Abstract
The Ras proto-oncogenic proteins, prototypes of the small GTPases, work as molecular switches: they are active when bound to GTP and inactive when bound to GDP. A variety of evidence suggested that the Ras paradigm is not fully valid for the Rho-family of small GTPases. Indeed, permanent activation is not sufficient but it is rather the continuous oscillation between the GDP-bound and GTP-bound conformations (namely the GDP/GTP cycling or GTPase flux), that is required for Rho-GTPases to perform their biological functions and properly coordinate actin cytoskeleton reorganization. In our recent study, we show that Rac1 needs to cycle between the GDP and GTP states in order to efficiently control cell motility. Similarly, it was previously reported that GDP/GTP cycling is required by RhoA for cytokinesis and by Cdc42 for cell polarization. The future challenge is to understand why the GTPase flux is so important for the biological actions of Rho GTPases.
Cell motility is an essential process involved in a large variety of biological phenomena. Different molecules and pathways have been linked to cell migration but very little is known about how they are integrated in time and space to regulate the motility process.1 It is well established that in motile cells the Rac1 GTPase drives the formation of the actin polymerization network at front protrusions by interacting with its effectors, such as the pentameric Wave complex and the Pak1 kinase.2 Other works, including those of our laboratory, had identified a role for the RalB GTPase in regulation of cell motility, via its downstream effector, the octameric exocyst complex.3-5
In our recent study6 we report a first physical and functional link connecting the actions of RalB and Rac1. We showed that the Ral-effector exocyst directly binds to a negative regulator of Rac1: the SH3BP1 protein. SH3BP1 belongs to the RhoGAP (GTPase Activating Protein) family7; it catalyzes the hydrolysis of Rac1-bound GTP to GDP, leading to inactive GDP-bound Rac1 conformation. We propose that the exocyst acts as a “molecular taxi” which participates in the transport and recruitment of SH3BP1 to the front of motile cells. We found that SH3BP1 localizes at the leading edge and regulates cell motility by downregulating Rac1 specifically at this cellular location. Interfering with this spatial Rac1 regulation, by depleting SH3BP1 using RNA interference or by expressing the constitutively active Rac1 G12V mutant, had a dramatic impact on the cells, inducing “anarchic” protrusions and consequently inefficient motility.
Our work indicates that Rac1 inactivation at the leading edge is necessary for cell migration. On the other hand, it is well known that, for a cell to move, Rac1 needs to be activated at the same cellular location by GEF (Guanine Nucleotide Exchange Factor) proteins which catalyzes the exchange of the GDP to GTP.7 The necessity of both Rac1 activation and Rac1 inactivation for motility implies that a continuous Rac1 GDP/GTP cycling occurs at the leading front. These findings support the concept that GTPases are more complex than simple “ON-OFF switches” which execute their biological functions only when locked in the “ON” GTP-bound state.8
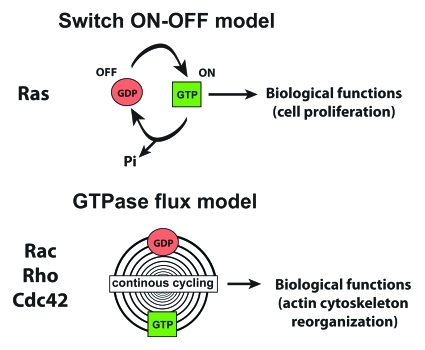
Indeed, it is well established and widely accepted that for the prototype Ras proteins (H-, N-, K-Ras) the GTP-loading suffices for their biological activities, i.e., binding to effectors and stimulation of down-stream signaling pathways. Accordingly, mutations that lock Ras in the GTP-bound state and constitutively activate oncogenic pathways are commonly found in human cancers.9 However, a variety of evidence suggested that other small GTPases, particularly those of the Rho family (Rho/Rac/Cdc42), require a continuous GDP/GTP cycling in order to perform their functions. In other words, GTP-loading is still necessary for binding to effectors, but is not sufficient per se for efficient execution of the Rho/Rac/Cdc42-controlled biological processes which are mainly related to actin cytoskeleton reorganization. Thus, the Ras paradigm does not hold for Rho-family GTPases (Fig. 1).
Figure 1.
Schematic comparison of the switch ON-OFF and GTPase flux models for the biological actions of small GTPases.
