SUMMARY
Longevity variability is a common feature of aging in mammals, but the mechanisms responsible for this remain largely unknown. Using microarray datasets coupled with Prediction analysis of microarrays (PAM), we identified a set of 252 cardiac transcripts predictive of relative lifespan in Wistar and Fisher 344 rats. PAM “tests” of rat heart transcriptomes from a third longer lived Fisher × Norway Brown rat strain validated the predictive value of this gene subset. The expression patterns of these genes were highly conserved, and corresponding promoter regions were employed to identify common cis–elements and trans-activating factors implicated in their control. Specifically, four transcription factors (Max, Ets2, Erg, and Msx2) present in heart displayed longevity-dependent, strain-independent changes in abundance, but only ETS2 had an expression profile that directly correlated with the relative lifespan gene set. In heart, ETS2 was prevalent in CMs and showed a high degree of myocyte-to-myocyte variability predominantly in adult rat hearts prior to the exponential increase in the rate of mortality. Exclusively in this group, elevated ETS2 significantly overlapped with TUNEL staining in heart myocytes. In response to sympathetic stimuli, ETS2 is also up-regulated, and functionally, adenovirus mediated over-expression of ETS2 promotes AIF-mediated, caspase-independent programmed necrosis exclusively in CMs that can be fully inhibited by the PARP-1 inhibitor DPQ. We conclude that variations in ETS2 abundance in hearts of adult rodents and the associated loss of CMs, contribute at least partially, to the longevity variability observed during normal aging of rats through activation of programmed necrosis.
Keywords: Aging, Mortality, Rat, microarrays, PAM, transcription factors
Introduction
Identification of genes that promote increased lifespan is central to modern research efforts in gerontology. During the past decade, researchers have identified numerous genes and gene variants that increase lifespan in simple model systems like S cerevisiae, C elegans, and D melanogaster (Biteau et al., 2011; Partridge, 2011), and subsequent analyses in mammalian systems support a genetic basis for longevity. Lifespan is, however, species- and strain-dependent, and longevity is only partially under genetic control (Campisi and Yaswen, 2009; Rikke et al., 2010; Sinclair and Michan, 2007). Consequently, dietary restriction, which generally promotes longevity across a variety of mammalian species (Kapahi and Katewa, 2010), can also reduce lifespan in a strain-dependent manner (Nelson et al., 2010). Longevity in mammals, in the absence of predation or disease, is therefore a consequence of a variety of gene actions and metabolic processes, and it is currently impossible to know a priori whether a specific gene product will promote increased lifespan (Martin, 2002; Zahn et al., 2007). This uncertainty is complicated by the fact that some longevity-associated genes may function in an organ specific manner.
To distinguish between organ-specific and conserved organismal pathways associated with lifespan, we previously compared transcriptomes from tissues in age-matched mice (Zahn et al., 2007). Transcriptomic data showed that organs, like thymus, displayed large transcriptional differences between young and old animals; whereas, others, like liver, showed few to no changes in expression with age. An intermediate profile was observed in mouse heart and highly vascularized tissues, and consistent with studies from other species, inflammatory response genes were broadly implicated (Saban et al., 2002). A number of age-associated changes were, however, observed in heart that were unique from those reported in skeletal and smooth muscle (Lee et al., 2002; Spindler et al., 2006). Despite these quantitative differences among similar tissue types, it is currently unclear how cardiac gene expression affects lifespan variability. This lack of understanding represents a potentially serious shortcoming to aging research, particularly since heart is largely devoid of neoplasms, is essentially disease-free in rodents (i.e., almost no myocardial infarcts), and is highly responsive to caloric restriction (Ruden et al., 2007; Spindler et al., 2006). Moreover, heart muscle produces energy primarily from mitochondrial respiration, and its high metabolism leads to production of reactive oxygen species (ROS) that contribute broadly to aging processes (Wallace, 2001). Cardiomyocytes (CMs) are, however, protected from the most deleterious effect of ROS by antioxidant enzymes implicated in longevity (Dai and Rabinovitch, 2009; Sheydina et al., 2011). Thus, longevity-associated genes expressed in heart may strongly influence lifespan variability.
To further elucidate the molecular basis of normal lifespan variability in mammals, we postulated that CMs express unique sets of genes linked to survival. To test this hypothesis, we investigated gene expression in hearts from three rat stains with different absolute lifespans. We report the identification of a unique gene set predictive of mortality and one transcription factor (TF) directly implicated in CM loss. This TF is up-regulated heterogeneously in CMs in vivo and specifically promotes programmed necrosis in vitro, which contributes, at least partially, to the cardiac component of longevity heterogeneity seen in rats.
Results
Microarray Analyses and Prediction of Putative Longevity-associated Genes
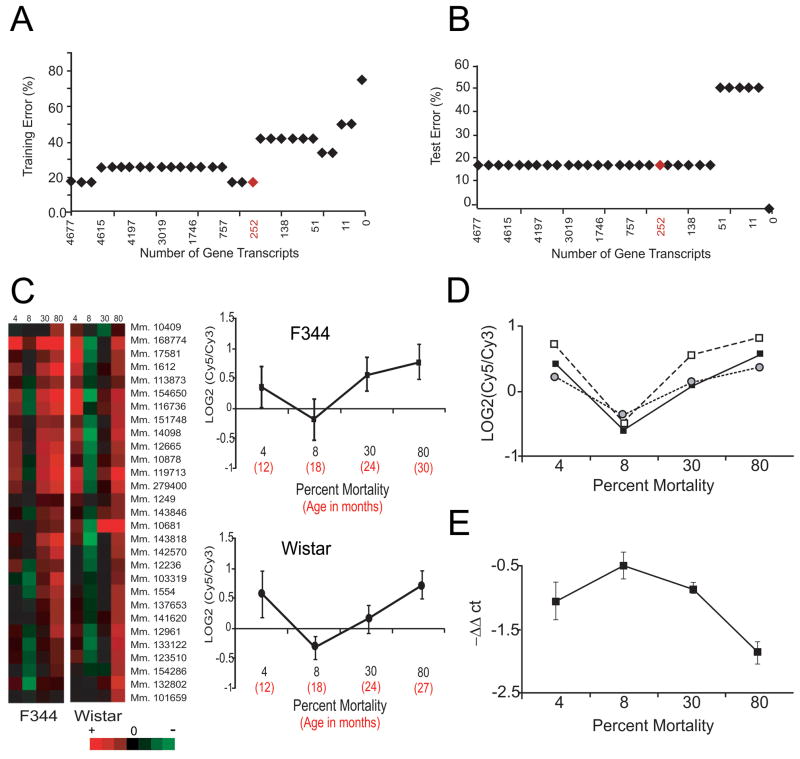
To identify transcripts with altered abundance predictive of relative lifespan, we generated transcriptomic profiles of male Fisher (F) 344 and Wistar rat hearts as a function of age (Figs. 1 and S1A). Instead of merely reporting significant differences and fold-changes in transcript abundance, we “corrected” the absolute variable “age” into “relative lifespan” and reorganized all microarray data into mortality groups (i.e., Classes) based on the cumulative probability of dying at a specified age within each rat cohort (Figs. 1 and S1B, Table 1). We then analyzed the reorganized and combined data from both strains, using a variant of the nearest shrunken centroid classification, called Prediction Analysis of Microarrays (PAM), which was originally developed to classify and predict diagnostic characteristics of cancer samples. We specifically employed PAM to predict mortality groups based on gene expression profiles and relative mortality curves (Schaner et al., 2003; Sorlie et al., 2003). PAM “training” was then used to identify gene transcripts whose centroid was stable within samples of the same Class from F344 and Wistar rats, and PAM cross-validation was used to statistically identify a minimal set of gene transcripts (n=252) that best characterized each Class (Fig. 2A). PAM Test errors were also calculated for prediction accuracy (Fig. 2B).
