Abstract
Objective
The objective of this study is to define a role for S1PR3 in intimal hyperplasia.
Methods and Results
A denudation model of the iliac-femoral artery in wild-type and S1PR3-null mice was used to define a role for S1PR3 in the arterial injury response because we found in humans and mice that expression of S1PR3 is higher in these arteries when compared to carotid arteries. At 28 days after surgery, wild-type arteries form significantly larger lesions than S1PR3-null arteries. BrdU labeling experiments demonstrate that upon injury, wild-type arteries exhibit higher medial as well as intimal proliferation than S1PR3-null arteries. Because S1PR3 expression in vitro is low, we expressed S1PR3 in S1PR3-null SMCs using retroviral-mediated gene transfer to study S1PR3 effects on cell functions and signaling. SMCs expressing S1PR3, but not vector-transfected controls, respond to S1P stimulation with activation of Rac, Erk and Akt. SMCs expressing S1PR3 also grow migrate more.
Conclusion
In humans and mice, S1PR3 expression is higher in iliac-femoral arteries compared to carotid arteries. S1PR3 promotes neointimal hyperplasia upon denudation of iliac-femoral arteries in mice, likely by stimulating cell migration and proliferation through activation of signaling pathways involving Erk, Akt and Rac.
Keywords: sphingosine-1-phosphate, neointima, restenosis, smooth muscle cells, vascular biology
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that plays a role in vascular pathologies including coronary artery disease1, atherosclerosis2–6, and intimal lesion formation after arterial injury.2, 7–9 S1P is present at submicromolar concentrations in plasma where it is bound mainly to albumin and high density lipoproteins.10 To what extent blood-borne S1P is recognized by vascular cells in normal arteries is unknown. In diseased arteries or upon arterial injury, S1P may be released from platelets and also locally produced by growth factor or cytokine-mediated induction of sphingosine kinase, which phosphorylates sphingosine to generate S1P.11 S1P binds to five G protein-coupled receptors, S1PR1-S1PR5. S1PR1, S1PR2 and S1PR3 are ubiquitously expressed, whereas S1PR4 and S1PR5 are mainly expressed in immune cells and brain, respectively.12
Restenosis is a significant complication of surgical and percutaneous procedures to restore arterial blood flow. Arterial lesions are characterized by intimal hyperplasia and negative remodeling of the artery, both causing luminal narrowing. In animal models of neointimal lesion formation injury-induced medial proliferation is followed by an accumulation of intimal cells.13, 14 The role for S1P in arterial lesion formation may be complex since cellular responses to S1P depend on which receptor is expressed, given that they couple to different G-proteins.12 We have recently demonstrated that S1PR2 is an inhibitor of neointimal lesion growth induced by carotid ligation by comparing the injury response in wild-type and S1PR2-null mice.15 This observation is in agreement with in vitro experiments showing that S1PR2 mediates the inhibition of SMC migration15–17 and the induction of genes of the differentiated SMC phenotype.7, 18 In the rat carotid artery, application of an antagonist for both S1PR1 and S1PR3 reduces intimal lesion size following denudation injury.9 S1PR1 is also required for recruitment of mural cells to the developing endothelial tube19, so it may promote injury-induced intimal hyperplasia by stimulation of medial cell migration. Only recently the role of S1PR3 in intimal hyperplasia has been addressed. During the preparation of this manuscript, it was reported that deletion of S1PR3 increases lesion size in carotid arteries following ligation injury.2 Here, we report that deletion of S1PR3 has the opposite effect in denuded iliac-femoral arteries, in which it decreases neointimal lesion size. Possible reasons for S1PR3 playing opposing roles in the arterial injury response using different models will be discussed.
Methods
Please see supplemental material for more detailed descriptions of methods.
Animals and human tissue
S1PR3-null mice and wild-type mice were kindly provided by Dr. Richard L. Proia (NIH, Bethesda, MD).20 Heterozygous mice were bred to generate S1PR3-null and wild-type littermates. Genotypes were verified by polymerase chain reaction analysis using specific primers (supplemental material). To obtain human specimens, patients with arterial occlusive disease in the carotid or iliac-femoral artery were recruited. Tissue was taken from the artery around the site of the incision or disobliteration and immersed immediately in RNAlater (Qiagen). This study was conducted in accordance with rules and regulation of the ethics commission of the General Medical Council, Hamburg, Germany.
Iliac-femoral artery denudation
Adult male mice were anesthetized with an intraperitoneal injection of xylazine and ketamine cocktail (8.8 mg/kg xylazine and 130 mg/kg ketamine). The popliteal artery was exposed and two ligatures (6-0 surgical silk) were placed around it. The artery was dilated by local administration of 1% lidocaine and the distal ligature was tied. A small incision was then made between the two ligatures and the vessel was denuded by using a 7-0 monofilament loop catheter. At the appropriate time point, mice were euthanized with Beuthanasia-D (500 mg/kg, i.p.) and iliac-femoral arteries were perfusion-fixed with 4% paraformaldehyde, embedded in paraffin. Measurements of cross sectional areas were performed on tissue sections following staining with hematoxylin and eosin using computer-assisted image analysis (NIH image).
