Scholar]
- 17.Doty RL, Perl DP, Steele JC, Chen KM, Pierce JD, Jr., Reyes P, et al. Odor identification deficit of the parkinsonism-dementia complex of Guam: equivalence to that of Alzheimer's and idiopathic Parkinson's disease. Neurology. 1991;41:77–80. doi: 10.1212/wnl.41.5_suppl_2.77. [DOI] [PubMed] [Google Scholar]
- 18.Ahlskog JE, Waring SC, Petersen RC, Esteban-Santillan C, Craig UK, O'Brien PC, et al. Olfactory dysfunction in Guamanian ALS, parkinsonism, and dementia. Neurology. 1998;51:1672–7. doi: 10.1212/wnl.51.6.1672. [DOI] [PubMed] [Google Scholar]
- 19.Berendse HW, Roos DS, Raijmakers P, Doty RL. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J Neurol Sci 2011; Jun 24 [Epub ahead of print] [DOI] [PubMed]
- 20.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730–5. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doty RL, Perl DP, Steele JC, Chen KM, Pierce JD, Jr., Reyes P, et al. Olfactory dysfunction in three neurodegenerative diseases. Geriatrics. 1991;46(Suppl 1):47–51. [PubMed] [Google Scholar]