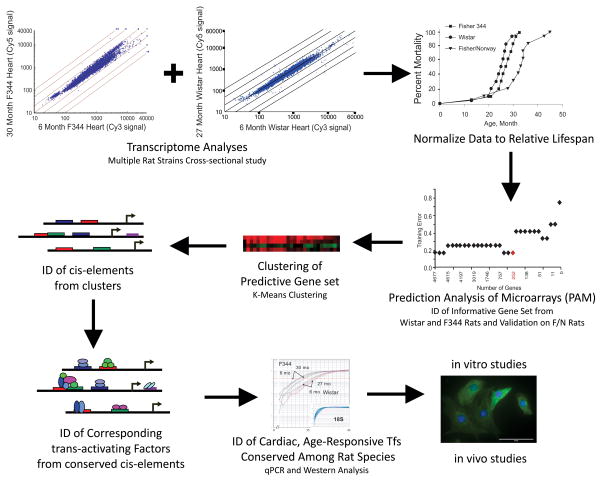
Figure 1. Study Design Scheme.
This study was designed to employ microarray datasets, normalized to “relative lifespan” to identify gene transcripts predictive of longevity groups. Data were clustered, cis- and trans-factors identified, and data analyzed as a function of mortality using 3 rat strains. Resulting data were used to identify strain-independent and age-dependent TFs that mimicked and possibly regulated the expression of the informative gene set. Functional assays were performed to determine how one factor could contribute to longevity variability in rodents.
Table 1.
Age and Relative Mortality groups
| Rat Strain | Microarray Studies | Otherstudies | Mortality Group |
|---|---|---|---|
| Fisher 344 (F344) | 1–3 day (NNCMs) | ||
| 3 months (ACM) | 0% | ||
| 6 months | 6 | 0 | |
| 12 | 12 | 4 | |
| 18 | 18 | 8 | |
| 24 | 24 | 30 | |
| 30 | 30 | 80 | |
| Wistar (W) | 6 months | 6 months | 0% |
| 12 | 12 | 4 | |
| 18 | 17/18 | 8 | |
| 23/24 | 23/24 | 30 | |
| 27 | 27 | 80 | |
| Fisher × Brown Norway (F/N) | 2 months | 0% | |
| 6 months | 6 | 0 | |
| 12 | 4 | ||
| 16 | 8 | ||
| 24 | 20 | ||
| 32 | 30 | ||
| 36 | 36 | 80 |
Microarrays were performed on pooled rat heart samples(n=4)obtained at the indicated ages. Comparisons were performed with strain-matched 6 month old rats, when mortality caused by aging was assumed to approach 0%. Other refers to assays performed on tissue or cell samples obtained from neonatal rat cardiomyocytes (NNCMs), adult cardiomyocytes (ACMs) or rat fibroblast (rFbs)at the indicated age groups. These include qRT-PCR(F344, W and F/N rats), protein analyses(F344 and F/N rats)or immunohistochemistry(F/N)as described in the text.
Figure 2. Prediction Analysis of Microarrays (PAM) and RNA analyses.
PAM is a software package that performs sample classification from gene expression data and permits discrimination between groups. (A) Graphical output from PAM training showing that the smallest set of gene transcripts predictive of survival groups (Classes) from the combined and reorganized microarray data of F344 and Wistar rats consisted of 252 transcripts (in red). (B) The calculated PAM Test Error for the predictive gene set showed that each mortality group based on relative lifespan (Class) could be determined accurately on average 82% of the time from gene expression data comprised of the 252 gene transcripts identified in 2B. (C) Selected expression profiles of predictive gene transcripts identified by PAM in F344 and Wistar rats. To the left are heatmaps illustrating how the predictive gene transcripts from two rat strains decreased in abundance between the 4% and 8% relative mortality groups, but increased between 8 and 80% relative mortality groups. The expression data for one individual transcript (Mm.279400, G protein-coupled receptor kinase 5) from both Wistar and F344 rats are shown to the right. D) Expression profiles of representative transcripts selected from each K-means Cluster [Cluster 1 - RAD21 homolog (black squares), Cluster 2 – Shroom (white squares), Cluster 3 - RAR-related orphan receptor alpha (grey circles)] are graphically illustrated to further illustrate the conserved expression pattern of this informative gene set. E) qRT-PCR data of Ets2 (mean ± SEM for all 3 rat strains) were normalized to GAPDH and are reported as -ΔΔCt values. Transcriptomic data (qPCR and microarray) were statistically compared by the Kolmogorov-Smirnov test, and no difference could be found between the inverse of the Ets2 profile and the informative gene sets shown in Figs. 2C or 2D.
A defining feature of all 252 transcripts identified by PAM as a function of mortality group was the synchronized shape (Fig. 2C). Graphically, transcripts in the group of animals alive at 80% mortality in each respective strain (Table 1) had an abundance that was equal to or greater than that identified in cohorts at 4% relative mortality (i.e., when mortality is essentially absent). For the 8% relative mortality group when 92% of the cohort is predicted to survive, transcripts were less abundant than that found in the 4% relative mortality group, but only 30 of 252 transcripts were significantly reduced (p<0.05). The slope of transcript abundance between 8 and 80% relative mortality was always positive, suggesting that survivors in each Class up-regulated these genes as a function of age. The 252 predictive gene set proved highly diverse and included TFs, receptors, signaling molecules, chaperones, and metabolic factors (Table S2). None of the genes corresponded to worm or fly homologues of known longevity-regulating genes (e.g., daf2, clk-1, isp-1, sir2, hsf); however, one insulin-responsive gene (insulin induced gene 2, Insig2) implicated in fatty acid synthesis (Yabe et al., 2003), one ubiquitin associated gene implicated in p53 degradation (ubiquitin protein ligase E3A, Ube3a)(Scheffner et al., 1993), and two heat shock proteins, (Hspa9a, which binds and sequesters p53 (Wadhwa et al., 2002)), and Hspd1 (chaperonin)) were present.
