Abstract
Studies on lymph node metastasis of soft tissue sarcomas are insufficient because of its rarity. In this study, we examined the expressions of vascular endothelial growth factor (VEGF)-C and VEGF-D in soft tissue sarcomas metastasized to lymph nodes. In addition, the effects of the two molecules on the barrier function of a lymphatic endothelial cell monolayer against sarcoma cells were analyzed.
We examined 7 patients who had soft tissue sarcomas with lymph node metastases and who had undergone neither chemotherapy nor radiotherapy before lymphadenectomy. Immunohistochemistry revealed that 2 of 7 sarcomas that metastasized to lymph nodes expressed VEGF-C both in primary and metastatic lesions. On the other hand, VEGF-D expression was detected in 4 of 7 primary and 7 of 7 metastatic lesions, respectively. Interestingly, 3 cases that showed no VEGF-D expression at primary sites expressed VEGF-D in metastatic lesions.
Recombinant VEGF-C at 10−8 and VEGF-D at 10−7 and 10−8 g/ml significantly increased the random motility of lymphatic endothelial cells compared with controls. VEGF-D significantly increased the migration of sarcoma cells through lymphatic endothelial monolayers.
The fact that VEGF-D induced the migration of fibrosarcomas through the lymphatic endothelial monolayer is the probable reason for the strong relationship between VEGF-D expression and lymph node metastasis in soft tissue sarcomas. The important propensities of this molecule for the increase of lymph node metastases are not only lymphangiogenesis but also down-regulation of the barrier function of lymphatic endothelial monolayers, which facilitates sarcoma cells entering the lymphatic circulation.
Keywords: lymphatic endothelial cells, lymph node, metastasis, motility, VEGF-C, VEGF-D
Introduction
Vascular and lymphatic systems are crucial for the metastasis of cancer cells. In particular, angiogenesis is well known to be important for tumor formation, progression and metastasis [1]. Soft-tissue sarcomas mainly metastasize via the blood stream to the lung [2]. Clinical trials using bevacizumb, which is a humanized anti-vascular endothelial growth factor (VEGF) antibody that suppresses angiogenesis, have already started for the treatment of soft tissue sarcomas [3, 4].
On the other hand, lymphatic metastasis is a relatively rare event in soft-tissue sarcomas [5], although several sarcomas exhibiting histologic patterns that resemble epithelial or endothelial cancers often metastasize via lymphatic vessels [6–8]. Due to its rarity, studies on lymph node metastasis of soft tissue sarcomas are insufficient, although the prognosis of soft tissue sarcomas with lymph node metastases is quite severe, with 5-year survival rates reported to be less than 30% [7, 9].
During the last decade, there has been an increase of reports on the relationship between lymphangiogenesis promoted by tumor cells and lymphatic metastasis [10–12]. VEGF-C and VEGF-D are known to regulate lymphangiogenesis [13, 14] via their receptors, VEGF receptor (VEGFR)-2 and VEGFR-3 [15–17]. As expected from their propensity to induce lymphangiogenesis, the expressions of VEGF-C and VEGF-D correlated with lymph node metastasis in various kinds of tumors [18–20]. In some tumors, however, VEGF-D expression inversely correlated with lymph node metastasis [10, 18], meaning that the roles of VEGF-D in lymph node metastasis currently remain controversial.
As for lymphangiogenesis-related molecules in soft tissue sarcomas metastasizing to lymph nodes, Lahat et al. reported that there was no correlation between the expression of VEGF-C and peritumoral lymphatic vessel density, which has been considered to correlate with lymphatic spread of the tumors in various human malignant neoplasms [1–3, 15–17, 21]. They did not, however, analyze VEGF-D expression and the molecules expressed in metastatic lymph node lesions.
In this study, we examined the expressions of VEGF-C and VEGF-D in soft tissue sarcomas metastasized to lymph nodes. In addition, the effects of the two molecules on the monolayer of lymphatic endothelial cells in vitro were analyzed.
Materials and Methods
Patients
Patients with a musculoskeletal malignant tumor treated in our hospital between 2000 and 2010 were analyzed. Among them, seven patients with soft tissue sarcomas had undergone surgery for both primary lesions and lymph node metastases. Histopathological findings confirmed that partial lymph node structures, such as marginal sinus, remained in 5 of 7 cases although most of the lymph nodes were invaded by tumors. In the rest of the cases, lymph node structures were completely replaced by tumor cells. None of the seven patients had undergone chemotherapy or radiotherapy before lymphadenectomy. Specimens of primary tumors and lymph node metastases were taken from the 3 male and 4 female patients (mean age 65.3 years). The sites of the primary tumors were 2 thighs, 1 lower leg, 1 knee, 1 buttock, 1 elbow, and 1 back. As a control, specimens from 6 patients (1 male and 5 females) with tumors metastasizing to non-lymph node sites, which were diagnosed radiologically, were examined (mean age 66.3) and the primary tumors originated from 5 thighs and 1 buttock.
Cell line and reagents
Human lymphatic endothelial cells (HLEC) were obtained from ScienCell Research Laboratories (Carlsbad, CA). Cells were cultured in endothelial cell medium (ScienCell Research Laboratories) with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Human fibrosarcoma cell line HT-1080 was obtained from the American Type Culture Collection (ATCC) and human osteosarcoma cell line HuO9 was kindly provided by Dr. Hotta (Niigata University, Niigata, Japan). These two tumor cell lines were cultured in DMEM with 10% fetal bovine serum.
