Abstract
Several genome-wide association and candidate gene studies have linked chromosome 15q24–q25.1 (a region including the CHRNA5-CHRNA3-CHRNB4 gene cluster) with alcohol dependence, nicotine dependence and smoking-related illnesses such as lung cancer and chronic obstructive pulmonary disease. To further examine the impact of these genes on the development of substance use disorders, we tested whether variants within and flanking the CHRNA5-CHRNA3-CHRNB4 gene cluster affect the transition to daily smoking (individuals who smoked cigarettes 4 or more days per week) in a cross sectional sample of adolescents and young adults from the COGA (Collaborative Study of the Genetics of Alcoholism) families. Subjects were recruited from families affected with alcoholism (either as a first or second degree relative) and the comparison families. Participants completed the SSAGA interview, a comprehensive assessment of alcohol and other substance use and related behaviors. Using the Quantitative trait disequilibrium test (QTDT) significant association was detected between age at onset of daily smoking and variants located upstream of CHRNB4. Multivariate analysis using a Cox proportional hazards model further revealed that these variants significantly predict the age at onset of habitual smoking among daily smokers. These variants were not in high linkage disequilibrium (0.28<r2<0.56) with variants that have previously been reported to affect risk for nicotine dependence and smoking related diseases in adults. The data suggests that an age-associated relationship underlies the association of SNPs in CHRNB4 with onset of chronic smoking behaviors in adolescents and young adults and may improve genetic information that will lead to better prevention and intervention for substance use disorders among adolescents and young adults.
Introduction
Cigarette smoking is a common addictive disorder. A recent Surgeon General's report indicates that one-third of people who have tried smoking become daily smokers [1]. In 2009, an estimated 20.6% (46.6 million) of Americans aged 18 years or older were current smokers (defined as those who reported that they smoked 100 cigarettes or more during their lifetime and currently smoke every day or some days [2]). Although antismoking campaigns have reduced cigarette use in recent years, the long-term decline of smoking came to a halt in 2010, especially among high school students. In 2010, both 8th and 10th graders showed evidence of an increase in smoking and approximately one in five high school students and adults in the United States continue to smoke regularly [1].
It is well established that the use of tobacco products or exposure to second-hand smoke has detrimental effects on physical health including an increased risk of heart disease, cancer, stroke, and chronic lung disease. In the United States, cigarette smoking accounts for 30% of all cancer deaths and for nearly 80% of deaths from chronic obstructive pulmonary disease [2], [3]. It is also the primary causal factor for early cardiovascular disease and deaths [2].
Many aspects of cigarette smoking behavior, including onset of smoking, smoking persistence, and nicotine dependence, cluster in families [4]. In addition to environmental factors (such as peer pressure), evidence from twin studies indicates that genetic factors contribute to the development of smoking and related addictive behaviors with heritability estimates ranging from 60% to 72% [5]–[7]. Genome wide association studies (GWAS) and candidate gene studies of nicotine dependence have identified several variants in the gene cluster encoding the α5, α3, and β4 nicotinic receptor subunits on chromosome 15 that alter risk for nicotine dependence, including an amino acid substitution (aspartic acid to asparagine at codon 398) in the α5 nicotinic receptor subunit gene (CHRNA5) and several non-coding variants [8]–[15]. GWAS using several lung cancer populations have also demonstrated association between lung cancer susceptibility and the same or highly correlated variants in the CHRNA5-CHRNA3-CHRNB4 gene cluster [16]–[18].
The association of genetic risk factors with nicotine dependence may be influenced by characteristics of smoking behavior such as the number of quit attempts, smoking frequency in adolescents, and age at onset (AAO) of daily smoking [19], [20]. A study using haplotype analysis with variants in the CHRNA5-CHRNA3-CHRNB4 gene cluster reported that variation in this cluster is associated with severity of nicotine dependence among long-term smokers who began daily smoking at age 16 or younger. Notably, this association was not present among those who began daily smoking after age 16 [21]. To further examine the impact of this gene cluster on the development of nicotine use, we tested whether variants within and flanking the CHRNA5-CHRNA3-CHRNB4 gene cluster affect AAO of daily smoking in a sample of adolescents and young adults from the COGA (Collaborative Study of the Genetics of Alcoholism) families.
Materials and Methods
Study subjects
The Human Studies Committee at the Washington University School of Medicine in Saint Louis approved the study. Approval number for our Collaborative Study on Genetics of Alcoholism: Molecular Biology of Alcoholism is 04-0057. A written informed consent was reviewed and obtained from family members.
