Abstract
The establishment and maintenance of epithelial cell polarity is essential throughout the development and adult life of all multicellular organisms. A key player in maintaining epithelial polarity is Crumbs (Crb), an evolutionarily conserved type-I transmembrane protein initially identified in Drosophila. Correct Crb levels and apical localization are imperative for its function. However, as is the case for many polarized proteins, the mechanisms of its trafficking and strict apical localization are poorly understood. To address these questions, we developed a liposome-based assay to identify trafficking coats and interaction partners of Crb in a native-like environment. Thereby, we demonstrated that Crb is a cargo for Retromer, a trafficking complex required for transport from endosomes to the trans-Golgi-network. The functional importance of this interaction was revealed by studies in Drosophila epithelia, which established Retromer as a novel regulator of epithelial cell polarity and verified the vast potential of this technique.
Three mutually antagonistic protein complexes have emerged as the key regulators of epithelial polarity, the PAR-3, Scribble, and Crb complexes.1 Crucially for their functions, these complexes localize to discrete sites of the plasma membrane: the Crb complex localizes apically to the adherens junction (analogous to the tight junction in vertebrate epithelia)2,3 whereas the Scribble complex localizes to the lateral and basal domains.4 Members of the PAR-3/Baz complex localize to the apical and sub-apical regions as well as to the adherens junction itself.5 Maintaining these distinct localizations depends not only on antagonism between these polarity complexes1 but also on polarized trafficking itineraries that deliver secreted and membrane proteins to specific membrane domains.6 Mechanisms of polarized traffic vary hugely depending on the cell type and the developmental stage. Extensive work in polarized cell systems has revealed several routes including vectorial and transcytotic pathways.7,8
It has been shown in many cases that the targeting information guiding a transmembrane protein to its sub-cellular destination can be encoded within its cytoplasmic domain, in the form of a short motif.9 Recognition of this motif by trafficking complexes allows decoding of this information, correct sorting of the cargo and transport to its subcellular destination.9,10 By consequence, the characterization of the interaction network of a transmembrane cargo can provide valuable information on the pathways through which it travels as well as the mechanisms of the underlying sorting events. With this rationale the Hoflack lab successfully characterized coat assembly of AP-1 mediated transport of the varicella zoster virus glycoprotein I11 and AP-3 mediated transport of LIMP-II12 using chemically synthesized peptides covalently coupled to liposomes via a hydrazone bond.13 These so-called proteo-liposomes were then used for recruitment, isolation and identification of potential interaction partners from brain cytosol. The main advantage of this system over conventional pull down assays or yeast two hybrid methods is the strongly enhanced sensitivity and specificity. This is achieved by presenting the cytoplasmic domain of the transmembrane cargo in a membrane context, which allows the isolation of sorting complexes that commonly require cues from both cargo and membrane.9 Furthermore, in this biochemical system experimental variables like the activation state of GTPases can be modified by the inclusion of GTP or GTPγS during protein recruitment.
In Pocha et al. 201114 we redesigned this system by coupling the recombinantly expressed and purified cytoplasmic domain of Crb 2 to liposomes via a thioether bond,14,15 thereby overcoming the restrictions imposed by chemical peptide synthesis. We found that Crb 2 specifically recruits Vps35 and Vps26b from brain cytosol, two subunits of an endosome-localized sorting complex termed the Retromer.16 The established and conserved function of this complex is to retrieve transmembrane receptors from the limiting endosomal membrane and to mediate their transport to the trans-Golgi-network (TGN).17 Loss of function of Retromer leads to missorting of its cargoes to late endosomes and lysosomes, resulting in the degradation and loss of cargoes.18,19 To test the functional significance of the Crb-Retromer interaction we inactivated Vps35 or Vps26 by either using a loss-of-function allele for Vps3520 or RNAi suppression of both genes in Drosophila. This produced a prominent loss of Crb protein in larvae, imaginal discs and the follicular epithelium, a common model epithelium for studying cell polarity. Additionally, loss of Vps35 induced two striking polarity defects: 1) multilayering of the follicular epithelium, indicative of gross defects in cell polarity, and 2) more specifically, loss of the Crb partner Stardust and a reduction in the levels of apically localized aPKC and Par6 (both members of the Par/Baz complex). The latter could be rescued by the overexpression of Crb, demonstrating that indeed the diminished levels of Crb caused by the inactivation Retromer are responsible for disrupted cell polarity. This study thus provides proof of principle that proteo-liposomes can be employed successfully to understand the trafficking properties of previously uncharacterized membrane proteins.