Goat polyclonal antibody to VEGF-C (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal antibody to VEGF-D (Santa Cruz Biotechnology) were used for immunohistochemistry and immunoblot to stain VEGF-C and VEGF-D, respectively. Mouse monoclonal antibody to VEGFR-2 (R&D Systems, Minneapolis, MN) and VEGFR-3 (R&D Systems) were used for immunofluorescence.
Recombinant proteins of humanVEGF-A121, B, C and D, platelet-derived growth factor (PDGF)-AA, fibroblast growth factor (FGF)-basic, and angiopoietin (ANG)-1 were obtained from PeproTech Inc. (Rocky Hill, NJ) and rabbit phosphoglucose isomerase (PGI) from Sigma (St Louis, MO).
Immunohistochemistry
The avidin-biotin-peroxidase complex (ABC) method was performed using the Vectastatin ABC Kit (Vector Laboratories, Burlingame, CA). Briefly, from a formalin-fixed, paraffin embedded-tumor tissue block, serial section slides of 4 µm were prepared, dried at 60°C for at least 60 min and incubated in a 0.3% hydrogen peroxide-methanol solution for 30 min to remove endogenous peroxidase. Next, the stained sections were incubated with pepsin for 10 min at room temperature and with the primary antibodies at a dilution of 1:50 overnight at 4°C. Subsequently, specimens were incubated with the corresponding biotinylated secondary antibodies. Freshly prepared ABC reagent was applied and incubated for 60 min. Finally, specimens were incubated with 0.02% 3,3-diaminobenzidine tetrahydrochloride solution containing 0.03% hydrogen peroxidase. Between each step, the sections were washed in PBS for 5 min, three times and then counterstained in Mayer's hematoxylin. Sections were scored as positive if ≥10% of the neoplastic cells stained positively.
Immunoblotting
HT-1080 and HuO9 cells expanded on 10-cm dishes were lysed in radioimmunoprecipitation assay buffer (20 mmol/L Tris, 150 mmol/L NaCl, 1% Triton X-100, 1% NP40, 10 mmol/L EDTA, and 25 mmol/L sodium deoxycholate) and centrifuged at 15,000 × g for 5 min. Supernatants were subjected to SDS-PAGE and transferred to a PVDF membrane, which was probed with anti-VEGF-C or anti-VEGF-D polyclonal antibody, and immunoreactive protein was detected.
Immunofluorescence
Cells were seeded on coverslips coated with bovine albumin, fixed with 4% formaldehyde and permeabilized with ice-cold methanol for 5 min at −20°C. The cells were blocked with 3.0% bovine serum albumin (BSA)/PBS for 30 min, and then labeled with primary antibodies, followed by incubation with Alexa Fluor 488 secondary antibody in the dark. To detect nuclei, the cells were co-stained with 4′,6′-diamidino-2-phenylindole. Fluorescent images were analyzed using a KEYENCE BZ-8100 fluorescence microscope (KEYENCE, Osaka, Japan).
Phagokinetic Track and Transwell Motility Assay
For the random motility assay, uniform carpets of gold colloids were prepared on glass coverslips coated with bovine serum albumin as previously described. The coverslips were placed in 35-mm tissue culture dishes, and 2000 HLEC were plated onto coverslips coated with gold colloids. After 24-h incubation with 10−11 to 10−7 g/ml cytokines, the area of migrated cells was calculated. The phagokinetic tracks of at least 30 individual cells were measured.
The migration assay for HLEC was performed using Transwell supports with 8.0-µm pore polycarbonate membrane inserts (Corning Life Sciences, Acton, MA). A 100 µl sample of medium with 1% fetal bovine serum (FBS) including cell suspension (1 × 105 cells/ml) was added to the upper compartment, and 600 µl medium with FBS and 10−10 to 10−7 g/ml recombinant proteins was added to the lower compartment. After 24-h incubation at 37 °C, the membranes were fixed with 70% ethanol for 1 h, stained with 0.4% trypan blue, and washed with distilled water. Cells that migrated through the membrane were counted after the cells on the upper surface of the membrane had been swiped with cotton swabs.
Transendothelial Migration Assay
The transendothelial migration assay for sarcoma cells was performed using Transwell seeded HLEC. First, 5×104 HLEC in culture medium were added to each insert in a 24-well plate and cultured for 48h until cells formed a monolayer. Next, 100 µl medium with 1% fetal bovine serum (FBS), including sarcoma cells (1 × 105 cells/ml) treated with CellTracker Green Fluorescent Probe (Lonza, Walkersville, MD) according to the instructions, was added to the upper compartment, and 600 µl medium with FBS and 10−8 and 10−7 g/ml recombinant proteins was added to the lower compartment. After 24-h incubation, the membranes were fixed with 70% ethanol for 1 h. Cells that migrated through the endothelial monolayer and the membrane were counted under a KEYENCE BZ-8100 fluorescence microscope.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL). Phagokinetic track motility, Transwell motility and transendothelial migration assays were analyzed using analysis of variance (ANOVA) and the significance of individual differences was evaluated using the Dunnett C or Tukey test if ANOVA was significant.