Concerning the Cdc42 protein, several findings pointed out the importance of GTP hydrolysis and GDP/GTP cycling for its biological activities: expression of the fast-cycling F28L mutant, but not that of the GTPase-deficient Q61L, induced cellular transformation;10 modulation of cell proliferation and transformation could be achieved by biochemical manipulations of Cdc42 ability to oscillate with the proper frequency between its GTP-bound and GDP-bound forms;11 in the yeast Saccharomyces cerevisiae, elegant genetics studies showed that Cdc42p cycling is critical for cell polarization12,13 and cell fusion.14
Regarding RhoA, the GAP activity of MgcRacGAP (member of the RhoGAP family) was reported to be necessary both at early steps and throughout the cytokinesis for the spatiotemporal regulation of this GTPase.15 This last work disproved the assumption that RhoA is sequentially switched ON (by GEFs) early in cytokinesis and then switched OFF (by GAPs) to disassemble the cytokinesis apparatus. The authors proposed instead that GEFs (e.g., Ect2) and GAPs (e.g., MgcRacGAP) act simultaneously, so that RhoA undergoes a rapid “flux” through the GTPase cycle during the entire duration of the cytokinesis process. The authors also anticipated that GTPase flux may drive other actomyosin dynamic activities.15
In the same line, our own work showed that the GAP activity of SH3BP1 is necessary for the spatiotemporal regulation of Rac1 throughout motility, supporting the notion of a general GTPase flux model for Rho-family GTPases. We can now view the regulation of cell motility under a new perspective: in motile cells Rac1 proteins are constantly cycling through the actions of GEFs (putatively βPix, Dock3, Asef, Tiam1) and the GAP SH3BP1; the balance between GDP-loaded and GTP-loaded Rac1 molecules can be shifted in both directions by modulation of both GEF and GAP activities, in a spatially and temporally controlled manner. In particular, the formation, maturation or disassembly of new adhesions is accompanied by signaling cascades that may affect the activity and/or the localization of these GEF/GAP regulators; for example, the GEF βPix is part of a trimeric complex, the PIX-PAK-GIT complex, which, via the GIT component, directly interacts with paxillin at focal adhesion sites.16 A third group of Rac regulators, the GDIs (Guanine Nucleotide Dissociation Inhibitors), is also involved in the local control of active Rac1 concentration via quite complex mechanisms.17-19
It is tempting to further speculate that the Rac1 GTPase flux may participate in the regulation of the motility periodic processes, such as the turnover of focal adhesions or the protrusion-retraction cycles occurring at the leading edges.20,21 However, by using a novel photo-activatable Rac1 construct,22 it was shown that not-cycling GTP-locked Rac1 can drive cell motility if its activity (i.e., capacity to bind effectors) is locally unmasked by the light; this was observed both with cultured isolated cells22 and with entire group of cells (the border cells) in the Drosophila ovary.23 This means that the spatial control of Rac1-GTP interactions with its effectors is sufficient to artificially direct cell movements, but our data indicate that physiological and efficient motility requires a fine spatio-temporal tuning of the GDP/GTP cycle of Rac1.
Many more questions are still pending. Kinetically, we have no clue on the speed of the GDP/GTP cycling of Rac1 during motility, the same is true for RhoA in cytokinesis; theoretically, the kinetic parameters could be measured by using FRET-based biosensors of the same type we used in our work, but this would require single-molecule resolution, which is technically challenging. Biochemically, we are far from understanding how the four groups of partners (GEFs, effectors, GDIs and GAPs) can rapidly alternate and integrate their respective interactions with the GTPase; the GDP/GTP flux is likely dictated by a complex and dynamic balance of various factors, including local concentrations, steric hindrance and competition effects. Functionally, we still do not know why the GTPase flux is so crucial for the action of Rho GTPases in actin cytoskeleton remodeling. It could be a mechanism to quickly shift the GDP/GTP state equilibrium and transiently generate spatially-constrained bursts of GTPase-dependent signals. Alternatively, the oscillation between GDP and GTP forms could insure the shuttle of the GTPase between different cellular localizations or different binding partners. All these questions need to be addressed in the future to better characterize the properties and the relevance of the GTPase flux of Rho-family GTPases.
Acknowledgments
We thank Carine Joffré, Adriana Santos and Pascal Silberzan for critically reading the manuscript. This work was supported by grants ANR IntegRal, ARC4845 and Association Christelle Bouillot (J.C.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17772
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–94. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Oxford G, Owens CR, Titus BJ, Foreman TL, Herlevsen MC, Smith SC, et al. antagonistic relatives in cancer cell migration. Cancer Res. 2005;65:7111–20. doi: 10.1158/0008-5472.CAN-04-1957. [DOI] [PubMed] [Google Scholar]
- 5.Rossé C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol. 2006;26:727–34. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrini MC, Sadou-Dubourgnoux A, Aoki K, Kunida K, Biondini M, Hatzoglou A, et al. SH3BP1, an Exocyst-associated RhoGAP, inactivates Rac1 at the front to drive cell motility. Mol Cell. 2011;42:650–61. doi: 10.1016/j.molcel.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–9. doi: 10.1016/S0962-8924(00)01832-8. [DOI] [PubMed] [Google Scholar]
- 9.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 10.Lin R, Bagrodia S, Cerione R, Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr Biol. 1997;7:794–7. doi: 10.1016/S0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- 11.Fidyk N, Wang J-B, Cerione RA. Influencing cellular transformation by modulating the rates of GTP hydrolysis by Cdc42. Biochemistry. 2006;45:7750–62. doi: 10.1021/bi060365h. [DOI] [PubMed] [Google Scholar]
- 12.Irazoqui JE, Gladfelter AS, Lew DJ. Cdc42p, GTP hydrolysis, and the cell’s sense of direction. Cell Cycle. 2004;3:861–4. doi: 10.4161/cc.3.7.993. [DOI] [PubMed] [Google Scholar]
- 13.Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5:1062–70. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 14.Barale S, McCusker D, Arkowitz RA. Cdc42p GDP/GTP cycling is necessary for efficient cell fusion during yeast mating. Mol Biol Cell. 2006;17:2824–38. doi: 10.1091/mbc.E05-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–7. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur J Cell Biol. 2006;85:265–74. doi: 10.1016/j.ejcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Moissoglu K, Slepchenko BM, Meller N, Horwitz AF, Schwartz MA. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell. 2006;17:2770–9. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, et al. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009;20:2049–59. doi: 10.1091/mbc.E07-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chianale F, Rainero E, Cianflone C, Bettio V, Pighini A, Porporato PE, et al. Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI. Proc Natl Acad Sci USA. 2010;107:4182–7. doi: 10.1073/pnas.0908326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machacek M, Danuser G. Morphodynamic profiling of protrusion phenotypes. Biophys J. 2006;90:1439–52. doi: 10.1529/biophysj.105.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, et al. A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol. 2011;13:371–81. doi: 10.1038/ncb2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–7. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]