Abstract
Regulated secretion is a fundamental cellular process in many different types of eukaryotic cells with Ca2+-triggered exocytosis taking centre stage. Elevations of the cytoplasmic Ca2+ concentration ([Ca2+]c) regulate multiple steps from vesicle fusion with the plasma membrane to fusion pore dilation and subsequent retrieval of spent vesicles. The general view is that the rise in [Ca2+]c initiates during the pre-fusion stage and either results from Ca2+-influx via Ca2+ channels in the plasma membrane or from release from intracellular Ca2+-stores. However, there is increasing evidence that exocytosis of secretory vesicles triggers additional, localised Ca2+ signals via insertion of vesicle-associated Ca2+ channels into the cell surface. These restricted Ca2+ signals following fusion are ideally suited for regulating the post-fusion fate of individual secretory vesicles. In invertebrates they have been shown to trigger compensatory endocytosis. Recently we have reported that exocytosis of lamellar bodies in alveolar type II epithelial cells results in a localized Ca2+-influx via vesicular P2X4 receptors which regulates fusion pore expansion and vesicle content release. This finding expands the emerging picture that post-fusion Ca2+ entry via vesicle-associated Ca2+ channels plays a central role for regulated exocytosis.
Keywords: calcium, exocytosis, fusion pore, endocytosis, secretion, vesicle
Ca2+ is considered a key element for multiple steps during regulated exocytosis. It is well established that a series of Ca2+-dependent steps during the pre-fusion stage is essential for fusion of exocytic vesicles with the plasma membrane.1-3 More recently, it became obvious that Ca2+ also plays a central role during the post-fusion phase, from regulating fusion pore dilation4-9 to coupling exocytosis to compensatory endocytosis.10,11 The current view is that these elevations of the [Ca2+]c initiate during the pre-fusion stage and last into the post-fusion phase. In neurons and neuro-endocrine cells, this ch is mediated by the close proximity and the spatio-temporal pattern of Ca2+ signals xocytosis. results from activation of voltage-gated Ca2+ channels in the plasma membrane,12 whereas in non-excitable secretory cells Ca2+ is also released from intracellular stores.13,14 However, in slow and non-excitable secretory cells in particular, additional mechanisms to rise [Ca2+]c during the post-fusion stage might be important. Ca2+ is readily cleared from the cytoplasm and the initial [Ca2+]c peak will abade before onset of the post-fusion phase.15,16 But what would be an efficient mechanism for creating such post-fusion Ca2+ signals? If such a rise in Ca2+ is restricted in time and space to the onset and site of vesicle fusion, respectively, it would be ideally suited to selectively affect this fused vesicle. A global rise in [Ca2+]c would not only affect regulation of the post-fusion fate of the fused vesicle, but would have a much wider impact, even triggering a new round of vesicle fusions.
Recent reports suggest that the secretory vesicles might harbour the remedy to this problem themselves. In invertebrates it has been shown that vesicular calcium channels are inserted into the cell surface upon exocytotic activity leading to calcium influx through these channels triggering compensatory endocytosis.17,18 In sea urchin eggs these channels have been identified as P-type voltage-gated Ca2+ channels17 that are activated in response to a depolarized plasma membrane upon vesicle fusion. For the newly identified “Flower” channel in fruit fly nerve terminals18 it remains to be determined whether it is also voltage sensitive or sensitive to extracellular signaling factors. In both instances the Ca2+ channels located on exocytic vesicles play a pivotal role during the post-fusion stage and guarantee a steady-state between the amount of membrane inserted and the amount that is retrieved. In neurons clearing the fused vesicle membrane from the release site and replenishing of the vesicular pool is critical for maintaining exocytosis.11
Ca2+ channels localized in the membranes of the secretory vesicles that respond to changes in the membrane potential or the extracellular chemical milieu upon fusion are ideally suited for generating a localized Ca2+ signal timed to the onset of fusion and constrained to microdomains around individual vesicles. However, despite the „advantages“ of such selective Ca2+ signalling, evidence for a similar mechanism in mammalian systems was still missing.
Miklavc et al.19 have recently described a “fusion-activated“ Ca2+-entry (FACE) mechanism in alveolar type II (ATII) epithelial cells. We found that the fusion of lamellar bodies, large storage organelles for lung surfactant, with the plasma membrane was followed by a transient rise of localized [Ca2+]c originating at the site of the lamellar body fusion. FACE followed initial fusion pore opening with a delay of 200-500 ms. Now we provided evidence that FACE is mediated by vesicle-associated P2X4 purinergic receptors, ligand-gated ion channels with high Ca2+ permeability.20 In contrast to the findings in invertebrates, we could not observe a significant increase in endocytic activity following FACE in ATII cells. However, we found that FACE facilitates fusion pore expansion and subsequent surfactant release, demonstrating direct regulation of the immediate post-fusion phase of exocytosis in mammalian cells by vesicle-associated Ca2+ channels. This finding expands the emerging picture that post-fusion Ca2+ entry via vesicle-associated Ca2+ channels plays an important role in regulated exocytosis and secretion.