The predictive value of the informative gene set was validated by testing its ability to classify a mortality group (i.e, Class) in a 3rd rat strain (Fisher × Brown Norway (F/N) rats) that has a ~50% longer lifespan than F344 or Wistar rats (Figs. 1 and S1B). Specifically, we utilized PAM to determine the degree of similarity among 36 month old F/N rat hearts (80% relative mortality) and the previously modelled F344 and Wistar rat microarray datsets. We postulated that if the “survival” gene set centroid were stable within samples of the same Class of Wistar and F344 rats (i.e., independent of strain and random variability), then the analysis should correctly predict the survival group of the third strain based wholly on the conserved expression profile of the test sample. The results from this analysis indicated that the 252 survival gene set expression pattern identified in F344 and Wistar rats could in fact accurately predict (with a posterior probability equal to unity, i.e., 100% accurate) the 80% relative mortality in 3 of 4 test groups of F/N rats, consistent with the 82% prediction accuracy determined from PAM Tests (Fig. 2B). These data lead us to conclude that this gene set is in fact predictive of relative mortality (i.e., survival) groups in rat hearts, independent of strain and random variability.
Identification of common cis-elements and trans-regulatory factors
Conserved expression profiles of “survival” genes among strains suggest that genetic components (i.e., cis- elements and trans-regulatory factors) account for the unique expression pattern displayed by this predictive gene set. We therefore identified putative TF binding sites (i.e., cis-regulatory elements) in the corresponding rodent gene promoters (Dohr et al., 2005). After a Gap test and K-means clustering to sort predictive gene transcripts into 3 gene groups (Fig. 1, Tables S3 and S4), we identified common cis-element families in at least 80% of the proximal promoter regions (−500 to +100 relative to the transcription start site) within each Cluster (Table S5, Fig 2D). In Cluster 1, 80% of the genes contained TF-binding sites for Ets, Creb, Nkx/Dlx, E-box and Irf family members. In Cluster 2, conserved cis-elements for Stat-, Ets- and Egr- TF families were identified in 90% of the genes; and in Cluster 3, Creb-family and Ets family transcription binding sites were common. Only Ets-family members were conserved among all three clusters.
Cis-elements are by themselves uninformative and some of the putative binding sites were not more prevalent in these gene promoters than that predicted for the genome at large. We, therefore, performed qRT-PCR analyses to identify potential binding factors that were significantly altered as a function of age in the left ventricles (n=4–6) of Wistar and F344 rats. For this, we examined the same five age groups employed for microarray analyses (Table 1). The abundance of many of the transcripts was unaltered (e.g., Ets1); however, transcripts encoding 12 trans-regulatory factors demonstrated significant changes as a function of age (Table S6). Among these, several transcripts showed strain-dependent changes (F344: Stat-1 decreased, Atf-2 increased; Wistar: Tcf-4, Tcf-12, and Mycn decreased, Nfil-3 increased), while six others (Erg, Ets2, Msx2, Max, Srebp-2 and Irf-1) demonstrated significant decreases (negative slope i.e., beta coefficient) with age (i.e., R squared values), independent of strain. We plotted the expression of these 6 transcripts as a function of relative mortality after normalization (ΔΔCt values). The plots indicated that Irf-1 correlated with the profile of the predictive 252 transcripts identified earlier (Fig. S1C); whereas, the profiles for Ets2, Max and Srebp-2 inversely correlated with transcripts from the predictive gene set.
When these analyses were extended to F/N rats corresponding to the same mortality groups (Table 1), 6 of 24 putative trans-regulatory factors showed significant changes in transcript abundance, but only four of these mRNAs (Ets2, Erg, Max and Msx2) had significantly altered expression in all 3 strains (Table S6). Of these, only Ets2 demonstrated a significant correlation (as an inverse relationship) with each gene cluster from each mortality group for all three rat strains (Fig. 2E). When compared with the 4% relative mortality group, Ets2 transcript abundance was always higher in the 8%, but lower in the 30 and 80% relative mortality groups. Statistically, this profile did not differ (p=0.7) from the inverse of Ets2 expression for any of the 252 predictive gene transcripts. The other three TFs were positively correlated with gene expression in one or two rat strains, but not all three (Fig. S1D).
ETS2 in F344 and F/N Rat Hearts with Aging
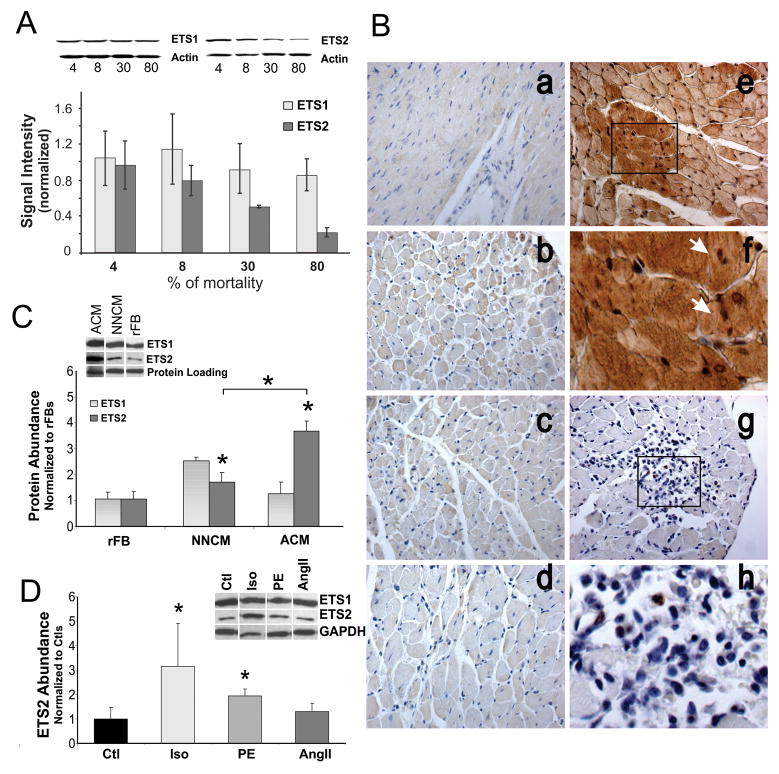
Having identified one TF putatively implicated in the regulation of the 252 informative gene set, we examined its expression in whole heart and isolated cells. Western analysis of protein homogenates from F344 hearts isolated from the 4, 8, 30 and 80% mortality groups revealed that ETS2 abundance, but not ETS1, decreased as a function of age (Fig. 3A). Quantitatively, ETS2 abundance varied considerably in the 8% mortality groups hearts, and this variability was 2-fold greater than that observed in any other group. ETS2 immunostaining also demonstrated substantial cell to cell variability in the 8% mortality group of F/N rats (Fig. 3B(b)), but in 4, 20 and 30% mortality groups, ETS2 immunostaining was relatively homogeneous (Fig. 3B(a,c,d)). Unexpectedly in the 8% mortality group, TUNEL staining was also observed in a subset of highly positive ETS2 CMs, as TUNEL positive, non-CMs did not stain strongly for ETS2 and no correlation with TUNEL staining could be seen in the other mortality groups (Fig. 3B(e–h)). Consistent with the data obtained from F344 rats, the number of TUNEL positive cells significantly increased in the 8 and 30% mortality groups for F/N rats (19.44±4.72% versus 37.87±6.26, p<0.01, n=3); however the number of TUNEL positive cells that co-stained with ETS2 significantly decreased (60.71±11.42% versus 4.6±11.3, p<0.05, n=3) during this same time frame. Co-staining was even less common in the 80% mortality group (<2%). Moreover, in a model of myocardial infarction with apoptotic CMs, we could not demonstrate any significant increase in ETS2 abundance following ischemia at any time point examined (n=3, p>0.05). These data suggest that any possible transcriptional effects of ETS2 in heart may be CM specific and that its up-regulation was not associated with injury or disease.