The dataset included in this study is a cross sectional sample of the COGA subjects who have had at least one assessment between the years 1989–2008 when they were between the ages of 12 and 25 years. Subjects were recruited from families affected with alcoholism (either as a first or second degree relative) and the comparison families in COGA. The assessment used SSAGA interviews (versions I through 4 and CSSAGA) comprehensive assessments of alcohol and other substance use and related behaviors [22], [23]. The sample consists of 693 families with size ranging from 4–27 (avg. 4.82) and 374 individuals without siblings.
Juvenile Diagnoses
All outcome variables related to cigarette use are lifetime measures constructed from each individual's sequential set of direct interviews. The criteria for each of the ever/never measures were examined on each of the interviews in turn and were assessed independently of any other interview(s). Once the criteria for a given measure were met, the variable was coded as present (even if the criteria were not met on a subsequent assessment). The corresponding AAO variable was set to the age reported on and/or computed from the interview on which the subject first met criteria for the lifetime measure.
Parental Diagnoses
Parental diagnoses were constructed from each parent's structured set of assessments. This set of assessments consisted of all available direct interviews. However, if there were no direct interviews available, then the sequential set of family-history assessments specific to that parent were substituted. The criteria for each of the ever/never measures were examined on each of the assessments in turn, independently of any other assessment. Once the criteria for a given measure were met, then variable was coded as present and the corresponding AAO variable was set to the age reported.
Smoking measures
Daily smokers are individuals who smoked cigarettes 4 or more days per week. Age at onset for daily smoking was the AAO from the first interview in which the subject reported daily smoking. Habitual smokers are individuals who smoked at least one pack a day for 6 months or more. Non-habitual smokers are individuals who smoked more than 100 cigarettes lifetime but did not meet the criteria for habitual smoking. Sample characteristics are shown in Table 1.
Table 1. Characteristics of study subjects who are daily smokers.
| All Daily Smokers | Habitual Smokers | Non-Habitual Smokers | |
| Number of subjects (% Males) | 1312 (53.04) | 384 (57.81) | 889 (51.29) |
| European Americans (% Males) | 951 (49.94) | 324 (55.55) | 597 (47.23) |
| African Americans (% Males) | 234 (62.39) | 27 (62.96) | 201 (62.68) |
| Unknown ethnicity (% Males) | 127 (59.05) | 33 (75.75) | 91 (52.74) |
| Mean age at interview (years) | 22.04±3.9 | 23.66±4.26* | 21.38±3.44* |
| Age range at interview (years) | 13–38 | 15–38 | 14–34 |
| Mean age at onset (years) | 16.18±2.62 | 15.73±2.93 | NA |
| Age range of onset (years) | 8–28 | 8–29 | NA |
Indicates t test (two tailed) p = 0.0001.
Specific SNPs/genes tested
We hypothesized that variants within and flanking the CHRNA5-CHRNA3-CHRNB4 gene cluster on chromosome 15q24–25.1 region that were associated with nicotine dependence and smoking related phenotypes may also affect AAO of daily/habitual smoking. We tested 10 SNPs (single nucleotide polymorphisms; Table 2) in this gene cluster that tagged variants associated with nicotine dependence and smoking related phenotypes [9], [10]. Genotyping was performed at the Genome Technology Access Center at Washington University School of Medicine in St. Louis (http://gtac.wustl.edu/) using an Illumina GoldenGate custom array as part of a larger set of 384 SNPs.
Table 2. QTDT analysis with age at onset of daily smoking as quantitative variable.
| QTDT Total1 | QTDT Orthogonal1 | |||||
| SNP | N | without rs169699682 | with rs169699682 | N | without rs169699682 | with rs169699682 |
| rs880395 | 1288 | 0.02 | 0.02 | 212 | 0.08 | 0.08 |
| rs7164030 | 1289 | 0.02 | 0.02 | 214 | 0.08 | 0.08 |
| rs16969968 | 1288 | 0.25 | - | 174 | 0.52 | - |
| rs578776 | 1289 | 0.17 | 0.18 | 218 | 0.66 | 0.66 |
| rs3743078 | 1289 | 0.17 | 0.19 | 192 | 0.14 | 0.13 |
| rs11634351 | 1289 | 0.007 | 0.006 | 188 | 0.03 | 0.04 |
| rs17487514 | 1288 | 0.007 | 0.006 | 146 | 0.004 | 0.005 |
| rs1996371 | 1288 | 0.003 | 0.002 | 186 | 0.02 | 0.03 |
| rs11857532 | 1287 | 0.02 | 0.02 | 245 | 0.02 | 0.02 |
| rs922692 | 1288 | 0.003 | 0.003 | 183 | 0.02 | 0.03 |
Age at interview in quartiles, gender, first principal component of stratification (pc1), parental smoking and parental drinking were included as covariates.
