Abstract
The sorting machinery in early endosomes is crucial for intracellular homeostasis and signal transduction and its disruption leads to the development of various diseases. In spite of its significance, the molecular mechanism underlying this machinery remains largely unknown. Actin filaments are implicated in intracellular trafficking, including membrane fission at endocytosis, membrane stretching at the Golgi complex, and maturation of endosomes. We have recently found that actin is required for receptor sorting in early endosomes and identified cortactin as a candidate for actin regulation in early endosomes. Inhibition of actin dynamics leads to enlargement of early endosomes and impairment of the sorting; the latter is also observed in cortactin-depleted cells. The endosomal localization of cortactin was enhanced by dynasore, a dynamin inhibitor that effectively inhibits endosomal sorting, indicating that cortactin is involved in the sorting machinery in early endosomes. Here we discuss the role of actin filaments in early endosomes and other molecules implicated in endosomal trafficking.
Keywords: early endosomes, actin, cortactin, WASH, membrane traffic
Cells sort internalized molecules into either recycling or degradation pathways using early endosomes.1,2 The sorting machinery is crucial for metabolism, intracellular signaling, and protection against infectious diseases; dysfunctions can induce various diseases.3,4 The sorting mechanism for the degradation of molecules is known to involve protein ubiquitination and the ESCRT machinery.5,6 The recycling pathway utilizes “geometrical sorting” of tubular endosomes, which have a high membrane surface-to-content ratio. However, in spite of its significance,7 the sorting mechanism for recycling molecules is yet to be elucidated.
While screening inhibitors that affect transport from early endosomes to recycling endosomes, we found that actin inhibitors effectively impaired endosomal traffic.8 These inhibitors did not significantly affect late endosome-to-lysosome traffic. When actin dynamics were inhibited, early endosomes fused with each other and induced the formation of large endosomes that contained molecules for both degradation and recycling. When the drug was removed, endosomal sorting restarted. Our results indicate that actin dynamics is required for the prevention of homotypic fusion and sorting in early endosomes.
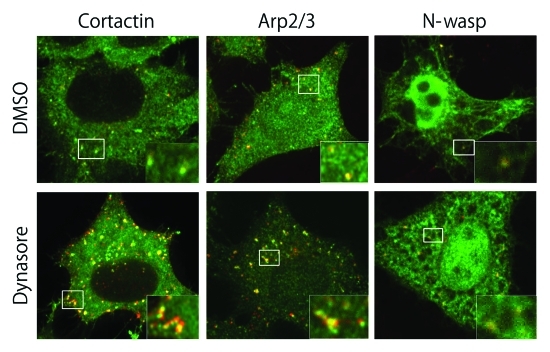
In addition, we determined that cortactin is required for transport from early endosomes. Cortactin is localized on early endosomes. When we blocked transport from early endosomes by treatment with dynasore,7 colocalization of cortactin and internalized transferrin significantly increased (Fig. 1). Moreover, in cortactin-depleted cells both receptor sorting and recovery from actin depolymerization were delayed, indicating that cortactin is essential in early endosomal transport.
Figure 1.
Cortactin and Arp2/3 are localized in early endosomes and their localization is enhanced by treatment with dynasore. HeLa cells were allowed to internalize Alexa555-trnasferrin (red) for 15 min and treated with 0.5% DMSO (upper panels) or 400 μM dynasore (lower panels) 5 min after internalization. They were stained with anti-cortactin, anti-Arp2/3, or exogenously expressed myc-N-wasp (green). The colocalization of cortactin and Arp2/3, but not of N-wasp, with internalized transferrin was significantly enhanced by dynasore.
Cortactin regulates actin dynamics through activation of the Arp2/3 complex.9,10 A similar mechanism is utilized by other activators of the Arp2/3 complex, termed nucleation-promoting factors, which include N-wasp, WAVE, JMY, WHAMM, and the recently identified WASH complex.11-15 N-wasp is also an actin regulator of endocytosis, and we found the partial localization of N-wasp in early endosomes; however, its localization is not enhanced by the treatment with dynasore (Fig. 1). Although we found that the N-wasp inhibitor wiskostatin affects early endosomal transport, its depletion by siRNA has no effect on transport (data not shown). Our results are in agreement with a recent report that found no significant localization of N-wasp or WAVE in early endosomes,16 suggesting that wiskostatin may inhibit other targets.17 Instead, the WASH complex has been identified as a crucial regulator of actin in early endosomes.15,18-20 The depletion of WASH results in the tubular extension of early endosomes, probably by inhibiting tubular fission. Moreover, its depletion impairs the recruitment of cortactin to endosomes. Our results imply that both WASH and cortactin may act cooperatively in actin dynamics in early endosomes.