Figure 3. ETS expression in vitro and in vivo.
(A) Immunoblot and data analysis for ETS1 and ETS2 in F344 rat heart from each relative mortality group (4, 8, 30 and 80%). (B) ETS2 immunostaining of heart tissues from F/N rats at a) 2, b) 16, c) 24 and d) 32 months of age. ETS2 (Brown) and TUNEL (Violet) co-staining of hearts from 16 month old animals are shown in e–g, with magnifications shown in f and h. Dark brown staining in e illustrates high levels of ETS2 shown in selected regions of myocardium. Arrows indicate TUNEL positive CMs (f). g and h show TUNEL positive non-CMs lacking strong ETS2 staining. (C) Immunoblot and data analysis for ETS1 and ETS2 protein abundance in rFB, NNCM, and adult left ventricular cardiomyocytes (AdCM), p<0.05. (D) Immunoblot and data analysis for ETS2 protein abundance in vehicle-treated NNCM (Ctls) and NNCMs treated for 24 hours with 5μM of ISO, PE and AngII, * p<0.01.
In isolated cells, we find that ETS2 is significantly more abundant in CMs than in fibroblasts, and its expression is age-dependent. Isolated CMs from 3 month old animals contain significantly higher levels of ETS2 than either NNCMs or rFBs (Fig. 3C). Moreover, pharmacological agonists known to affect CM growth and function transiently alter ETS2 abundance and expression exclusively in NNCMs. Phenylephrine (PE), isoproterenol (ISO), and to a lesser extent angiotensin II (AngII) resulted in specific morphological changes to NNCMs, but only PE and ISO caused the beating frequencies of the plated cells to increase. In these same cells, ETS2 abundance increased significantly by 3- and 1.6-fold in response to ISO (ANOVA, p=0.006, n=5) and PE (ANOVA, p=0.004, n=5), respectively relative to total protein within 24 hours of treatment (Fig. 3D). No significant change in ETS2 was observed in CMs after treatment with AngII. Similarly, no significant changes in either Ets1 or Ets2 mRNA or protein could be demonstrated in rFBs with any of these agonists at any time point examined. These data demonstrate that up-regulation of ETS2 occured in CMs but not FBs in response to sympathetic agonists, and that it’s expression is more prevalent in adult CMs than NNCMs.
Elevated ETS2 promotes cardiomyocyte cell death
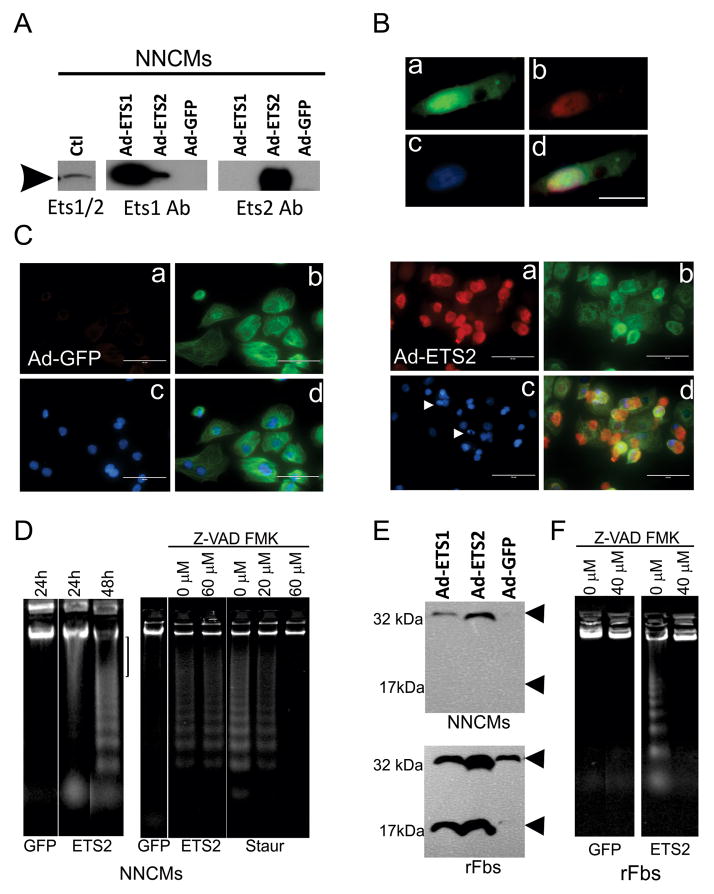
To determine how up-regulation of ETS2 affects cardiocytes, NNCMs and separately rFBs were infected with Adenovirus (Ad)-GFP (control), Ad-ETS1 and Ad-ETS2. Following infection, ETS1, ETS2 and GFP were significantly elevated (>50–100-fold) in both cell types (Figs. 4A, S1E). ETS1 and ETS2 were preferentially expressed in nuclei, and to a lesser extent in the cytoplasm of NNCMs and rFBs (Fig. 4B). Following infection, no significant change in cell morphology or function was observed in any group. The surface area did not significantly differ from Ad-GFP treated controls for NNCMs (97.6±14.8, n=5, p>0.05) or for rFBs (78.4 ± 14.4 versus control, n=5, p>0.05). The spontaneous beating rates of NNCMs were unchanged (68±7, n=5 cell preparations, p>0.05), and no significant difference in cell viability could be demonstrated among the infected cells (n=3, p>0.05). In contrast to the senescent phenotype observed in human fibroblasts with elevated levels of ETS2 (Ohtani et al., 2001), no increase in senescence-associated β-galactosidase staining or p16ink4a could be demonstrated in either rFBs or NNCMs (p>0.05, n=5). We did, however, observe an increase in the number of floating cells within 48h and 24h of Ad-ETS2 infection in rFBs and NNCMs respectively. The increased number of floating cells did not quantitatively differ from the Ad-GFP controls for the rFBs; but ETS2 led to a significant increase (n=3, p<0.001) in floating NNCMs relative to controls.
Figure 4. Over-expression of ETS factors in cardiac cells.