Nominal P values are shown uncorrected for multiple testing. The Bonferroni-corrected significance threshold is P = 0.005.
Data analysis
Association analyses with age at onset of daily smoking in daily smokers were conducted using the statistical software QTDT (Version 2.4.5, http: sph.umich.edu/csg/abecasis/QTDT; [24]). Among daily smokers both total and within-family (orthogonal model) evidence for association was assessed using age at onset of daily smoking as a continuous variable. The orthogonal model is the generalization of the Fulker model [24], allowing for families of any size (with or without parental genotypes) and was used in a variance components framework. QTDT total association test evaluates total evidence for association and is more powerful than QTDT orthogonal test in absence of population stratification. Therefore we first evaluated the evidence of population stratification by comparing the between and within components of association using QTDT stratification test.
The age at onset for habitual smoking among daily smokers was analyzed using Cox proportional hazards regression analysis (coxph) using the R statistical analysis package. This analysis can incorporate a clustered sandwich estimator to account for the familial correlation among observations. The age at onset measures for non-daily smokers were right-censored at each subject's age at their most recent assessment. The first principal component from the stratification analysis (pc1), age at interview, gender, maternal/paternal daily smoking and maternal/paternal regular drinking were included as covariates. The violation of the proportional hazards assumption was tested with non-zero slope of Schoenfeld residuals versus time using the survival analysis package in R.
Previous studies have demonstrated that a non-synonymous coding SNP, rs16969968 in CHRNA5, increases the risk for nicotine dependence [8]–[15]. To test whether rs16969968 underlies the association between variants upstream of CHRNB4 and AAO of daily smoking, we included rs16969968 as a covariate in QTDT and survival analysis.
Results
Variants located upstream of CHRNB4 are significantly associated with age at onset of daily smoking using QTDT analysis
In this data set, QTDT finds no evidence for stratification therefore we performed QTDT-total test to evaluate the total evidence for association. Several SNPs show significant association with AAO of daily smoking (Table 2). The most significantly associated variant is rs1996371, located upstream of the CHRNB4 gene (p = 0.002). Individuals homozygous for the risk allele had AAO at least one year earlier than individuals who are homozygous for the non-risk allele; the difference in AAO between these two groups was statistically significant (p = 0.0002; Table 3). Variants rs11634351 and rs922692 that are highly correlated with rs1996371 (r2 = 0.98 and r2 = 0.95, respectively) also show association with AAO of daily smoking. Conditional analysis showed that association of rs1996371 and correlated SNPs with AAO of daily smoking is independent of rs16969968 (Table 2). Conditional analysis using variants rs578776 and rs880395, which represent other distinct loci in this gene cluster produced similar results and confirmed the independent relationship of rs1996371 (p<0.005). Variants rs1996371 and rs922692 withstood the conservative Bonferroni correction (<0.005). Because most of the markers used in this study are highly correlated we also performed the less conservative correction of SNPs in LD with each other given by Nyholt et al [25]. This method provides the effective number of independent marker loci and in the present study this number was 5, giving an experiment-wide significance threshold of p<0.01. According to this less stringent criterion for correction, rs1996371, rs11634351, and rs922692 were significant after correction for multiple testing.
Table 3. Distribution of age at onset of daily smokers with reference to alleles of rs1996371.
| Genotype | Daily Smoker | Habitual Smoker | Non-Habitual Smoker |
| Mean age at onset (N) | Mean age at onset (N) | Mean age censored (N) | |
| AA | 16.41±2.6 (611)* | 15.67±3.09 (147) | 21.54±3.45 (465) |
| AG | 16.07±2.6 (518) | 15.82±2.92 (173) | 21.15±3.40 (330) |
| GG | 15.56±2.4 (162)* | 15.55±2.59 (64) | 21.42±3.48 (94) |
Indicates t test (two tailed) p = 0.0002.