For analysis of proliferating cells, mice were intraperitoneally injected with bromodeoxyuridine (BrdU, 30 mg/g body wt) at 1, 9, and 17 hours before euthanasia. Tissue sections were stained with BrdU antibody (Roche) and hematoxylin. Hematoxylin-positive (total) and BrdU-positive (replicating) cells were counted. All studies were performed in accordance with the guidelines for animal experimentation at the NIH and at the University of Washington.
Analysis of gene expression by real time PCR
Real time PCR was performed as previously described. 7 Specificity of primers was verified by dissociation curves, and gene expression was normalized to expression of GAPDH.
Isolation and culture of mouse carotid SMCs
Carotid SMCs were prepared and cultured as previously described. 21
Expression of S1PR3 in SMCs using retroviral-mediated gene transfer
Human S1PR3 cDNA was kindly provided by Timothy Hla (Cornell University, New York, NY22 and LXSH vector by Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) 23 S1PR3 cDNA was subcloned into the BamH1 site of the LXSH retrovirus backbone vector. PE501 early high-titer ecotropic packaging cells were transfected with S1PR3-LXSH plasmid using Lipofectamin-2000 transfection agent (Invitrogen). Viral particles in cell supernatant were used to infect a high-titer PA317 amphotropic packaging cell line. This step was performed twice to increase titers. Several clones were probed for S1PR3 expression and used to produce virus to transfect S1PR3-null carotid SMCs. Multiple SMC clones were probed for S1PR3 expression and clones strongly expressing S1PR3 were selected and expanded for experiments (S1PR3-SMCs). SMC clones expressing empty vector were used as controls (LXSH-SMCs).
Rac and RhoA assays
Activities of Rac1 and RhoA were measured using commercially available kits (Rac G-LISA, RhoA G-LISA, both from Cytoskeleton).
Cyclic AMP measurements
Cyclic AMP was measured using cAMP Biotrak enzyme-immunoassay system (GE Healthcare) as to the manufacturer’s instructions.
Analysis of phospho-Erk and phospho-Akt by Western blotting
Blots were probed with phospho-Erk and phospho-Akt (T308) antibodies over night at 4°C and then developed with ECL (GE Healthcare). Equal loading of protein was confirmed by re-probing blots for β-tubulin. All antibodies were from Cell Signaling Tech.
Proliferation Assay
Cell replication was measured using a metabolic labeling assay.24
Scratch assay
LXSH-SMCs and S1PR3-SMCs were plated into 12-well dishes at 100,000 cells per well. Twenty four hours later, a vertical scratch was made to the cell monolayer using a sterile 1 mL pipette tip. A perpendicular line was drawn onto the plate to mark a specific location and a picture was taken. Media was replaced with serum-free media and S1P (Cayman) or PDGF-BB (R&D) were added as indicated. After 24 hours, the scratch was imaged at the same position and cell free areas were quantified using Image J software (NIH). Migration was then expressed as percent closure of cell free area.
Statistics
As indicated in figure legends, data are presented as mean +/− S.D. or S.E.M. Significance of differences between 2 groups was calculated using Student’s t-test. Multiple comparisons using one data set were analyzed by applying a Bonferroni adjustment.25 A probability of P<0.05 is considered significant.
Results
Iliac-femoral arteries express significantly more S1P3R than carotid arteries
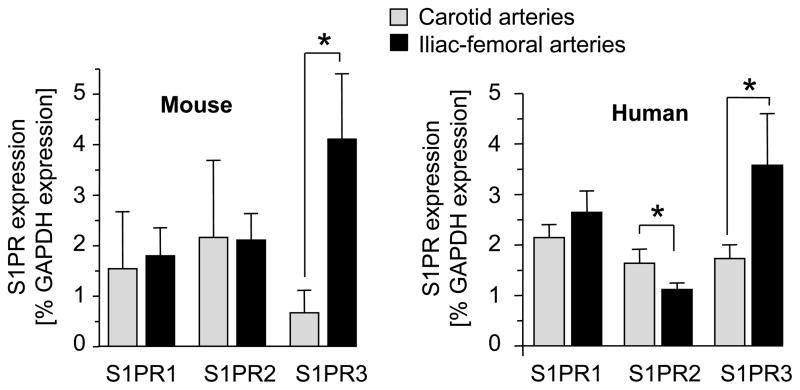
The C57BL/6 mouse is widely used to study the response to arterial injury. We and others have noticed that C57BL/6 mice can be considered “resistant” to neointimal lesion growth following injury of the carotid artery because injury produces no or only small lesions compared to mice of different genetic backgrounds.13, 26, 27 In contrast, C57BL/6 iliac-femoral arteries respond to injury with significant neointimal lesion formation.28 One possibility to explain this difference may lie in expression of S1PRs since we found that S1P3R is strongly expressed in iliac-femoral arteries compared to carotid arteries (Figure 1). Interestingly, this difference has also been observed in humans (Figure 1). We therefore chose denudation of the iliac-femoral artery to investigate a possible role for S1P3R in neointimal lesion growth.