(A) Adenovirus-mediated overexpression of ETS1 and ETS2 in CMs. In performing these studies, the ETS2 antibody (sc351, Santa-Cruz) proved highly specific for its intended target; however, two different Ets1 antibodies (sc350 and sc111, Santa-Cruz) demonstrated weak cross-reactivity with ETS2. (B). Images showing a) GFP expression, b) ETS2 immunostaining restricted primarily to the nucleus, c) DAPI nuclear stain and d) a composite image. (C) NNCMs were are infected with Ad-Ets2 for 24 hours. Images show a) ETS2 immunostaining, b) Troponin-T immunostaining, c) DAPI staining and d) a composite image. Arrows indicated condensed nuclei. Size marker=100μm. (D) ETS2 over-expression selectively promotes large-scale DNA in fragmentation in NNCMs 24 hours after infection. At 48 hours, internucleosomal fragmentation was observed. ETS2-mediated high molecular weight and internucleosomal fragementation could not be inhibited by pretreatment with 60μM Z-VAD-FMK; however, caspase-dependent apoptosis could be induced with 0.5 mM Staurosporine, which could be inhibited by pre-treatment with 60 μM Z- VAD-FMK. (E) Pro-caspase 3, but not cleaved Caspase 3 (175Asp) was detected in NNCMs infected with Ad-ETS1 or Ad-ETS2; however, infected rFBs contained elevated levels of cleaved Caspase3. (F) In rFBs, ETS2 over-expression, but not GFP, promotes DNA fragmentation that can be inhibited by 50μM Z-VAD-FMK.
These findings together with the presence of cells positive for both ETS2 and TUNEL staining in the 8% mortality groups suggested that CMs might be susceptible to ETS-activated programmed cell death. Indeed, condensed or fragmented nuclei were observed in 8±3.2%, 12±2.1%, 6±2.3%, and 1±0.5% of Ets1-, Ets2-, GFP-infected and non-infected NNCM’s respectively, but only ETS2 led to a significant increase (n=3, p=0.001). Propidium iodide (PI)-positive cells were not observed in rFBs, and no significant difference in PI staining could be demonstrated among NNCMs infected with Ad-ETS1, Ad-ETS2 and Ad-GFP. Fig. 4C shows Ad-ETS2 infected NNCM cells containing condensed nuclei associated specifically with cardiac TnT positively stained cells, excluding the possibility that fragmentation occurred in contaminating cells. Elevated ETS2 also caused a significant increase in damaged DNA relative to controls. Unexpectedly, we detected fragmentation of DNA in two distinct size classes: high molecular weight and the more typical internucleosomal fragmentation associated with programmed cell death. The former was most prevalent in NNCMs within 24 hours of ETS2 infection, but with longer incubation times (48 hours), the latter become prevalent (Fig. 4D). Using Z-VAD-FMK, a cell-permeant pan caspase inhibitor used to define caspase-independent cell death, we were unable to prevent fragmentation resulting from elevated levels of ETS2 in NNCMs even after varying pre-incubation times (1–3hours) and drug concentrations (10–80μM). In contrast, caspase-dependent DNA laddering (internucleosomal fragementation) was observed in non-infected cells treated with staurosporine (0.5μM, Sigma), that could be completely abolished by pretreatment (1 hour) with Z-VAD-FMK (Fig. 4D). DNA fragmentation was also observed in ETS2 infected rFBs that could be inhibited by Z-VAD-FMK. Moreover, cleaved active Caspase 3 (Asp175) was only observed from infected rFBs (n=6, t-test, p <0.05, Fig 4E), and it was undetectable in protein extracts derived from Ad-ETS2 infected NNCMs (Fig. 4E). Elevated levels of ETS2 therefore promote caspase-independent cell death exclusively in CMs.
Programmed Necrosis induced by ETS2
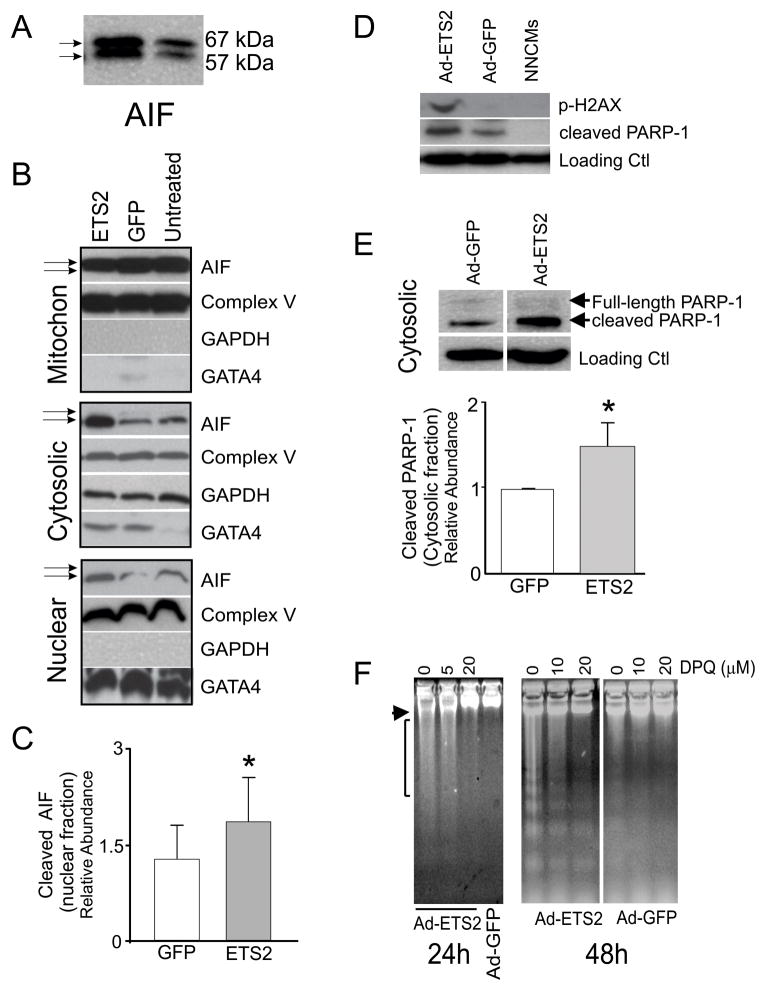
Caspase-independent forms of cell death can be induced by endonuclease G (Endo G), by apoptosis-inducing factor (AIF), or by high temperature requirement protein A2 (HtrA2/Omi) (Bae et al., 2008; Siu et al., 2007). All three proteins were detected in infected NNCMs; however, no significant change in abundance for any of these factors could be demonstrated following ETS2 over-expression (Fig. S1F, p>0.05). Unexpectedly, both active (57kDa) and inactive forms of AIF (67kDa) were observed in adenovirus infected NNCMs (Fig. 5A), and elevated levels of ETS2 specifically caused AIF to translocate from the mitochondrial fraction (inactive) to the cytoplasmic and nuclear fractions (active)(Fig 5B, see arrows). No translocations of Endo G or HtrA2 from mitochondrial to cytosolic or nuclear compartments were observed (not shown). Quantitatively, AIF content increased by ~2-fold in the nuclear fraction of NNCM infected with Ad-ETS2 compared with controls (p=0.02, n=4), and active AIF was readily observed in the cytoplasm and nuclei of these cells (Fig. 5B,C). Thus AIF, but not other activators of caspase-independent forms of cell death, is activated in response to ETS2 over-expression in NNCMs.
Figure 5. ETS2 over-expression promotes programmed necrosis in cardiomyocytes.