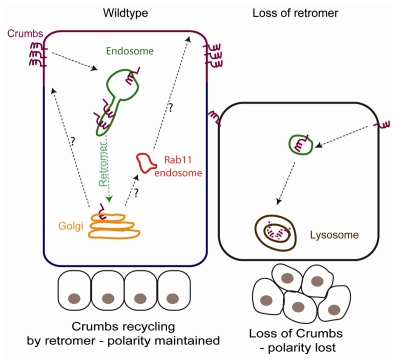
Interestingly, our findings highlight the fact that Crb localization at the apical pole of polarized cells is far from static. Crb undergoes endocytosis, reaching endosomes, from which it is retrieved by Retromer. The next step(s) in the trafficking of Crb in polarized cells remains largely unknown. Whether it traverses the TGN on its way back to the plasma membrane or other endosomal compartments, particularly Rab 11-positive recycling endosomes, will need to be explored in future studies (Fig. 1). Previously it has been suggested that Crb traffics via Rab 11-positive recycling endosomes on its journey to the plasma membrane.21,22 However, it is currently unclear whether only newly synthesized Crb traffics through Rab11 positive endosomes or if Crb recycling from the plasma membrane converges with newly synthesized Crb in this compartment.
Figure 1.
Trafficking of Crumbs in wildtype and Retromer-deficient epithelial cells. In wildtype cells Crb is located at steady-state at the apical domain (purple) and excluded from the basolateral domain of the plasma membrane (blue). It undergoes endocytosis and is retrieved from endosomes by Retromer. The pathway(s) by which it is recycled to the apical domain remain(s) uncertain. One possibility is that Crb is transported to the TGN from where it is sorted to the apical domain, either directly or indirectly via Rab11 positive recycling endosomes (indicated by black arrows). Alternatively, Retromer may mediate a more direct sorting of cargoes to the apical domain. Loss of Retromer leads to reduced retrieval of Crb from endosomes. Crb trapped in endosomes is degraded in lysosomes, resulting in strongly reduced Crb levels and striking defects in epithelial polarity and integrity.
One point still to be resolved is the functional significance of Crb recycling. Is the retrograde transport of Crb a method of regulating plasma membrane levels of Crb? Or is there a particular significance in the recycling of Crb by Retromer rather than other recycling machineries? The latter question raises new possibilities with regards to the function of Crb itself. It has been shown in various systems that polarized molecules depart from the TGN in a specific manner, i.e., sorting of polarized transport can occur at the level of the Golgi.7 The transport of Crb back to this site may suggest a role for Crb in co-transporting other apically destined proteins on its way to the apical membrane. This might be an attractive hypothesis given the observed defects in apicalization in the absence of Crb.3,23 Alternatively, it is conceivable that Crb, like many other Retromer cargoes, is acting as a transport receptor for secreted proteins that are sorted in the TGN. In this case Crb would travel back to the TGN to bind soluble cargo(es) that require specific secretion from the apical surface. Exploring these possibilities in future studies will strongly enhance our understanding of Crb and its role in epithelial polarization.
Acknowledgments
We thank Dr. E. Knust for helpful discussions and critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17658
References
- 1.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–74. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–99. doi: 10.1016/0092-8674(90)90189-L. [DOI] [PubMed] [Google Scholar]
- 3.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 4.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–8. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 5.Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–23. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–45. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fölsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–81. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–66. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aridor M, Traub LM. Cargo selection in vesicular transport: the making and breaking of a coat. Traffic. 2002;3:537–46. doi: 10.1034/j.1600-0854.2002.30804.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–14. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 11.Baust T, Czupalla C, Krause E, Bourel-Bonnet L, Hoflack B. Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc Natl Acad Sci USA. 2006;103:3159–64. doi: 10.1073/pnas.0511062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baust T, Anitei M, Czupalla C, Parshyna I, Bourel L, Thiele C, et al. Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell. 2008;19:1942–51. doi: 10.1091/mbc.E08-02-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourel-Bonnet L, Pécheur EI, Grandjean C, Blanpain A, Baust T, Melnyk O, et al. Anchorage of synthetic peptides onto liposomes via hydrazone and alpha-oxo hydrazone bonds. preliminary functional investigations. Bioconjug Chem. 2005;16:450–7. doi: 10.1021/bc049908v. [DOI] [PubMed] [Google Scholar]
- 14.Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E. Retromer controls epithelial cell polarity by trafficking the apical determinant Crb. Curr Biol. 2011;21:1111–7. doi: 10.1016/j.cub.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Crottet P, Meyer DM, Rohrer J, Spiess M. ARF1.GTP, Tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol Biol Cell. 2002;13:3672–82. doi: 10.1091/mbc.E02-05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–22. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–7. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeth JF, Sawyer JK, Wilner A, Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS ONE. 2009;4:e7634. doi: 10.1371/journal.pone.0007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, et al. Trafficking of Crb3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–63. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wodarz A, Grawe F, Knust E. CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech Dev. 1993;44:175–87. doi: 10.1016/0925-4773(93)90066-7. [DOI] [PubMed] [Google Scholar]