Figure 1. Expression of S1PRs in mouse and human carotid and iliac-femoral arteries.
Total RNA from carotid and iliac-femoral arteries from C57BL/6 mice and human samples was prepared and subjected to real time PCR analysis for expression of S1PR1, S1PR2 and S1PR3. For mouse samples, 8–12 arteries were pooled. Data (mean +/− SEM, N=3 pools for mouse arteries, N=8 for human iliac-femoral arteries and N=5 for human carotid arteries) are presented as percent of GAPDH expression. *P<0.05
S1PR3 promotes intimal hyperplasia
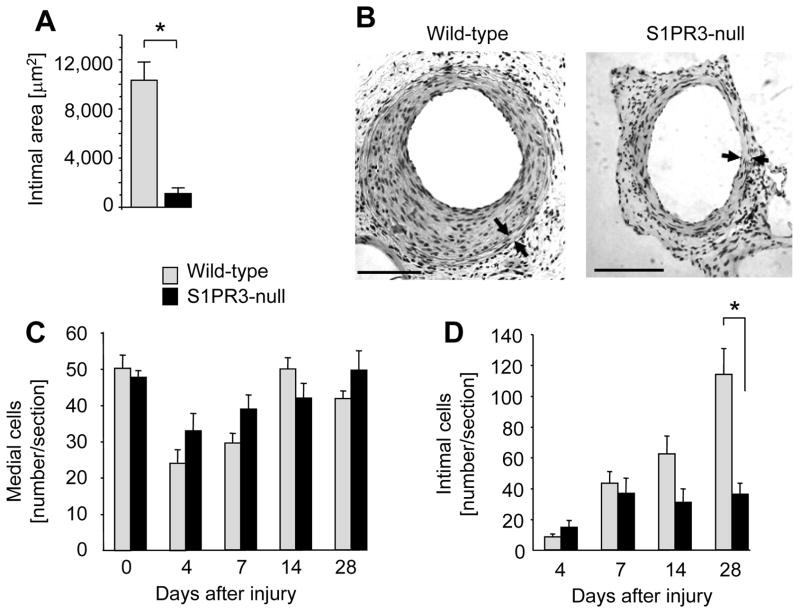
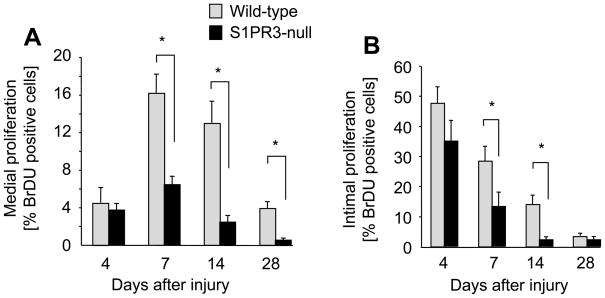
To define a role for S1PR3 in the arterial injury response, we compared medial and intimal areas at various time points following denudation of iliac-femoral arteries in wild-type and S1PR3-null mice. Lack of S1PR3 expression decreases neointimal lesion size at 28 days post injury by 90% (Figure 2A and B). In both wild-type and S1P3R-null mice, medial cell numbers decline at 4 days post injury and recover by day 14 (Figure 2C). In contrast, intimal cell numbers continuously increase in wild-type animals throughout the time course (4–28 days) but stay constant after day 7 in S1PR3-null mice resulting in a significantly higher number of intimal cells in wild-type animals at day 28 post injury (Figure 2D). BrdU labeling experiments show that wild-type medial as well as intimal SMCs proliferate more compared to S1PR3-null at days 7 and 14 post injury (Figure 3A and B). For medial SMCs, this difference is still significant at day 28 post injury (Figure 3A). The kinetics of proliferation after injury are similar in wild-type and S1PR3-null arteries. Medial proliferation is low at day 4, peaks at day 7 and then declines. Intimal proliferation is the highest at day 4 and then declines (Figure 3A and B). These data suggest that in response to injury, S1PR3 expression promotes cell proliferation in the media as well as in the developing intima. We also measured expression of S1PRs at 4 and 7 days after injury. Unexpectedly, expression of S1PR3 decreases to only 10% compared to uninjured controls at day 4 post surgery, but then recovers to 25% at day 7 (supplemental Figure 1). In both wild-type and S1PR3-null mice, injury stimulates expression of S1PR1 (at day 7) and S1PR2 (at day 4 and day 7). To address the question whether S1PR3 affects the inflammatory response to injury, we measured expression of the common leukocyte antigen CD45 and CD14, a marker for monocytes, macrophages and dendritic cells. There was no difference in expression levels of CD45 or CD14 between wild-type and S1PR3-null arteries. Expression of both markers is induced by injury (approximately 10-fold at day 4), but with different kinetics in that CD45 expression further increases between day 4 and day 7, whereas CD14 expression does not (supplemental Figure 2).