(A) CMs infected with Ad-ETS2 contain two forms of AIF (apoptosis inducible factor): 67kD full-lengh and 57kD cleaved (active). (B) Mitochondrial, cytoplasmic and nuclear enrichment analyses of AIF in untreated CMs or cells infected with Ad-ETS2 or Ad-GFP. Fractionation experiments show that Ad-ETS2 overexpression causes cleaved AIF to translocate from mitochondrial to cytosolic and nuclear compartments. Fractionation enrichment was verified by GATA4 (nuclear), Complex V (mitochondria) and GAPDH (Cytosol). Some mitochondrial associated Complex V was, however, present in each fraction. (C) Relative abundance of AIF in the nuclear fraction of Ad-ETS2 infected NNCMs, after normalization to take into account complex V/mitochondrial contaminants (*t-test, p=0.02, n=4). (D) Ad-ETS2 promotes the release of cleaved PARP-1 and phosphorylation of H2AX in NNCMs. (E) From fractionation studies and after normalization for complex V/mitochondrial contaminants, cleaved PARP-1 abundance significantly increased in the cytosolic fraction after Ad-ETS infection. (F) DPQ treatment completely abolished DNA damage associated with ETS2 overexpression. Overnight pre-incubations prior to Ad-ETS2 infection were performed, and 20 μM DPQ completely abolished all high molecular weight DNA damage 24 and 48 hours post-infection.
Activation of AIF and the associated cell death is considered to be a form of programmed necrosis and is thought to occur following activation of Poly (ADP-ribose) polymerase 1 (PARP-1) (Kung et al., 2011; Moubarak et al., 2007). Consistently, we observed that cleaved PARP-1 significantly increases in cytoplasm in response to elevated levels of ETS2 (p=0.001, n=3, Fig. 5D–E), and its elevation correlates with AIF translocation from mitochondria. To confirm that PARP-1 activation of AIF mediates the ETS2 response in NNCMs, we pre-treated the ETS2 infected cells with a second generation PARP-1 inhibitor - DPQ (3,4-dihydro-5-(4-(1-piperidinyl)butoxy)-1(2H)-isoquinolinone. Pre-incubation with 5 or 10μM DPQ partially inhibited the DNA damage observed 24 hours after ETS2 infection, while treatment with 20 μM DPQ completely abolished the high molecular weight laddering (Fig. 5F). At 48 hours post-infection, high molecular weight fragmentation could be abolished by DPQ, and although some DNA laddering was still observed, it was markedly reduced (n=3). Pre-treatment with Z-VAD-FMK did not alter this result. Very few direct downstream targets of AIF have been identified; however, nuclear AIF was recently found to associate with histone H2AX to promote phosphorylation of Ser139 and activate a DNA degrading complex (Artus et al., 2010). Indeed, we observed that elevated ETS2 promotes phosphorylation of H2AX at Ser139 (Fig. 5D), but not on histone H2B at Ser13 (not shown). ETS2 over-expression therefore specifically promotes phosphorylation of H2AX, and programmed necrosis initiated by this TFs is mediated principally through AIF after PARP-1 activation.
Discussion
The present study took advantage of gene expression data and PAM to identify gene transcripts predictive of relative lifespan. The gene set identified here showed “stable” expression with a unique profile that had a positive slope in animal cohorts from 8% to 80% mortality. Theoretically, this profile could be achieved either by transcriptional or post-transcriptional mechanisms. To discriminate between these possibilities, we utilized a component of PAM to “test” (i.e., predict) the 80% mortality group of 36-month old F/N rats based on the aggregate transcriptomic analysis of Wistar and F344 rats. Three PAM “tests” indicated that the expression profile of the 252 predictive gene set in F/N rats significantly correlated with the 80% mortality group of the other two rat strains (with a posterior probability equal to unity). The fact that this transcriptomic-based determination could identify a group in a third rat strain with a longer lifespan demonstrates that the normal variation in transcript abundance among myocardial samples is less important than conserved biological mechanisms responsible for controlling changes in gene expression that occur as a function of lifespan, and not just absolute age.
The presence of conserved biological mechanisms indicates that some lifespan-regulatory pathways are functional in rodent heart and that transcriptional mechanisms may be implicated in longevity variability. Consistent with this view, several of the 252 genes that accurately predict longevity (based on mortality curves) among the three rat strains were analogous to previously described longevity factors that influenced the activity of insulin-signaling pathways, stress response systems, chromatin structure and energy metabolism (Barger et al., 2003; McCarroll et al., 2004; Zahn et al., 2007). The correlation of this predictive gene set with longevity could, however, have been a reactive component of longevity traits instead of a causal component, and some of the transcripts identified were probably non-CM in origin. For example, cell cycle related components were present (e.gs., Ccnd2, Ches1, Cdc2l5, Cul3) but some of these would not have been expected in non-proliferating CMs. In contrast, very low expression of apoptosis related genes (e.gs., Casp9, Trp53inp1, Birc6, Rad21) in the 8% mortality group may be critical to long-term survival. These findings also argue that the expression profiles of these genes within a given rat strain are not principally controlled by a single TF as the majority of putative age-dependent, cardiac TFs were not conserved across strains or with age.
While this interpretation was potentially confounded by cell type variability, differences between RNA and protein abundance and post-transcriptional regulatory mechanisms, we identified at least one TF (ETS2) whose expression was strongly, but inversely correlated with the 252 “stable” gene set. We show that ETS2, which is principally expressed in CMs, shows age-dependent patterns of expression, is responsive to sympathetic (PE and ISO) stimuli, and functionally, promotes programmed necrosis of CMs. In adult hearts from the 8% mortality groups of 3 rat strains, ETS2 shows considerable cell-to-cell variability, and when present at high levels, TUNEL co-staining is observed. No such correlation was observed in any other mortality group examined. Mechanistically, ETS2 mediated loss of CMs involves activation of the PARP1-AIF axis to activate programmed cell necrosis, and possibly secondary activation of apoptosis. Since the PARP1-AIF axis is intricately involved with necrotic signaling, we conclude that ETS2 specifically activates this signaling pathway in CMs to promote cell death in vitro and in vivo (Hong et al., 2004; Kung et al., 2011). With that said, the adenovirus system that we employed to over-express ETS1 and ETS2 led to very high levels (~50–100-fold) of expression. This leads to questions about whether the changes in cell behavior observed are relevant to the action of the protein at more physiological in vivo levels and some caution should be taken when interpreting these data. Future experiments with a more highly regulated system will be required to address this issue. Despite these limitations, our findings provide a partial explanation of why CM and precursor cell loss occurs in young adults by 12 months of age (Anversa et al., 1990; Chimenti et al., 2003), prior to when rat mortality increases exponentially.