Variant rs1996371 and highly correlated SNPs affect age at onset among daily smokers using survival analysis
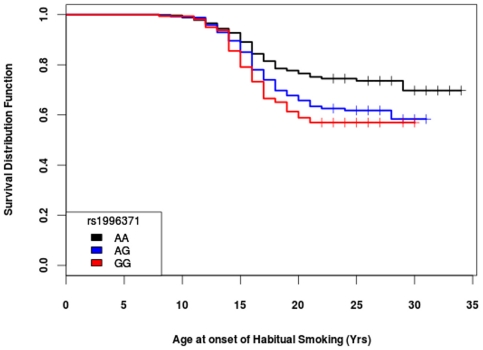
We further explored the effects of rs1996371 on smoking behaviors by testing whether this SNP modifies the AAO of habitual smoking among subjects who are already daily smokers. For non-habitual smokers, the AAO measures were right-censored at each subject's age at their most recent assessment. Our Cox-proportional analysis using AAO of habitual smoking censored at non-habitual smokers demonstrated that the individuals who have a minor allele of rs1996371 and highly correlated variants have 1.2 times more chance of becoming habitual smokers with every one-year increase in their age (Table 4). We tested the proportional hazard assumption for all covariates using Schoenfeld residuals and no violation was detected. The strongest effect of association with rs1996371 is between 14 and 21 years (Figure 1). At age 21, 43% of individuals with 2 copies of the minor allele of rs1996371 are habitual smokers while 25% of individuals homozygous for the major allele are habitual smokers (Figure 1). We also performed the same analysis using rs16969968 as a covariate and confirmed that the effect of rs1996371 and correlated variants on AAO of habitual smoking is independent of the influence of rs16969968 (Table 4). The effect remained significant after controlling for other variants in this gene cluster (HR≃ 1.23, P<0.005) and Bonferroni correction for multiple testing. Some earlier studies showed that gender is a major factor influencing smoking behaviors. In our stratified analysis, although power was reduced, rs1996371 and correlated SNPs significantly predicted risk for habitual smoking with increasing age in females and males (females: rs1996371, p = 0.05, HR: 1.25; males: rs1996371, p = 0.02, HR:1.2).
Table 4. Cox-proportional hazard analysis using age at onset of habitual smoking censored at non-habitual smokers.
| without rs16969968 as a covariate1 | with rs16969968 as a covariate1 | |||||
| Marker | N | Hazard ratio (95%CI) | p value2 | N | Hazard ratio (95%CI) | p value2 |
| rs11634351 | 1273 | 1.23 (1.07–1.43) | 0.005 | 1288 | 1.23 (1.05–1.44) | 0.008 |
| rs17487514 | 1273 | 1.14 (0.97–1.34) | 0.11 | 1288 | 1.13 (0.95–1.35) | 0.15 |
| rs1996371 | 1272 | 1.24 (1.07–1.43) | 0.004 | 1288 | 1.23 (1.05–1.44) | 0.008 |
| rs11857532 | 1271 | 1.14 (0.99–1.32) | 0.05 | 1288 | 1.14 (0.98–1.32) | 0.09 |
| rs922692 | 1272 | 1.24 (1.07–1.43) | 0.004 | 1288 | 1.23 (1.06–1.44) | 0.008 |
Analysis was performed within family clusters using additive model. Age at interview in quartiles, gender, pc1, parental smoking and parental drinking were included as covariates.
Nominal P values are shown uncorrected for multiple testing. The Bonferroni-corrected significance threshold is P = 0.005.
Figure 1. Genotypes of rs1996371 significantly predict age at onset of habitual smoking.
Discussion
People who start smoking at a young age are more likely to have higher lifetime amounts of tobacco smoked and consequently are at greater risk of becoming nicotine dependent [19]. Variants flanking and within the CHRNA5-CHRNA3-CHRNB4 gene cluster region affect the risk for nicotine dependence [8]–[15], and are also involved in the transition toward heavy smoking in mid-adulthood and in smoking persistence [26]. Studies using haplotype analysis reported that common haplotypes in this gene cluster are associated with nicotine dependence in adults especially among those who began daily smoking before age 16 [21]. These haplotypes also linked to nicotine withdrawal and smoking cessation phenotypes though the associations were not influenced by AAO of daily smoking [27]. In this study, we conducted family-based association tests using AAO of daily smoking as a quantitative phenotype. We identified an association between AAO of daily smoking and a group of correlated variants located upstream of CHRNB4 gene in a cohort of young adults, that was independent of the effect of rs16969968.