Results
Pathological diagnoses
Pathological diagnoses of the tumors metastasized to lymph nodes were 3 undifferentiated pleomorphic sarcomas, 1 clear cell sarcoma, 1 myxoid liposarcoma, 1 myxoid-type extraskeletal myxoid chondrosarcoma and 1 epithelial sarcoma (Table 1) and those metastasized to non-lymph node sites (4 to lungs, 1 to spine and 1 to subcutaneous) were 2 undifferentiated pleomorphic sarcomas, 1 myxofibrosarcoma 1 myxoid liposarcoma and 1 extraskeletal myxoid chondrosarcoma (Table 2).
Table 1.
Patient with lymph node metastasis
| Patien t No. |
age |
sex |
pathological diagnosis |
site |
primary | LN metastasis | ||
|---|---|---|---|---|---|---|---|---|
| VEGF-C | VEGF-D | VEGFC | VEGFD | |||||
| 1 | 52 | F | clear cell sarcoma | elbow | − | − | − | + |
| 2 | 85 | F | extraskeletal myxoid | buttock | − | − | − | + |
| 3 | 68 | M | myxoid liposarcoma | back | + | + | + | + |
| 4 | 62 | F | undifferentiated pleomorphic | knee | − | − | − | + |
| 5 | 66 | M | epithelial sarcoma | thigh | + | + | + | + |
| 6 | 64 | M | undifferentiated pleomorphic | lower leg | − | + | − | + |
| 7 | 60 | M | undifferentiated pleomorphic | thigh | − | + | − | + |
Table 2.
Patient with metastasis to non-lymph-node sites
| Patient No. |
age |
sex |
pathological diagnosis |
site |
primary |
site of metastasis |
|
|---|---|---|---|---|---|---|---|
| VEGF-C | VEGF-D | ||||||
| 8 | 81 | F | undifferentiated pleomorphic sarcoma | thigh | − | − | lung |
| 9 | 82 | F | leiomyosarcoma | thigh | − | − | lung |
| 10 | 63 | F | myxofibrosarcoma | thigh | − | − | lung |
| 11 | 72 | M | extraskeletal myxoid | thigh | + | + | lung |
