Abstract
αB-crystallin (αB) is an archetypical small heat shock protein whose physiological function is not clearly defined. The interest in this protein arises from its well-established but poorly understood association with a myriad of neurodegenerative diseases, cancer and cardiomyopathies. The discovery of the secretion of αB from human adult retinal pigment epithelial cells (ARPE19) via exosomes not only points to the involvement of this protein in lateral transfer of information between cells in the visual system but also to the status of this protein as a potential ligand that may activate or modulate immune and stress responses, normal growth and oncogenic pathways. Retinal pigment epithelium (RPE) is a single layer of polarized cells that supports photoreceptor physiology and function. We have initiated investigations on understanding the origin of the elevated levels of αB in extracellular sub-retinal proteolipid deposits (known as "drusen") associated with the death of photoreceptor neurons in age-related macular degeneration (AMD). Here we discuss the potential implications of the presence and transport of αB in exosomes across cell membranes in RPE.
Keywords: αB-crystallin, Small heat shock protein, Secretion, Exosome, Retinal Pigment Epithelium, HSP70, Lipid rafts
The presence of αB in cholesterol rich membrane microdomains (lipid rafts)1 provides a physiological basis for the historically recognized, yet unexplained association of this protein with membranous components.2 Importantly lipid rafts are involved in exosome biogenesis.3 Although known to be a soluble protein4 we have demonstrated that αB is a Golgi-membrane associated protein in many tissue and cells including the RPE.1,5 It follows, therefore, that the association of αB with perinuclear Golgi is related to trans Golgi network where lipid rafts are assembled.
αB and exosome biogenesis in ARPE
Based on the assumption that exosomes carry cell-specific cargo for extracellular destinations,6 it is likely that the protein composition of an exosome may suggest clues about its biogenesis. Among the stress proteins, large heat shock proteins (e.g., HSP70, hsc70) are known to be present in exosomes; they may have a role in chaperoning various proteins to exosomes directly or via lipid rafts.7-9 The role of small heat shock proteins in exosome biogenesis, if any, is unknown. The reported anti-aggregation properties10 of αB, in particular its promiscuousness in these interactions may yet have an important role in exosomal information transfer.
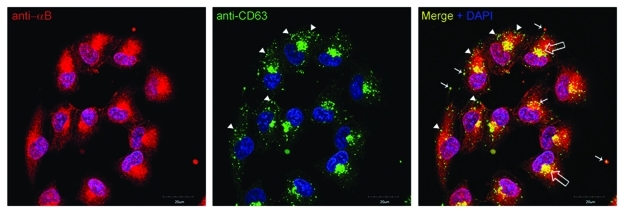
Exosomes contain a high concentration of tetraspanins,11 proteins known for enabling interactions among cytosolic and membrane proteins and facilitating membrane fission and fusion. The tetraspanin, CD63 is commonly employed to characterize exosomes. In ARPE cells co-localization of tetraspanin CD63 and αB in some multivesicular bodies (MVBs)/exosomes and perinuclear Golgi is easily visible (Fig. 1). However, it is interesting to note that αB (red, Figure 1) does not co-localize in CD63 positive MVBs/exosomes in the peripheral regions of the cell (green in Figure 1, Merge). This observation is in agreement with the differential location of tetraspanin enriched domains and the lipid rafts where αB resides. The relationship between the two remains to be sorted out.
Figure 1.
A confocal z-section (2.5 µm depth) of ARPE19 cells labeled with anti-αB (red) and tetraspanin (CD63) (green). Note co-localization in the perinuclear Golgi (open arrows) and vesicular staining (yellow, thin arrows).Interestingly, the co-localization seems to be more prevalent nearer the perinuclear Golgi (indicated by thin arrows). Note that the green (CD63) and Red (αB) do not go together in the periphery of the cells (arrow heads). Not all vesicles labeled with anti CD63 label with anti αB, indicating that the role of CD63 in αB-containing exosomes may be either transitory or selective. Nuclei are stained with DAPI (blue); scale bar = 20 µm.