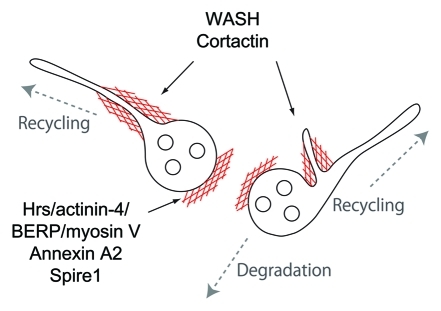
We revealed that the inhibition of actin dynamics induces the formation of enlarged early endosomes and impairs receptor sorting. However, cortactin-depleted cells do not form enlarged endosomes, implying that other factors, such as Hrs/actinin-4/BERP/myosinV, may regulate endosomal fusion by regulating actin dynamics.21 Cortactin and WASH may affect receptor sorting in early endosomes (Fig. 2). However, actin itself appears to be involved in sorting for the regulated recycling pathway, including beta2-adrenergic receptors.16 Actin may have multiple roles in regulating endosomes, including fusion, membrane segregation, and receptor sorting. Interestingly, GOLPH3 binds to both phosphatidylinositol and actomyosin, providing tensile force to stretch the Golgi structure.22,23 A similar mechanism may create membrane tubulation in early endosomes because tubular membrane carriers are located in the Golgi and early endosomes. Future studies are necessary to elucidate how membrane deformation and cytoskeletal remodeling are coordinated for the sorting mechanism.
Figure 2.
Actin-associated proteins implicated in endosomal transport. The large globular region, which contains molecules for degradation, involves the Hrs/actinin-4/BERP/myosin V complex (CART), Annexin A2, and Spire1. Tubular extension of endosomes, which are destined for the recycling pathway, involves WASH and cortactin.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17628
References
- 1.Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 3.Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic. 2002;3:781–90. doi: 10.1034/j.1600-0854.2002.31103.x. [DOI] [PubMed] [Google Scholar]
- 4.Aridor M, Hannan L. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1:836–51. doi: 10.1034/j.1600-0854.2000.011104.x. [DOI] [PubMed] [Google Scholar]
- 5.Henne WM, Buchkovich NJ, Emr SD. The ESCRT Pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 7.Mesaki K, Tanabe K, Obayashi M, Oe N, Takei K. Fission of tubular endosomes triggers endosomal acidification and movement. PLoS ONE. 2011;6:e19764. doi: 10.1371/journal.pone.0019764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohashi E, Tanabe K, Henmi Y, Mesaki K, Kobayashi Y, Takei K. Receptor sorting within endosomal trafficking pathway is facilitated by dynamic actin filaments. PLoS ONE. 2011;6:e19942. doi: 10.1371/journal.pone.0019942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–61. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 11.Stradal TEB, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–11. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 13.Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134:148–61. doi: 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuchero JB, Coutts AS, Quinlan ME, Thangue NBL, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol. 2009;11:451–9. doi: 10.1038/ncb1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derivery E, Sousa C, Gautier J, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–23. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, et al. Sequence-Dependent Sorting of Recycling Proteins by Actin-Stabilized Endosomal Microdomains. Cell. 2010;143:761–73. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerriero CJ, Weisz OA. N-WASP inhibitor wiskostatin nonselectively perturbs membrane transport by decreasing cellular ATP levels. Am J Physiol Cell Physiol. 2007;292:C1562–6. doi: 10.1152/ajpcell.00426.2006. [DOI] [PubMed] [Google Scholar]
- 18.Derivery E, Gautreau A. Evolutionary conservation of the WASH complex, an actin polymerization machine involved in endosomal fission. Commun Integr Biol. 2010;3:227–30. doi: 10.4161/cib.3.3.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David R. Endocytosis: WASHed and sorted. Nat Rev Mol Cell Biol. 2010;11:6–7. doi: 10.1038/nrm2819. [DOI] [PubMed] [Google Scholar]
- 20.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton. 2010;67:193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Q, Sun W, Kujala P, Lotfi Y, Vida TA, Bean AJ. CART: an Hrs/actinin-4/BERP/myosin V protein complex required for efficient receptor recycling. Mol Biol Cell. 2005;16:2470–82. doi: 10.1091/mbc.E04-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011;21:113–21. doi: 10.1016/j.tcb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dippold HC, Ng MM, Farber-Katz SE, Lee S-K, Kerr ML, Peterman MC, et al. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–51. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]