| 12 | 51 | F | myxoid liposarcom | thigh | − | − | spine |
| 13 | 49 | F | undifferentiated pleomorphic sarcoma | buttock | − | − | subcutaneous |
Expression of VEGF-C and VEGF-D
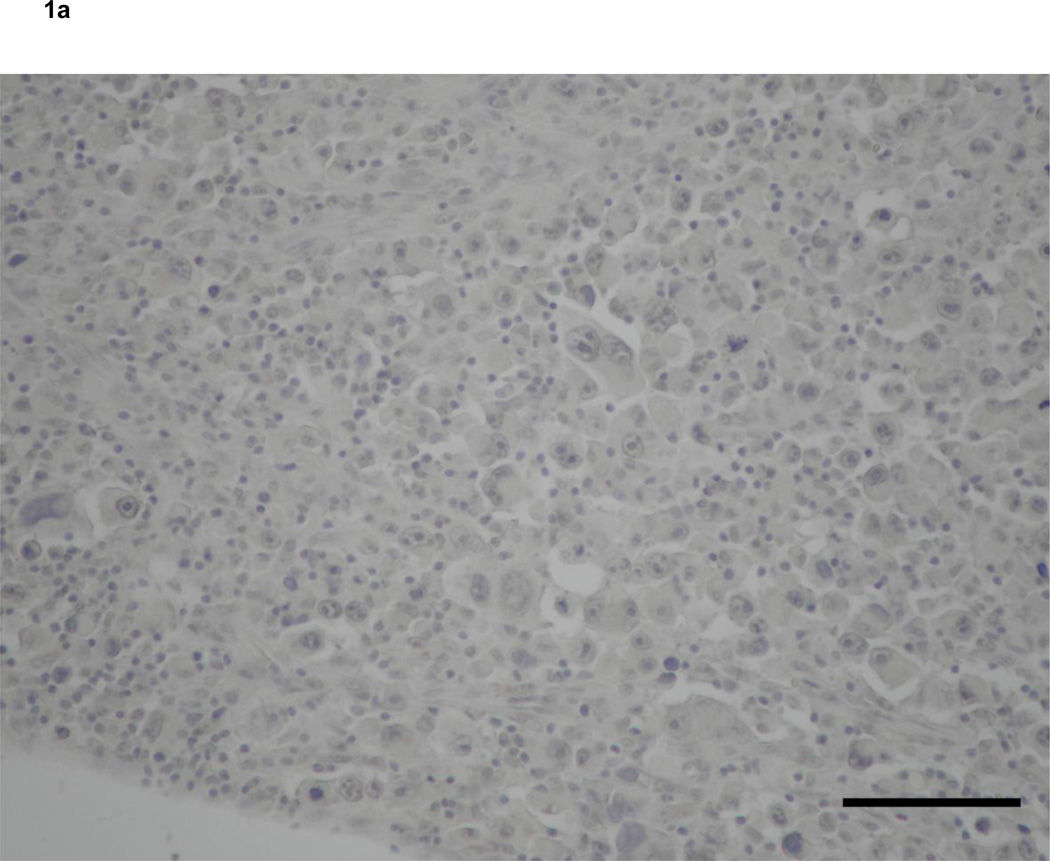
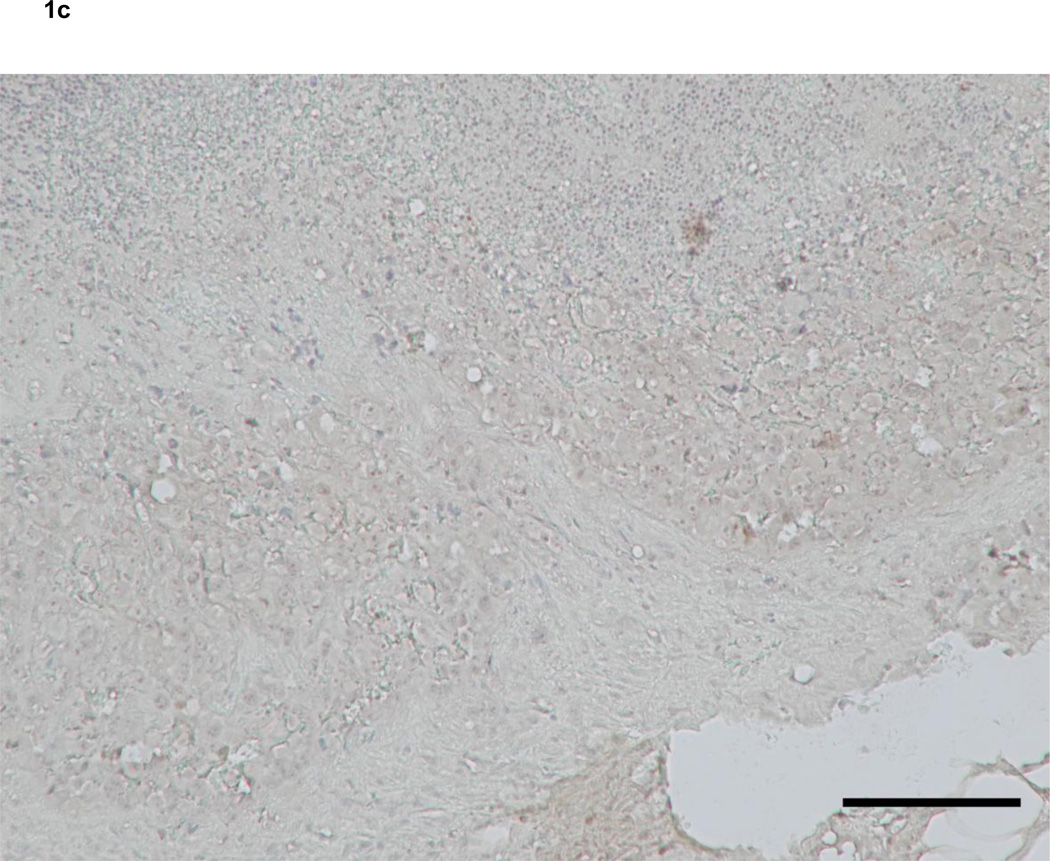
Immunohistochemistry revealed that 2 of 7 soft tissue sarcomas that metastasized to lymph nodes expressed VEGF-C both in primary and metastatic lesions (Fig. 1a, c). On the other hand, VEGF-D expression was detected in 4 of 7 primary and 7 of 7 metastatic lesions of soft tissue sarcomas that metastasized to lymph nodes, respectively (Table 2). VEGF-D was located in the cytoplasm of tumor cells and hardly at all in lymphocytes (Fig. 1b, d, f). Interestingly, three cases that did not express VEGF-D at primary sites showed VEGF-D expression in metastatic lesions (Fig. 1e, f). In addition, four cases in which primary lesions were VEGF-D-positive more strongly expressed this protein at metastatic than primary sites (Fig. 1b, d). In control cases, which were soft tissue sarcomas metastasized to non-lymph node sites, only 1 of 6 primary tumors expressed both VEGF-C and -D.
Figure 1. Expression of vascular endothelial growth factors in soft tissue sarcomas.
Primary (a, b) and metastatic (c, d) lesions from a 60-year-old man with MFH. Neither the primary nor metastatic tumor expressed VEGF-C (a, c). The metastatic lesion (d) expressed VEGF-D more strongly than the primary lesion (b).
Primary (e) and metastatic (f) lesions from an 85-year-old woman with myxoid-type extraskeletal chondrosarcoma. The metastatic lesion (f) expressed VEGF-D, although the primary lesion (e) did not.