Both apoptosis and necrosis are prominent causes of cell death in major cardiac disease syndromes. CM necrosis is thought to be the major pathological lesion in acute myocardial infraction, but the relative frequencies of necrosis and apoptosis remain unclear (Kung et al., 2011). Moreover, there is almost no data on the role of the PARP-1-AIF axis during myocardial development or aging, and only recently have investigators described a role for PARP1 and AIF in myocardial infarction. Under normal circumstances, AIF acts as an oxidoreductase, but when released from the mitochondria, it can activate programmed necrosis. The mechanism whereby PARP-1 induces AIF release from mitochondria is controversial and may involve sequential activation of calpains and Bax or release induced by PAR polymer (Moubarak et al., 2007). PARP-1 over-activation can also cause a decrease in NAD+ levels, which may affect sirtuin activity, and excessive ATP consumption as well as increased ROS production that can contribute to caspase-mediated apoptosis (Kung et al., 2011). AIF is of particular interest to the aging heart, because it is activated in response to oxidative stress, ischemia-reperfusion and heart failure (Bae et al., 2008), and as shown here, adrenergic agonist up-regulation of ETS2. Moreover, the harlequin (Hq) mouse strain, which contains a proviral insertion in the AIF gene and shows reduced AIF expression in all tissues, including heart, is a model of premature aging (Klein et al., 2002). Phenotypically, Hq mice are characterized by mitochondrial dysfunction and an increased risk of oxidative stress-induced heart disease (Crabbe and Hill, 2010). Thus dysregulation of AIF can cause cardiac abnormalities that may contribute to the aging phenotype.
Genotoxic stress causes a senescence associated secretory phenotype (Coppe et al., 2010) that contributes to the aging process by promoting accumulation of oxidative stress damage, structurally disorganized mitochondria, energy production deficients, ROS production (Sheydina et al., 2011), and cell loss especially in older individuals. Until now, however, no mechanism has been described that adequately accounts for CM cell loss reported in young adults in the absence of injury. Here, we demonstrate for the first time that elevated ETS2 promotes CM programmed necrosis in vitro that may contribute to cell loss in heart. It is however important to note that we only made this determination after normalizing all of the expression data to relative mortality groups. Multiple groups have reported expression changes in heart with age (Sheydina et al., 2011; Volkova et al., 2005), but the majority of significant changes are not conserved across strains. For example, Park et al compared seven mouse strains (129sv, B6, B6C3F1, BALB/c, C3H, CBA, DBA) aged 5 and 25 months using microarrays (Park et al., 2009), and only one gene product (coenzyme A hydratase 1) decreased, while five others (Complement component 4, chemokine ligand 14, Scr family associated phospho-protein 2, phenylalanine hydrolase, and Sp100-rs) increased among all 7 strains of mice. Our data suggest that this lack of conservation across strains may be due to an analysis of transcript abundance with absolute age as opposed to relative lifespan. Thus taking into account relative versus absolute lifespan may be critical to unraveling mechanisms responsible for lifespan variability in mammals.
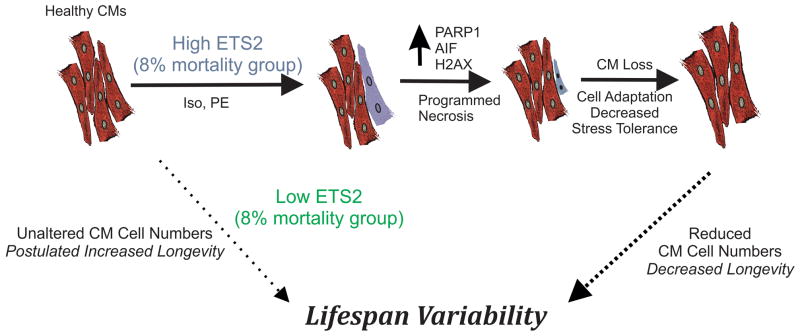
In summary, we have employed transcriptomic and bioinformatic analyses to identify a set of genes predictive of relative mortality across rat strains. A putative regulator, ETS2, was then found to have an expression pattern that was a mirror image of the predictive gene set expression profile. In vitro, we show for the first time that ETS2 promotes programmed cell necrosis through activation of PARP-1 and AIF and that its actions are CM specific. In vivo, transient, but elevated levels of ETS2 correlate directly with TUNEL staining in hearts exclusively from the 8% mortality groups. Since CMs are poorly regenerative (Perino et al., 2008) and ETS2 upregulation promotes CM cell death, we suggest that surviving contractile cells in adult heart experience increased stresses. The long-term consequences of this stress affect CM function, contribute to reduced contractile function, reduce stress tolerance and promote further cell loss (Sheydina et al., 2011). This self-sustaining cycle may ultimately contribute to increased mortality with age and reduced longevity as described in the model presented in Fig. 6. Conversely, low levels of ETS2 in CMs are predicted to be compatible with CM preservation and prolonged lifespan, however, this remains to be demonstrated. The findings that expression of this TF are heterogeneous among CMs in vivo and that its over-expression promotes cell death underscores how organ-specific and time-dependent changes in gene expression contribute to longevity variability in mammals.
Figure 6. ETS2 and Programmed Necrosis in Cardiomyocytes.
Model of ETS2 action in CMs and how its expression in heart may contribute to mammalian longevity variability through loss of CMs following activation of programmed necrosis. Solid arrows indicate pathways analyzed in this study; whereas, dotted arrows indicate hypothetical pathways implicated by our data.
Experimental Procedures
Comprehensive details for all experimental models, methods and protocols are provided in the Supplement.
Male rats [Fisher 344 (F344), Wistar, and Fisher × Brown Norway (F/N) rats] with different absolute lifespans were grouped based on the predicted life expectancy. This reorganization allowed an estimation of survival based on age-specific death rates (i.e., probability of dying at a given relative age). The probability of dying at 6 months of age for each strain was taken as approaching 0%, and transcriptomic analyses were performed on survivors from each cohort where the probability of dying at 4%, 8%, 30% and 80% was calculated from mortality curves (Figs. 1 and S1, Table 1). Microarrays were performed with probes generated from F344 (n=4/age group) and Wistar rat (n=3/age group) heart mRNA. Four independent pooled samples from 6- and 36-month-old F/N rat ventricles were also analyzed for PAM “tests” (Sorlie et al., 2001). Transcripts encoding TFs were quantitatively assessed by qRT-PCR using primers described in Table S1. Cardiocytes (CMs and FBs) were prepared from neonatal and adult rat hearts. Full-length mouse Ets1 cDNA (1463 bp) was amplified by PCR and replication-defective recombinant adenoviruses encoding ets1 (ETS1) or ets2 (ETS2) together with green fluorescent protein were constructed by homologous recombination as described (Luo et al., 2007). All cells were infected with adenovirus and switched to maintenance medium for 24 h prior to data collection. Antibodies used in this study are listed in the supplement. Nuclear fragmentation/condensation was detected in live cells after addition of Hoechst 33342, and necrotic cells were excluded by co-staining with PI. For the analysis of internucleosmal fragmentation, genomic DNA was isolated as described (Li et al., 2007). Cellular subfractions from CMs were isolated (mitochondrial, cytoplasmic, and nuclear) as described with modifications (Kim et al., 2010).