Figure 2. S1PR3 promotes intimal hyperplasia.
Wild-type (wt) and S1PR3-null iliac-femoral arteries were denuded and animals sacrificed at the indicated time points. Arteries were perfusion-fixed and tissue sections stained with H&E. (A) For the 28 day time point, intimal areas were measured using NIH Image software. Data are shown as mean +/− SEM for wt (N=9) and S1PR3-null (N=6). *P<0.001 (B) A typical cross-section of wild-type and S1PR3-null arteries at 28 days after injury is shown. Arrowheads mark the external and internal elastic lamina. The bar indicates 100 μm. The number of cells in media (C) and intima (D) at was quantitated by counting multiple sections that were 250 μm apart. Data (mean +/− SEM, N=7–13 animals per group) are presented as number of cells counted per section. *P<0.05
Figure 3. Injury-induced proliferation is increased in wild-type arteries compared to S1PR3-null arteries.
Wild-type (wt) and S1PR3-null iliac-femoral arteries were denuded and injected with BrdU (30 μg/g body weight) at 17, 8 and 1 hour prior to sacrifice at the indicated time points. Arteries were perfusion-fixed and tissue sections stained with anti-BrdU (Roche). The number of positive cells in media (A) and intima (B) was quantitated by counting multiple sections that were 250 μm apart. Data (mean +/− SEM, N=7–13 animals per group) are presented as percent BrdU positive cells. *P<0.05
S1PR3 activates Rac, Akt and Erk, but does not inhibit S1PR2-dependent activation of Rho
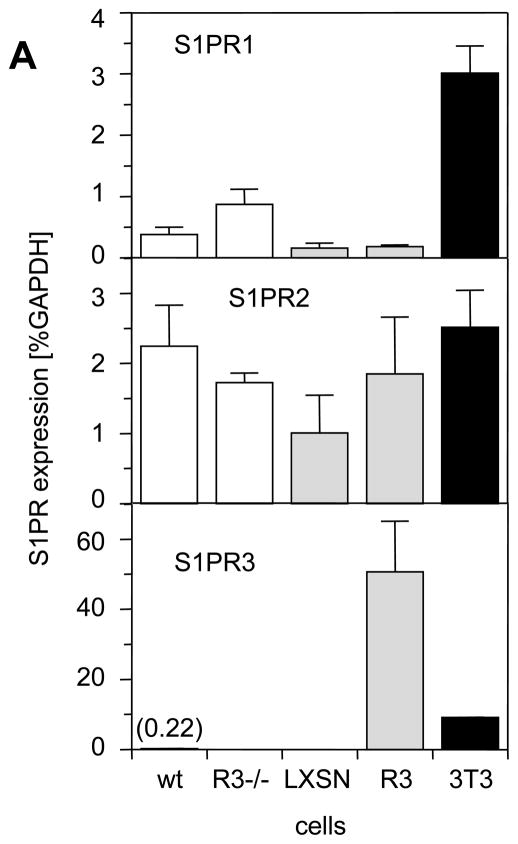
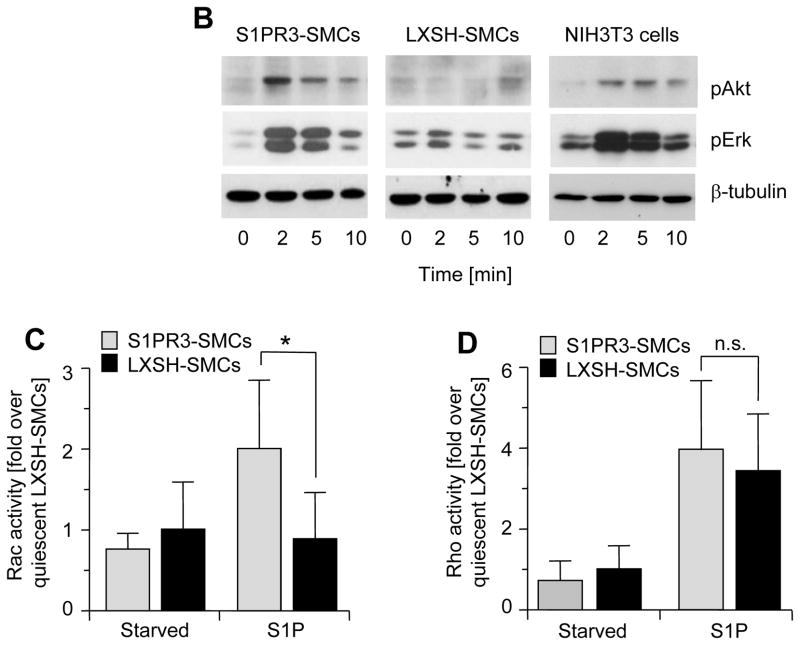
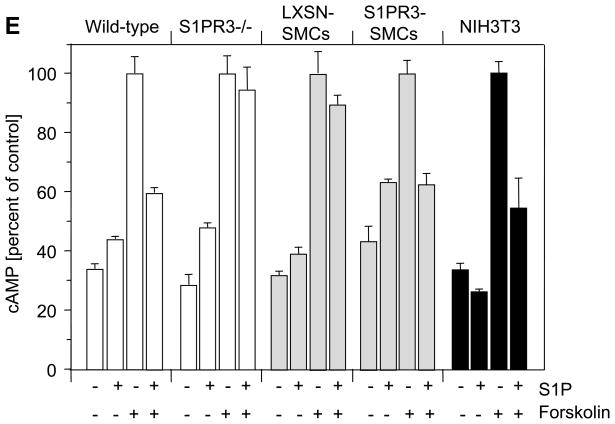
To define S1P-induced signaling pathways in SMCs that are mediated by S1PR3, we attempted to prepare SMCs from iliac-femoral arteries. However, we never succeeded in growing sufficient numbers of cells to perform in vitro experiments. We then prepared SMCs from carotid arteries derived from wild-type and S1PR3-null mice and measured S1PR1, S1PR2 and S1PR3 message levels. We included NIH3T3 cells as a potential positive control for S1PR3 expression because fibroblasts may express more S1PR3 than SMCs.29, 30 Indeed, compared to NIH3T3 cells, wild-type SMCs express significantly less S1PR1 and S1PR3 (Fig. 4A), whereas S1PR2 expression is similar in both cell types. Consistent with higher expression of S1PR1 and S1PR3, only NIH3T3 cells but neither wild-type nor S1PR3-null SMCs respond to S1P with phosphorylation of Erk and Akt (Figure 4B, negative data for SMCs not shown). We therefore decided to over-express S1PR3 in S1PR3-null carotid SMCs to investigate the possibility that high expression of S1PR3 in iliac-femoral arteries stimulates Erk/Akt-dependent signaling pathways. We used retroviral gene transfer to generate multiple SMC clones strongly expressing S1PR3 (S1PR3-SMCs). Carotid S1PR3-null SMCs were also transfected with empty vector (LXSH-SMCs) and used as controls. 