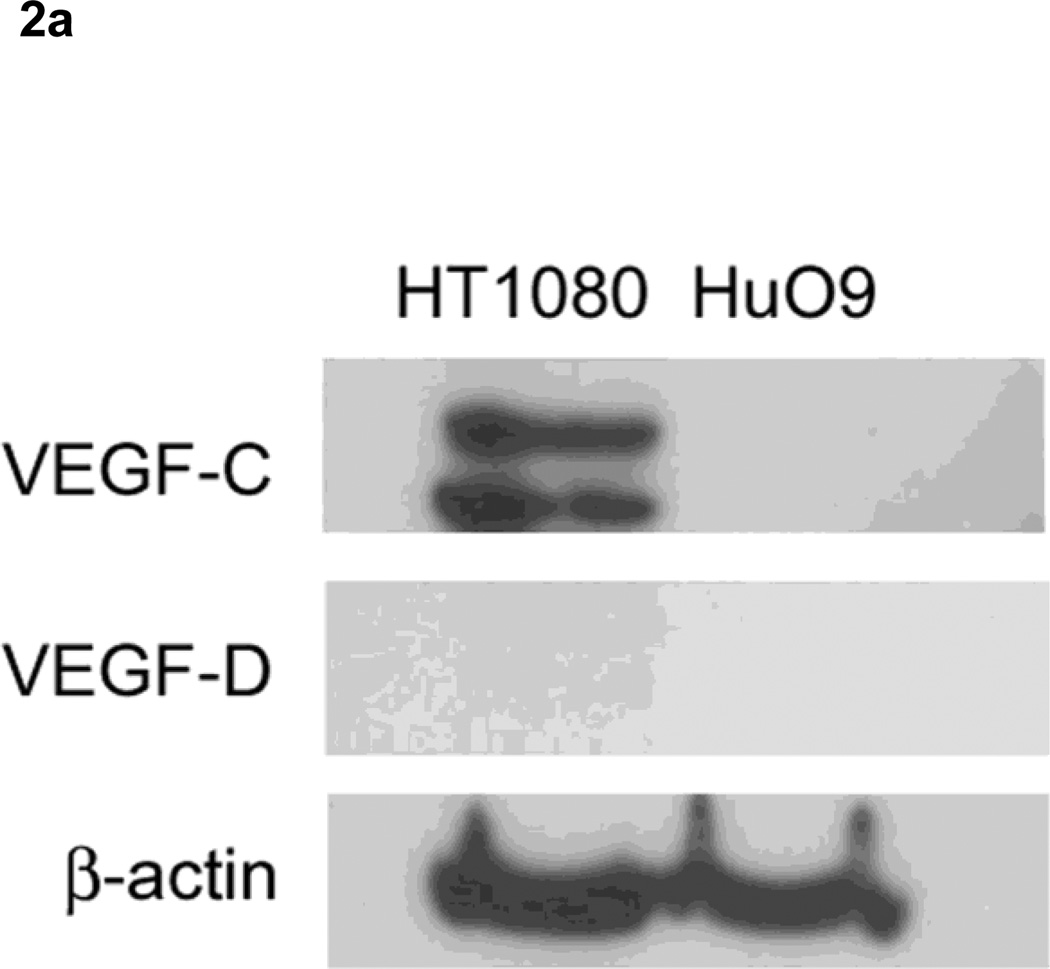
In immunoblotting, anti-VEGF-C antibody recognized two bands in the lysate of HT1080 cells, but not of HuO9, which is compatible with a report by Joukov et al. that the main form of both endogenous VEGF-C produced by HT1080 cells was a doublet of 29/31 kDa [22], Meanwhile, neither cell line expressed VEGF-D (Fig. 2a). Immunofluorescence results showed that both HT1080 cells (Fig. 2b, c) and HuO9 cells (Fig. 2d, e) expressed VEGFR-3 (Fig. 2b, d) and slight VEGFR-2 (Fig. 2c, e).
Figure 2. Expression of vascular endothelial growth factor receptors in soft tissue sarcoma cell lines.
Immunoblotting revealed that HT1080 cells, but not HuO9, expressed VEGF-C (29/31kDa), while neither cell line expressed VEGF-D.
Immunofluorescence revealed that both HT1080 cells (b, c) and HuO9 cells (d, e) expressed VEGFR-3 (b, d) and slight VEGFR-2 (c, e). Bars on photographs indicate 100µm.
Effect of angiogenic and lymphangiogenic molecules on the motility of HLEC
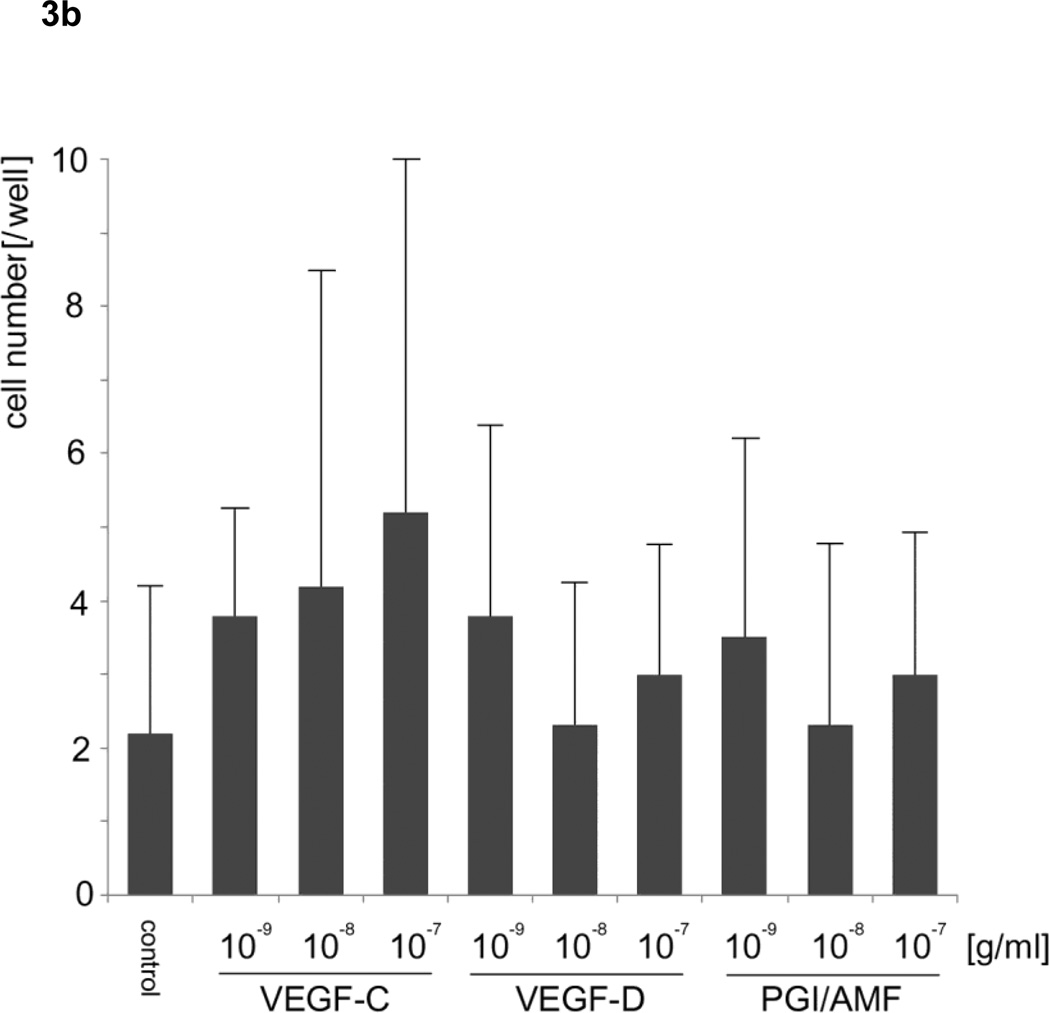
To examine whether VEGF-C and -D can stimulate the motility of HLEC, we utilized two independent motility assays: 1) phagokinetic track assay for measuring overall random motility (Fig. 3a) and 2) migration assay utilizing Transwell for measuring directed motility capabilities (Fig. 3b). Recombinant VEGF-C at 10−8 and VEGF-D at 10−7 and 10−8 g/ml significantly increased