Data are expressed as means ± S.D. The Student’s t-test and ANOVA with repeated independent measurement were employed to determine statistical significance, where p<0.05 were considered significant. Statistical procedures were done in PASW 18.0.
Supplementary Material
Acknowledgments
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Author Contributions
Conception: KRB
Study Design: AS/MV/EGL/KRB
Data Acquisition: AS/MV/LJ/OJ/JZ/HJT/MGP/MW/YZ/KRB
Data Analysis/interpretation: AS/MV/LJ/KRB
Drafting article: AS/MV/LJ/KRB
Final approval: AS/MV/LJ/OJ/JZ/HJT/MGP/MW/YZ/EGL/KRB
Supporting Information List:
1) Experimental Procedures
2) Table S1: List of primers for TFs studied by Q-PCR
3) Table S2: Set of 252 gene transcripts that putatively predicts survival based on PAM analyses.
4) Table S3: Analysis of variance for K-mean clustering of 252 “survival” genes.
5) Table S4: Cluster classification according to Database for Annotation, Visualization and Integrated Discovery
6) Table S5: “Gene2Promoter” analysis for main functional group genes from cluster 3.
7) Table S6: Linear regression analysis of qRT-PCR data with age as an independent variable.
8) Supplementary Figure Legend
Contributor Information
Anna Sheydina, Email: annasheydina@gmail.com.
Maria Volkova, Email: maria.volkova@yale.edu.
Liqun Jiang, Email: jiangli@grc.nia.nih.gov.
Ondrej Juhasz, Email: JuhaszO@grc.nia.nih.gov.
Jing Zhang, Email: Zhangji@grc.nia.nih.gov.
Hyun-Jin Tae, Email: taeh@mail.nih.gov.
Maria G. Perino, Email: perinowork@gmail.com.
Mingyi Wang, Email: MingyiW@grc.nia.nih.gov.
Yi Zhu, Email: zhuy54@gmail.com.
Edward G. Lakatta, Email: LakattaE@grc.nia.nih.gov.
Kenneth R. Boheler, Email: bohelerk@grc.nia.nih.gov.
References
- Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte Cell Loss and Myocyte Cellular Hyperplasia in the Hypertrophied Aging Rat-Heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- Artus C, Boujrad H, Bouharrour A, Brunelle MN, Hoos S, Yuste VJ, Lenormand P, Rousselle JC, Namane A, England P, et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010;29:1585–1599. doi: 10.1038/emboj.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Yalamarti B, Kang PM. Role of caspase-independent apoptosis in cardiovascular diseases. Front Biosci. 2008;13:2495–2503. doi: 10.2741/2861. [DOI] [PubMed] [Google Scholar]
- Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Hwangbo D, Jasper H. Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol. 2011;46:349–354. doi: 10.1016/j.exger.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Yaswen P. Aging and cancer cell biology, 2009. Aging Cell. 2009;8:221–225. doi: 10.1111/j.1474-9726.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin KE, Desprez PY, Campisi J. A Human-Like Senescence-Associated Secretory Phenotype Is Conserved in Mouse Cells Dependent on Physiological Oxygen. Plos One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe RA, Hill KA. Heart tissue of harlequin (hq)/Big Blue (R) mice has elevated reactive oxygen species without significant impact on the frequency and nature of point mutations in nuclear DNA. Mutat Res. 2010;691:64–71. doi: 10.1016/j.mrfmmm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS. Cardiac Aging in Mice and Humans: The Role of Mitochondrial Oxidative Stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohr S, Klingenhoff A, Maier H, Hrabe de Angelis M, Werner T, Schneider R. Linking disease-associated genes to regulatory networks via promoter organization. Nucleic Acids Res. 2005;33:864–872. doi: 10.1093/nar/gki230. Print 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Dawson VL, Dawson TM. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Katewa SD. Dietary restriction and aging, 2009. Aging Cell. 2010;9:105–112. doi: 10.1111/j.1474-9726.2010.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Yang HJ, Youn H, Yun YJ, Seong KM, Youn B. Myricetin Inhibits Akt Survival Signaling and Induces Bad-mediated Apoptosis in a Low Dose Ultraviolet (UV)-B-irradiated HaCaT Human Immortalized Keratinocytes. J Radiat Res. 2010;51:285–296. doi: 10.1269/jrr.09141. [DOI] [PubMed] [Google Scholar]
- Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation down-regulates apoptosis-inducing factor. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- Kung G, Konstantinidis K, Kitsis RN. Programmed Necrosis, Not Apoptosis, in the Heart. Circ Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wei H, Chesley A, Moon C, Krawczyk M, Volkova M, Ziman B, Margulies KB, Talan M, Crow MT, Boheler KR. The pro-angiogenic cytokine pleiotrophin potentiates cardiomyocyte apoptosis through inhibition of endogenous AKT/PKB activity. J Biol Chem. 2007;282:34984–34993. doi: 10.1074/jbc.M703513200. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nature Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- Martin GM. The evolutionary substrate of aging. Arch Neurol. 2002;59:1702–1705. doi: 10.1001/archneur.59.11.1702. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, Kenyon C, Bargmann CI, Li H. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat Genet. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Murcia JMD, Susin SA. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Liao CY, Rikke BA, Johnson TE, Diaz V. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–495. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011;10:5–9. doi: 10.1111/j.1474-9726.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino MG, Yamanaka S, Li JL, Wobus AM, Boheler KR. Cardiomyogenic stem and progenitor cell plasticity and the dissection of cardiopoiesis. J Mol Cell Cardiol. 2008;45:475–494. doi: 10.1016/j.yjmcc.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. 2010;45:691–701. doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden DM, Rasouli P, Lu XY. Potential long-term consequences of fad diets on health, cancer, and longevity: Lessons learned from model organism studies. Technol Cancer Res Treat. 2007;6:247–254. doi: 10.1177/153303460700600312. [DOI] [PubMed] [Google Scholar]
- Saban MR, Nguyen NB, Hammond TG, Saban R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am J Pathol. 2002;160:2095–2110. doi: 10.1016/S0002-9440(10)61159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, Wang YC, Duran GE, Sikic TL, Caldeira S, et al. Gene expression patterns in ovarian carcinomas. Mol Biol Cell. 2003;14:4376–4386. doi: 10.1091/mbc.E03-05-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Sheydina A, Riordon DR, Boheler KR. Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond) 2011;121:315–329. doi: 10.1042/CS20110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Michan S. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Bae S, Bodyak N, Rigor DL, Kang PM. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J Mol Cell Cardiol. 2007;42:678–686. doi: 10.1016/j.yjmcc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR, Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- Volkova M, Garg R, Dick S, Boheler KR. Aging-associated changes in cardiac gene expression. Cardiovasc Res. 2005;66:194–204. doi: 10.1016/j.cardiores.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res. 2002;274:246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100:3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn JM, Poosala S, Owen AB, Ingram DK, Lustig A, Carter A, Weeraratna AT, Taub DD, Gorospe M, Mazan-Mamczarz K, et al. AGEMAP: a gene expression database for aging in mice. PLoS Genetics. 2007;3:e201. doi: 10.1371/journal.pgen.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.