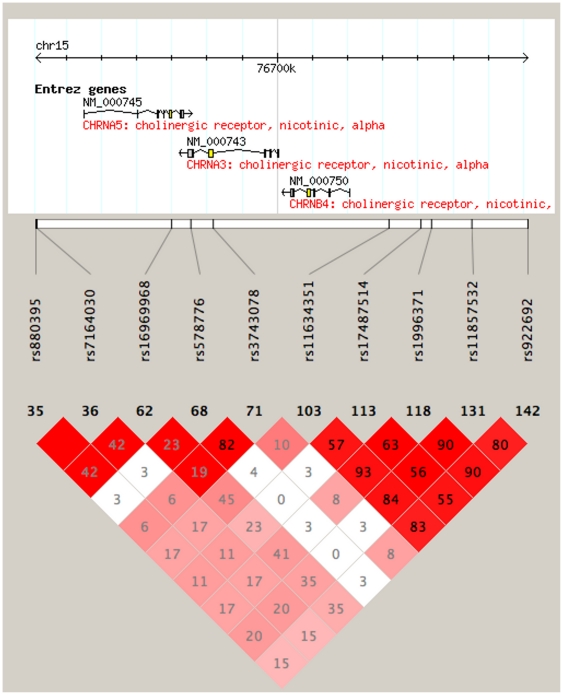
There are many environmental factors such as influence of peer group [28], lack of parental monitoring [29] and easy access to cigarettes can also influence the genetic liability to smoking behavior in young adults. Koopmans et al [30] reported that the total variance accounted by genetic influences on smoking initiation and cigarettes per day is 39% and 86%, respectively. The liability to ever smoking is largely independent from liability to continue to smoke. This could be due to the fact that while over 80% of individuals in some studies report that they have tried smoking cigarettes, only about one third of them will smoke beyond experimentation [31]. Hence genetic factors may have stronger impact on daily smoking (smoked ≥4 days/week) and habitual smoking (one pack a day for 6 months) rather than smoking initiation. Therefore in the present study we focused on subjects ascertained as daily smokers to find out the relationship between CHRNA5-CHRNA3-CHRNB4 variants and onset of daily/habitual smoking behaviors, believing that this selected group can explain more genetic variance than smoking initiation or exposure. We found that the rs1996371 and correlated variants located upstream of CHRNB4 gene were associated with age at onset of daily smoking. Since our sample is multi-ethnic we also restricted our analyses to the largest subgroup (European Americans) to ensure that the association was not a false positive due to heterogeneity in our dataset. The association remained in this subgroup. The association between age at onset of daily smoking and rs1996371 and correlated SNPs also remained statistically significant after correcting for multiple testing proposed by Nyholt et al [25], (rs1996371, p = 0.0058). The daily smokers who had at least one copy of the minor allele of rs1996371 had a mean AAO of daily smoking 1 year younger than individuals without the minor allele. The non-synonymous SNP, rs16969968 was not associated with age at onset of daily smoking. We further wanted to explore whether daily smokers with these variants are at increased risk of becoming habitual smokers in future. Our Cox-proportional hazard analysis indeed showed that smokers who have an early AAO of daily smoking and carry a minor allele of rs1996371 have approximately 1.2 times higher risk of becoming habitual smokers compared to daily smokers who carry the major allele. This association was further confirmed in stratified analysis in EAs (rs1996371, p = 0.03, HR: 1.17). In African Americans the variant was not statistically significant, most likely because of low power, but the effect was in the same direction (rs1996371, p>0.05, HR: 1.21). SNP rs1996371 has low correlation (0.28<r2<0.56 in Europeans using HapMap data, Figure 2) with variants (e.g. rs1051730, rs16969968) that were reported to affect risk for nicotine dependence and smoking related diseases in adults. Interestingly Schlaepfer et al reported a variant in the 3′UTR of CHRNB4 gene, rs1948, has been linked to early age of initiation for tobacco use in an ethnically diverse young adult cohort [32]. They also found that a variant, rs11634351 that is in low LD with rs1948 (r2 = 0.24) had significant association with age at first drink [32]. In current study we found that rs11634351 is associated with AAO of daily smoking and is in complete LD with rs1996371 (Figure 2). High comorbidity among smoking and drinking behaviors might explain this possible overlap [33].
Figure 2. Linkage disequilibrium between genotyped SNPs.
Number in each square represents the a pairwise LD relationship (r2) between the two SNP's in Caucasians using HapMap data and varying red color represent the linkage disequilibrium values for that pair as measured by D′ (bright red shows high D′).