the random motility of HLEC compared with controls in the phagokinetic track motility assay (analysis of variance; F15, 784 = 8.415, p <0.05). In the migration assay using Transwell, neither VEGF-C nor -D in the lower chamber significantly stimulated the directed motility of HLEC (analysis of variance; F9, 90 = 1.446, p = 0.30). These motility assays indicated that VEGF-C and -D stimulated random cell motility (chemokinesis) of HLEC rather than directed motility (chemotaxis). In these experiments, we used PGI because this molecule is known to be identical to autocrine motility factor (AMF), which stimulates the motility of tumor cells and human umbilical vein endothelial cells [23]. Against our expectations, PGI/AMF did not stimulate the random or directed motility of HLEC. In addition, VEGF-A and B, FGF-basic, PDGF-AA, and ANG-1 did not increase random motility (analysis of variance; F15, 144 = 0.469, p = 0.953) (Fig. 3c).
Figure 3. Effects of vascular endothelial growth factors on lymphatic endothelial cells.
a. Phagokinetic track assay for measuring random motility revealed VEGF-C at 10−8 and VEGF-D at 10−8 and 10−7 g/ml up-regulated the motility of HLEC.
b. Transwell assay revealed that VEGF-C and VEGF-D did not affect the directed motility of HLEC. Error bars indicate standard deviation.
c. VEGF-A and B, FGF-basic, PDGF-AA and ANG-1 did not affect random motility of HLEC compared with controls in the phagokinetic track motility assay.