20There was no significant difference in expression of S1PR1 or S1PR2 between LXSN- and S1PR3-SMCs (Fig. 4A). Compared to NIH3T3 cells, S1PR3-SMCs expressed approximately 5 times more S1PR3 message (Fig. 4A). Following stimulation with S1P, only S1PR3-SMCs but not LXSN controls showed phosphorylation of Erk and Akt (Figure 4B). As S1P positively regulates cell migration by activating Rac via S1PR1 and S1PR3, or negatively by activating Rho via S1PR216, 17, we measured S1P-induced activation of Rac and Rho in S1PR3-SMCs and LXSN controls. We found that Rac activation by S1P is S1PR3-dependent and that S1PR3 does not affect activation of Rho by S1P2R (Figure 4C and D). To address the question whether S1PR1 is signaling, we measured the effect of S1P on forskolin-induced cAMP levels. As S1PR1 couples to Gαi, S1P addition should reduce cAMP levels if S1PR1 is active. Forskolin led to increased cAMP levels in all cells (Figure 4E). Addition of S1P, however, only reduced cAMP levels in cells expressing S1PR3 (wild-type SMCs, S1PR3-SMCs and NIH3T3 cells) but not in cells lacking S1PR3 (S1PR3-null SMCs and LXSN controls; Figure 4E). This observation indicates that S1PR1 does not contribute to S1P responses in cultured SMCs.
Figure 4. Expression of S1PR3 is linked to S1P-induced activation of Rac1, Akt and Erk, but does not affect S1P-dependent activation of RhoA.
(A) Total RNA was prepared from carotid wild-type SMCs (wt), S1PR3-null SMCs (R−/−), LXSN-SMCs (LXSN, two clones), S1PR3-SMCs (R3, two clones) as well as from NIH3T3 cells (3T3) and analyzed for expression of S1PRs by real time PCR. Data (mean +/− SEM) are presented as percent of GAPDH expression. N=8 for both SMC types, N=4 for LXSN-SMCs, N=6 for S1PR3-SMCs, N=2 for NIH3T3 cells. Following stimulation of LXSH-SMCs (2 clones) and S1PR3-SMCs (2 clones) or NIH3T3 cells with S1P (1 μmol/L) or PDGF-BB (10 ng/mL) as indicated, cells were processed for Western blotting with antibodies against the phospho-forms of Akt and Erk as well as for measuring activities of Rac and Rho. (B) Typical blots for phospho-Akt and phospho-Erk are shown from one out of four experiments that yielded identical results. Blots were reprobed for β-tubulin to demonstrate equal loading. (C) Data for Rac activation were normalized to activity in quiescent LXSH-SMCs and are presented as mean +/− S.D. (N=5) *P<0.05 (D) Data for Rho activation were normalized to activity in quiescent LXSH-SMCs and are presented as mean +/− S.D. (N=4); n.s.=non significant (E) Cells were stimulated for 5 min with S1P (1 μmol/L) and forskolin (3 μmol/L) as indicated. Cyclic AMP was measured and data (mean +/− S.D.) expressed as percent of control (forskolin). The assay was performed in triplicates.