It will be interesting to see whether similar mechanisms are present in other secretory cells. At least in non-neuronal systems it is becoming evident that secretory output is not mainly adjusted by changing the number of vesicles fusing, but by regulating the release of vesicle contents via modulation of fusion pore dynamics and vesicle retrieval during the post-fusion stage.21 It is hence tempting to speculate that generation of Ca2+-microdomains around fused vesicles is a key element of the complex post-fusion vesicle behavior. It is also yet unclear what determines the outcome of post-fusion Ca2+ signals (i.e., fusion pore opening vs. compensatory endocytosis). Does it depend on the spatio-temporal characteristics of the local rise in Ca2+ and hence on the occurrence and type of vesicular Ca2+ channel involved? Is it dependent on the cell type and mode of fusion (rapid, bulk fusion of many small vesicles vs slow fusion of few large granules), which endows vesicles with different molecular machineries? Are biophysical properties of the vesicle (e.g., swelling upon fusion) effectors/affectors?22 What other functions for post-fusion Ca2+ signals can be identified?
It will be interesting to see what the answers to these questions are and further work will be needed to decode the mechanisms how vesicle-associated Ca2+ channels regulate the post-fusion stage of exocytosis and the functions thereof.
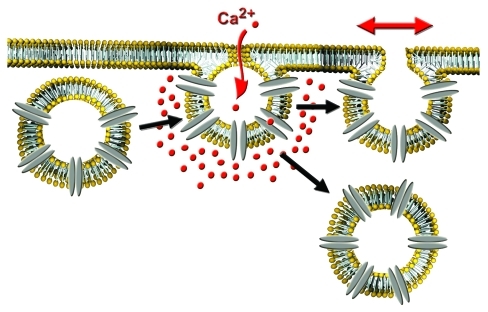
Figure 1.
Vesicle-associated Ca2+ channels regulate post-fusion stage of exocytosis. Insertion of vesicular calcium channels into the cell surface upon fusion of secretory vesicles with the plasma membrane results in localized Ca2+-entry and a rise in the Ca2+ concentration around the fused vesicle. This can either modulate fusion pore expansion and content release or promote compensatory endocytosis.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17935
References
- 1.Burgoyne RD, Morgan A. Calcium sensors in regulated exocytosis. Cell Calcium. 1998;24:367–76. doi: 10.1016/S0143-4160(98)90060-4. [DOI] [PubMed] [Google Scholar]
- 2.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Chacón R, Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Lett. 1995;363:221–5. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- 5.Haller T, Dietl P, Pfaller K, Frick M, Mair N, Paulmichl M, et al. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J Cell Biol. 2001;155:279–89. doi: 10.1083/jcb.200102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann J, Lindau M. A novel Ca2+-dependent step in exocytosis subsequent to vesicle fusion. FEBS Lett. 1995;363:217–20. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- 7.Jackson MB, Chapman ER. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu Rev Biophys Biomol Struct. 2006;35:135–60. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 8.Scepek S, Coorssen JR, Lindau M. Fusion pore expansion in horse eosinophils is modulated by Ca2+ and protein kinase C via distinct mechanisms. EMBO J. 1998;17:4340–5. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segovia M, Ales E, Montes MA, Bonifas I, Jemal I, Lindau M, et al. Push-and-pull regulation of the fusion pore by synaptotagmin-7. Proc Natl Acad Sci USA. 2010;107:19032–7. doi: 10.1073/pnas.1014070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel SS, Smith RM, Baibakov B, Ikebuchi Y, Lambert NA. Calcium influx is required for endocytotic membrane retrieval. Proc Natl Acad Sci USA. 1999;96:5019–24. doi: 10.1073/pnas.96.9.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, et al. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–10. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–30. doi: 10.1016/S0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 13.Coorssen JR, Schmitt H, Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 1996;15:3787–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Frick M, Eschertzhuber S, Haller T, Mair N, Dietl P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am J Respir Cell Mol Biol. 2001;25:306–15. doi: 10.1165/ajrcmb.25.3.4493. [DOI] [PubMed] [Google Scholar]
- 15.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 16.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 17.Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, et al. Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J Cell Biol. 2000;148:755–67. doi: 10.1083/jcb.148.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, et al. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–60. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miklavc P, Frick M, Wittekindt OH, Haller T, Dietl P. Fusion-activated Ca2+ entry: an “active zone” of elevated Ca2+ during the postfusion stage of lamellar body exocytosis in rat type II pneumocytes. PLoS ONE. 2010;5:e10982. doi: 10.1371/journal.pone.0010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, et al. Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1101039108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorn P. New insights into the control of secretion. Commun Integr Biol. 2009;2:315–7. doi: 10.4161/cib.2.4.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerberg J, Chernomordik LV. Membrane fusion. Adv Drug Deliv Rev. 1999;38:197–205. doi: 10.1016/S0169-409X(99)00029-0. [DOI] [PubMed] [Google Scholar]