Effect of VEGF-C and VEGF-D on the monolayer of HLEC
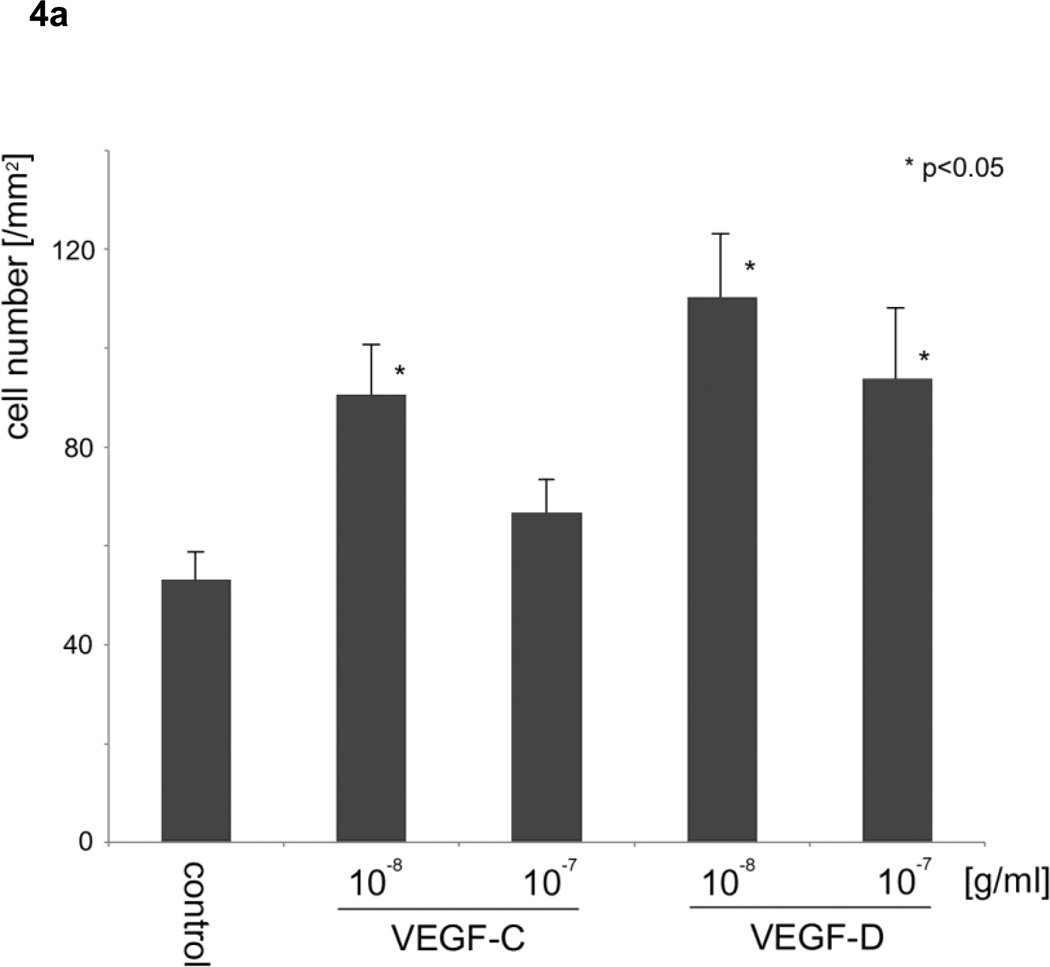
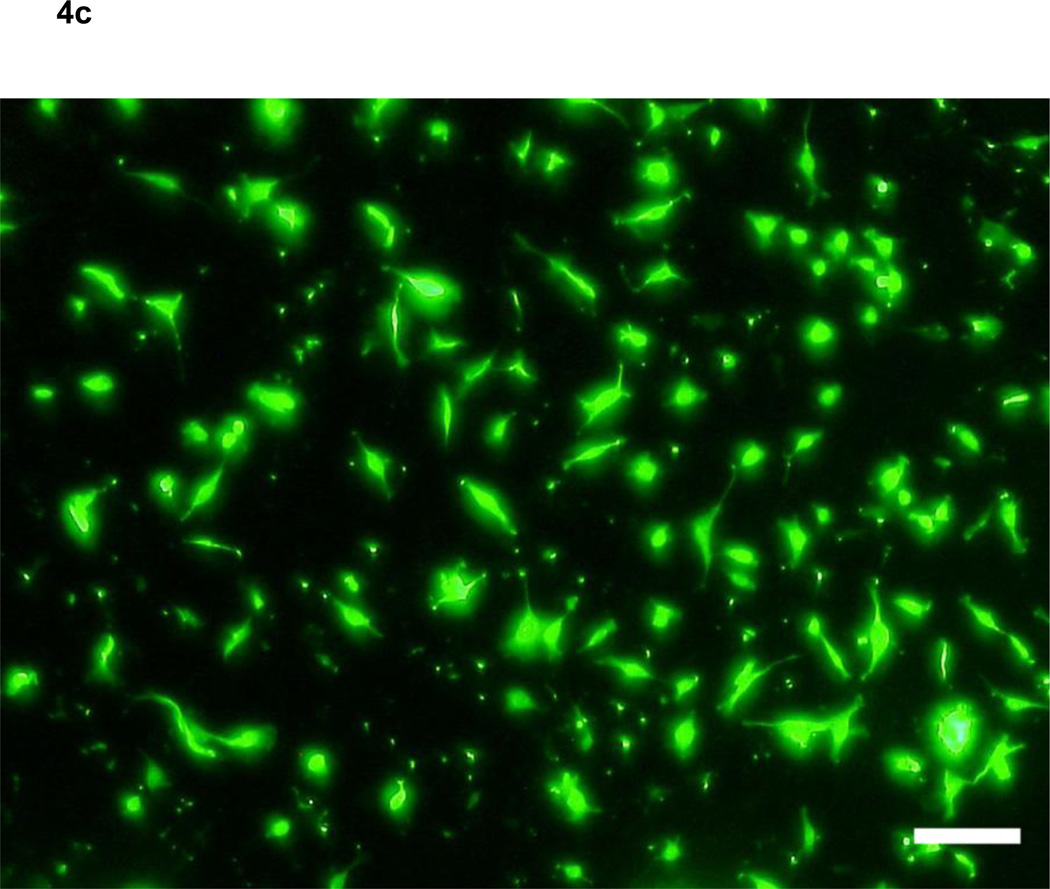
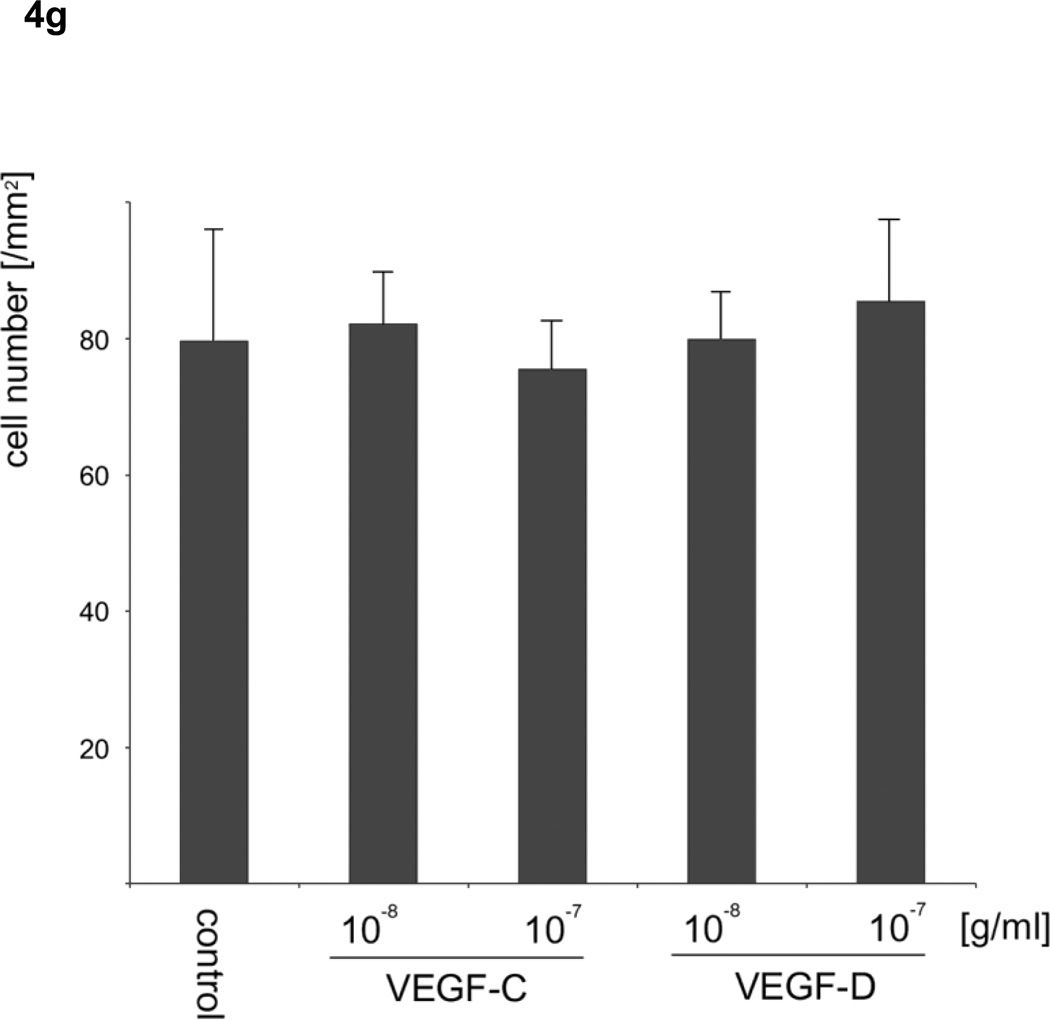
Based on the results of the motility assays of HLEC, we hypothesized that VEGF-C and -D affect the stability of the endothelium of lymph vessels by up-regulating the random motility of endothelial cells, and facilitate tumor cell migration into lymphatic systems. To verify the effect of VEGF-C and -D on the endothelium of lymph vessels for sarcoma cells, the numbers of sarcoma cells that migrated through an endothelial monolayer in medium including VEGFs were analyzed. VEGF-C at 10−8 and VEGF-D at 10−7 and 10−8 g/ml significantly increased the transendothelial migration of HT1080 cells (analysis of variance; F4, 20 =18.438, p <0.05) (Fig. 4a–d). Meanwhile, VEGF-D at 10−7 g/ml, but not VEGF-C, significantly increased the transendothelial migration of HuO9 cells (analysis of variance; F4, 20 =9.041, p <0.01) (Fig. 4e). A migration assay for sarcoma cells using Transwell without the endothelial monolayer was also performed, because there was a possibility that VEGF-C and -D stimulated not only the random motility of HLEC but also the directed motility of HT1080 and HuO9 cells, in which the expression of VEGFR3 was confirmed. Neither VEGF-C nor -D, however, increased