S1PR3 promotes proliferation and migration of SMCs
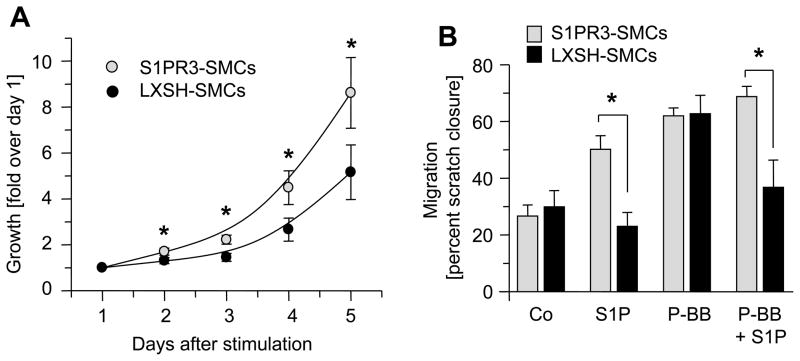
Since injury-induced neointimal lesion formation is significantly decreased in S1P3R-null arteries compared to wild-type controls, we tested the possibility that S1PR3 promotes SMC proliferation or migration. Cell proliferation was assessed by using a metabolic labeling assay in which mitochondrial dehydrogenase (mDH) activity is correlated to cell numbers.24 Measurements of mDH activity indicate that there are more S1PR3-SMCs than LXSH-SMCs at all time points after serum stimulation, suggesting S1PR3 promotes cell growth (Figure 5A). Cell migration was measured using scratch assays and migration expressed as percent “wound” closure. As expected from previous reports showing that S1PR2 is an inhibitor for migration15, 31, S1P inhibits PDGF-BB-induced migration of LXSN-SMCs (P<0.05, n=3, PDGF-BB vs. PDGF-BB+S1P) and S1P alone does not stimulate migration (Figure 5B). In contrast, in SMCs overexpressing S1PR3, S1P itself stimulates migration (P<0.05, n=3, control vs. S1P). Moreover, S1PR3 expression appears to abolish the inhibitory effect of S1PR2 as in S1PR3 SMCs, increases migration even in the presence of PDGF-BB (P<0.05, n=3 PDGF-BB versus PDGF-BB + S1P; Figure 5B). LXSH-SMCs and S1PR3-SMCs migrate equally well in presence of PDGF-BB indicating that S1PR3 expression has no general effect on cell migration (Figure 5B). In the presence of S1P, however, migration of S1PR3-SMCs is always greatly increased when compared to LXSN-SMCs (Figure 5B).
Figure 5. S1PR3 promotes SMC proliferation and migration.
(A). Growth curves of LXSH-SMCs (N=9, 5 different clones) and S1PR3-SMCs (N=11, 2 different clones) were measured using MTT assay. Data (mean +/− SEM, N=11 for S1PR3-SMCs, N=9 for LXSH-SMCs) are expressed as fold absorption at 560 nm over day 1. *P< 0.05 (B) Migration of S1PR3-SMCs (N=6, 2 clones) and LXSH-SMCs (N=6, 2 clones) was measured in the absence or presence of 1 μmol/L S1P, 10 ng/ml PDGF-BB or both using the scratch assay. Data (mean +/− SEM) are presented as percent of “wound” closure. *P<0.05
Discussion
The role of S1P in biological processes is complex because cells may express multiple S1PRs that couple to different G-proteins, which may mediate different, even opposing effects on cells. It has previously been shown that concomitant pharmacological inhibition of S1PR1 and S1PR3 attenuates lesion formation in the rat carotid artery denudation model suggesting a positive role for these receptors in intimal hyperplasia.9 Genetic deletion of S1P2R increases lesion size in mouse carotid arteries following ligation.15 The main reason that we chose the iliac-femoral artery as a model to define a role for S1PR3 in arterial stenosis was that S1P3R expression in mouse iliac-femoral arteries is much higher than in carotid arteries (Figure 1). Interestingly, this difference was also found in humans and may indicate the possibility of targeted pharmacological treatment to inhibit restenosis in iliac-femoral arteries by using S1PR3 antagonists. Following denudation of the iliac-femoral artery, wild-type mice develop much larger lesions than S1PR3-null mice and this correlates with higher medial as well as intimal proliferation in wild-type arteries (Figures 2 and 3). Notably, despite higher medial proliferation in wild-type arteries compared to S1PR3-null arteries, medial cell numbers do not significantly differ in the two arteries, whereas intimal cell numbers are higher in wild-type arteries. We therefore believe that proliferating medial cells migrate into the intima where they contribute to neointimal lesion formation. Given that S1PR3 expression promotes neointimal lesion formation, we were surprised to find that injury causes a downregulation of S1PR3 expression (see supplemental Figure 1). It is possible that protein levels are less affected by injury than RNA levels but we cannot comment on receptor protein levels because we have not found an antibody that detects endogenous S1PR3 levels in the vasculature. Nevertheless, genetic deletion of S1PR3 strongly inhibits neointimal lesion formation and we conclude that the receptor is functional despite decreased expression after injury. Given the recent observation that S1PR3 regulates the macrophage content in atherosclerotic lesions in ApoE-null mice2 and that S1P is critical for leukocyte egression from lymphatic organs32, we tested the possibility that S1PR3 affects the inflammatory response to injury by measuring the expression of the common leukocyte antigen, CD45, and CD14, a marker for macrophages, monocytes and dendritic cells. As expected, we found increased arterial expression of both CD14 and CD45 after injury, but there was no difference between wild-type and S1PR3-null arteries (supplemental Figure 2) suggesting that S1PR3 does not play a role in recruiting or retaining inflammatory cells in this model.