The most significant variant in this study (rs1996371) was also found to be associated with CHRNB4 mRNA expression in human brain (p = 0.01) [34]. There are a few functional studies that have reported the possible involvement of the CHRNB4 gene with drugs of abuse. Bruschweiler-Li et al. demonstrated that a 2.3-kb fragment of the CHRNB4 promoter region containing the CA box is capable of directing cell-type specific and developmentally regulated expression of a reporter gene in vivo [35]. There is also some indication that blockade of α3β4 nAChRs results in a reduction of opioid and stimulant self-administration, suggesting that nAChRs that contain the β4 subunit are involved in mediating withdrawal syndromes elicited by some drugs of abuse [36]. In a study using transgenic mice, Frahm and colleagues [37] showed that targeted overexpression of β4 leads to strong aversion to nicotine. This study further provided evidence that the medial habenula acts as a gate-keeper in the control of nicotine consumption and that the balanced contribution of β4 and α5 subunits is critical for this function. Thus, further studies elucidating the molecular mechanisms underlying expression of the β4 gene may improve our understanding of nicotine addiction and withdrawal as well as lung cancer, and other smoking-related diseases.
In this study we identified distinct variants in the CHRNA5-CHRNA3-CHRNB4 gene cluster that influence the age at onset of habitual smoking in young adults. These variants are not in LD with rs16969968 or its correlates that are associated with nicotine dependence. Earlier Schlaepfer and coworkers [32] also showed that variants other than rs16969968 or its correlates affect age at initiation of tobacco use at early age. These results show that there may be different mechanisms, which promote smoking behaviors at younger ages. The age dependent interplay of nicotinic receptors subtypes could explain the process of initiation to persistence of chronic smoking behaviors, but there remains a need for follow-up studies to confirm the hypothesis.
Acknowledgments
The Collaborative Study on the Genetics of Alcoholism (COGA), Co-Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes centers where data collection, analysis, and storage take place. The ten sites and Principal Investigators and Co Investigators are: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J.Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice, K. Bucholz); University of California at San Diego (M. Schuckit); Howard University (R. Taylor); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy); Virginia Commonwealth University (D. Dick).
Footnotes
Competing Interests: Dr. Bierut, Dr. Goate and Dr. Wang are listed as inventors on the patent “Markers for Addiction” (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. Bierut acted as a consultant for Pfizer, Inc. in 2008. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This national collaborative study is supported by the National Institutes of Health (NIH) Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.United States Department of Health and Human Services (USDHHS) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. . Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- 2.Centers for Disease Control (CDC) Current cigarette smoking among adults aged >18 years-United States. Centers for Disease Control (CDC); 2010. pp. 1135–1140. [Google Scholar]
- 3.Mokdad AH, Giles WH, Bowman BA, Mensah GA, Ford ES, et al. Changes in health behaviors among older Americans, 1990 to 2000. Public Health Rep. 2004;119:356–361. doi: 10.1016/j.phr.2004.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Archives of general psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, et al. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- 6.Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- 7.True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, et al. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 8.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 14.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Vikis HG, Wang D, Lu Y, Wang Y, et al. Familial aggregation of common sequence variants on 15q24–25.1 in lung cancer. J Natl Cancer Inst. 2008;100:1326–1330. doi: 10.1093/jnci/djn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John U, Meyer C, Rumpf HJ, Hapke U. Self-efficacy to refrain from smoking predicted by major depression and nicotine dependence. Addict Behav. 2004;29:857–866. doi: 10.1016/j.addbeh.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 20.O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003;25:219–225. doi: 10.1016/s0749-3797(03)00198-3. [DOI] [PubMed] [Google Scholar]
- 21.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of studies on alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 23.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA–a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 24.Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, et al. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry. 2011;69:650–660. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, et al. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, et al. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. 2010;105:2014–2022. doi: 10.1111/j.1360-0443.2010.03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, et al. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of abnormal psychology. 2007;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 31.Lessov-Schlaggar CN, Pergadia ML, Khroyan TV, Swan GE. Genetics of nicotine dependence and pharmacotherapy. Biochemical pharmacology. 2008;75:178–195. doi: 10.1016/j.bcp.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature neuroscience. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 34.Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruschweiler-Li L, Fuentes Medel YF, Scofield MD, Trang EB, Binke SA, et al. Temporally- and spatially-regulated transcriptional activity of the nicotinic acetylcholine receptor beta4 subunit gene promoter. Neuroscience. 2010;166:864–877. doi: 10.1016/j.neuroscience.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. European journal of pharmacology. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- 37.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]