Exosomes are assembled within the cell. Briefly, an exosome starts life as an intraluminal vesicle (ILV) in the late endosome.6 The assembly of ILVs entails invagination of the limiting membrane of the endosome creating a vesicle that must contain the proteins associated with the invaginating membrane and the immediate cytoplasm, now in its lumen. Repeated generation of such vesicles inside the endosome produces a MVB. The contents of the MVB, the exosomes or ILVs, are released out of the cell when it fuses with the plasma membrane. The invagination of the limiting membrane of the endosome involves participation of a multiprotein complex, ESCRT (Endosomal Sorting Complex Required for Transport), which recruits proteins from the membrane. Based on its anti-aggregation properties it is conceivable that αB associates with one of the ESCRT complexes to help sort specific set of proteins into MVBs. Although mechanistic details of this process are unknown the ESCRT machinery (which is on the cytoplasmic face of the late endosome) is known to use a mono-ubiquitination tag on a protein as a signal for its incorporation into a MVB.12 Once the proteins are directed to the inward budding ILVs, a de-ubiquitinating activity removes the ubiquitin tag from the cargo protein, now in the vesicle. Notably, αB is known to be a component of the of FBX-dependent ubiquitination of proteins13,14 and therefore, could act as a catalyst for protein recruitment into the ILVs. Thus αB expressing cells may produce exosomes with specific proteins. It is obvious that this process may be modulated by additional cues, both from inside and outside of the cell, as exemplified by MHC class II sorting during dendritic cell and T cell interactions.15
Exosomes and the RPE function
Exosome biogenesis is suggested to be directly associated with the endocytic pathway.6 This is interesting in light of the physiological preoccupation of the RPE cells in phagocytosis of the spent rod outer segments.16,17 This process operates on the apical face of this epithelium. Secretion of αB-containing exosomes is predominantly from the apical face of ARPE cells,1 which brings us to consider potential interactive links between these two processes. If αB has a role in exosome biogenesis (see above) then we would have established a molecular link between the physiology of RPE on one hand, and the functional health of neuroretina, on the other. This is relevant because αB is known to impart resistance to heat stress when expressed in cells in culture18 and is known to be present in the Inter photoreceptor matrix.19
Intestinal epithelial cells produce differentially loaded exosomes from their basal and the apical surfaces.20We would speculate that under normal conditions the polarized RPE produces exosomes from its basal and apical facets with differential molecular make up, thus catering to differential demands on the two sides of the interface of the blood-brain barrier. It is plausible that different physiological cues, or under pathological conditions αB is predominantly secreted from basal face of the RPE, which would result in the accumulation of αB in the “drusen.” It will be interesting to find conditions that cause reversal of the polarity of exosome secretion or cause miss-recruitment of αB into exosomes targeted for transport via the basal face. Importantly, lipid rafts may have a significant role in this polarized sorting.3
Finally, it is tempting to speculate that production of exosomes by RPE may be mechanistically related to the establishment of the “immune privilege”20 associated with the eye. This speculation is prompted by the reported production of a larger number of exosomes by tumor cells that have been shown to be immunosuppressive.21 The veracity of these speculations awaits experimental data. αB as a candidate exosomal gene product, however, promises a new insight into control and modulation of retinal pigment epithelial cell physiology.
Acknowledgments
This work was supported by National Eye Institutes Health, NIH, Bethesda, MD grants to SPB.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17610
References
- 1.Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261–9. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kibbelaar MA, Bloemendal H. Fractionation of the water-soluble proteins from calf lens. Exp Eye Res. 1979;29:679–88. doi: 10.1016/0014-4835(79)90024-1. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 4.Bhat SP. Crystallins, genes and cataract. Prog Drug Res. 2003;60:205–62. doi: 10.1007/978-3-0348-8012-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Gangalum RK, Schibler MJ, Bhat SP. Small heat shock protein alphaB-crystallin is part of cell cycle-dependent Golgi reorganization. J Biol Chem. 2004;279:43374–7. doi: 10.1074/jbc.C400371200. [DOI] [PubMed] [Google Scholar]
- 6.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–5. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 8.Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–75. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–9. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz J. The function of alpha-crystallin in vision. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 11.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559–62. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 12.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 13.Barbash O, Lin DI, Diehl JA. SCF Fbx4/alphaB-crystallin cyclin D1 ubiquitin ligase: a license to destroy. Cell Div. 2007;2:2. doi: 10.1186/1747-1028-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Engelsman J, Keijsers V, de Jong WW, Boelens WC. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J Biol Chem. 2003;278:4699–704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- 15.Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–42. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993;41:253–63. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- 17.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Hauck SM, Schoeffmann S, Deeg CA, Gloeckner CJ, Swiatek-de Lange M, Ueffing M. Proteomic analysis of the porcine interphotoreceptor matrix. Proteomics. 2005;5:3623–36. doi: 10.1002/pmic.200401223. [DOI] [PubMed] [Google Scholar]
- 20.Detrick B, Hooks JJ. Immune regulation in the retina. Immunol Res. 2010;47:153–61. doi: 10.1007/s12026-009-8146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 22.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–49. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]