Our data demonstrate that in carotid wild-type SMCs, S1PR3 reduces forskolin-induced cAMP levels (see Figure 4E) but does not promote activation of Erk or Akt (data not shown). SMCs expressing high levels of S1PR3, however, respond to S1P with activation of Erk and Akt. One possible explanation is that different thresholds exist for activation of these pathways. Another possibility is that S1PR3, which primarily couples to Gαi, may at high receptor densities also couple to Gαq, a strong activator of PLCβ, which mediates activation of Erk and Akt.33 Consistent with S1PR3-mediated activation of Erk and Akt is our observation that SMCs overexpressing S1PR3 grow faster and migrate better than S1PR3-deficient controls (Figure 5). Notably, S1P no longer inhibits PDGF-BB-induced migration in S1PR3 overexpressing cells (Figure 5B). As S1P-mediated inhibition of cell migration depends on S1PR2-dependent activation of Rho15, 31, we tested the possibility that S1PR3 expression inhibits Rho activation by S1P. As this is not the case (Figure 4D), S1PR3-mediated hyperactivation of Erk and Akt may simply counteract the inhibitory function of S1PR2-dependent activation of Rho. Thus, expression ratios between S1PR2 and S1PR3 (and possibly S1PR1) may be more indicative for the formation of neointimal lesions in iliac-femoral arteries than absolute expression levels of any individual S1P receptor.
While our studies were in progress, it was reported that S1PR3-null mice develop larger lesions in ligated carotid arteries than wild-type animals, suggesting an inhibitory role for S1PR3 in intimal hyperplasia.2 There are several possible reasons for opposite effects of S1PR3 in iliac-femoral arteries (this paper) and carotid arteries.2 One possibility is that there are colony-specific properties since the mice used by us and Dr. Levkau’s laboratory2 stem from different sources. To address this important point, we performed carotid ligation injury in our wild-type and S1PR3-null mice. These experiments confirmed the observations made by Dr. Levkau’s group that S1PR3-null animals develop significantly larger lesions at 4 weeks after injury (supplemental Figure 3). Another explanation for the opposing roles of S1PR3 is that in carotid arteries, where S1PR3 expression is low, S1PR3 mainly functions to inhibit production of cAMP, a long known inhibitor for SMC growth.34 Thus deletion of S1PR3 in these arteries might stimulate neointimal lesion growth. In contrast, in iliac-femoral arteries, S1PR3 is highly expressed and induces activation of Erk, Rac and Akt, all known to promote cell growth and migration.8–41 Thus, deletion of S1PR3 might reduce neointimal lesion formation. Finally, another possibility is that S1PR3 inhibits lesion growth in carotid arteries because of its function in endothelial cells, where S1PR3 is anti-inflammatory by activating endothelial nitric oxide synthase (eNOS) through Akt-dependent phosphorylation.35–37 Endothelial cells are present in the ligated carotid artery but removed from the iliac-femoral artery by the denudation procedure. In agreement with a lesion inhibitory role for S1PR3 through eNOS activation is the observation that eNOS-deficient mice grow much larger lesions following carotid ligation compared to wild-type controls.38
In conclusion, function of S1PR3 in the arterial injury response depends on the vascular bed and possibly expression levels and type of injury. Therefore, pharmacological approaches directed against S1PR3 to block restenosis in iliac-femoral arteries might have a negative impact on restenotic lesions in other vascular beds. Moreover, our finding that expression differences regarding S1PR3 also exist in humans indicates the possibility of artery-specific pharmacological treatment of restenosis.
Supplementary Material
Acknowledgments
We thank Richard Proia (NIH, Bethesda) for S1PR3-null mice, Tim Hla (Cornell University, New York) for human S1PR3 cDNA and Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle) for LXSH vector.
Sources of Funding:
This work was supported by NIH grant HL-088374, by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (M.W.) and by a predoctoral fellowship form the American Heart Association (ADW).
Footnotes
Disclosures:
None
References
- 1.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 2.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-Phosphate Receptor 3 Promotes Recruitment of Monocyte/Macrophages in Inflammation and Atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 3.Keul P, Tolle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The Sphingosine-1-Phosphate Analogue FTY720 Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 4.Klingenberg R, Nofer J-R, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-Phosphate Analogue FTY720 Causes Lymphocyte Redistribution and Hypercholesterolemia in ApoE-Deficient Mice. Arterioscler Thromb Vasc Biol. 2007;27:2392–2399. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 5.Nofer J-R, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EAL. FTY720, a Synthetic Sphingosine 1 Phosphate Analogue, Inhibits Development of Atherosclerosis in Low-Density Lipoprotein Receptor Deficient Mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, Takuwa N, Gonda K, Yamamoto Y, Ohkawa R, Nishiuchi T, Sugimoto N, Yatomi Y, Mitsumori K, Asano M, Kinoshita M, Takuwa Y. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Grabski AD, Shimizu T, Deou J, Mahoney WM, Jr, Reidy MA, Daum G. Sphingosine-1-Phosphate Receptor-2 Regulates Expression of Smooth Muscle Alpha-Actin After Arterial Injury. Arterioscler Thromb Vasc Biol. 2009;29:1644–1650. doi: 10.1161/ATVBAHA.109.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald RA, Pyne S, Pyne NJ, Grant A, Wainwright CL, Wadsworth RM. The sphingosine kinase inhibitor N,N-dimethylsphingosine inhibits neointimal hyperplasia. Br J Pharmacol. 2009;159:543–553. doi: 10.1111/j.1476-5381.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-Phosphate Receptor Subtypes Differentially Regulate Smooth Muscle Cell Phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 11.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacology & Therapeutics. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touchard AG, Schwartz RS. Preclinical Restenosis Models: Challenges and Successes. Toxicologic Pathology. 2006;34:11–18. doi: 10.1080/01926230500499407. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA. Sphingosine 1-Phosphate Receptor 2 Negatively Regulates Neointimal Formation in Mouse Arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 18.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 21.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91:845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 22.Lee M-J, Thangada S, Paik J-H, Sapkota GP, Ancellin N, Chae S-S, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-Mediated Phosphorylation of the G Protein-Coupled Receptor EDG-1 Is Required for Endothelial Cell Chemotaxis. Molecular Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 23.Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Jaccard J, Wan CK. LISREL approaches to interaction effects in multiple regression. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 26.Inoue S, Nakazawa T, Cho A, Davastan F, Shilling D, Daum G, Reidy M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: A role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46:756–763. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler Thromb Vasc Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 28.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse Model of Femoral Artery Denudation Injury Associated With the Rapid Accumulation of Adhesion Molecules on the Luminal Surface and Recruitment of Neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 29.Gil PR, Japtok L, Kleuser B. Sphingosine 1-phosphate mediates chemotaxis of human primary fibroblasts via the S1P-receptor subtypes S1P and S1P and Smad-signalling. Cytoskeleton (Hoboken) 2010;67:773–783. doi: 10.1002/cm.20486. [DOI] [PubMed] [Google Scholar]
- 30.Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol Heart Circ Physiol. 2007;292:H2698–2711. doi: 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith ZG, Dhanasekaran DN. G Protein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 34.Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med. 1997;3:775–779. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 35.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roviezzo F, Bucci M, Delisle C, Brancaleone V, Di Lorenzo A, Mayo IP, Fiorucci S, Fontana A, Gratton JP, Cirino G. Essential requirement for sphingosine kinase activity in eNOS-dependent NO release and vasorelaxation. FASEB J. 2006;20:340–342. doi: 10.1096/fj.05-4647fje. [DOI] [PubMed] [Google Scholar]
- 37.Tolle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schonfelder G, Schafers M, Lipinski KvW, Jankowski J, Jankowski V, Chun J, Zidek W, Giet MVd. Immunomodulator FTY720 Induces eNOS-Dependent Arterial Vasodilatation via the Lysophospholipid Receptor S1P3. Circ Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L-N, Wilson DW, da Cunha V, Sullivan ME, Vergona R, Rutledge JC, Wang Y-X. Endothelial NO Synthase Deficiency Promotes Smooth Muscle Progenitor Cells in Association With Upregulation of Stromal Cell-Derived Factor-1{alpha} in a Mouse Model of Carotid Artery Ligation. Arterioscler Thromb Vasc Biol. 2006;26:765–772. doi: 10.1161/01.ATV.0000207319.28254.8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.