the number of HT1080 cells (Fig. 4f) and HuO9 cells (Fig. 4g) migrated through the pores of the Transwell® membrane compared with the control (analysis of variance in HT1080 cells: F4, 20 = 0.771, p = 0.557, analysis of variance in HuO9 cells: F4, 20 = 0.500, p = 0.736).
Figure 4. Effects of vascular endothelial growth factors on the monolayer of lymphatic endothelial cells and sarcoma cells.
VEGF-D at 10−8, 10−7 g/ml and VEGF-C at 10−7 g/ml significantly augmented the transendothelial migration of HT1080 cells through a lymphatic endothelial monolayer compared with the control (a). Representative images of control (b), VEGF-C (c) and VEGF-D (d) at 10−8 g/ml are shown. VEGF-D at 10−7 g/ml, but not VEGF-C, significantly increased the transendothelial migration of HuO9 cells (e). Neither VEGF-C nor -D increased the number of HT1080 cells (f) and HuO9 cells (g) migrating through the pores of the Transwell® membrane compared with the control. (N=5) Error bars indicate standard deviation. Bars on photographs indicate 100µm.
Discussion
Immunohistochemistry in the current study revealed that all seven metastatic lesions of soft tissue sarcomas that metastasized to lymph nodes showed VEGF-D expression. It is noteworthy that three VEGF-D-negative cases at primary sites showed VEGF-D expression in metastatic lesions and the rest of the cases presented stronger VEGF-D expression in the metastatic lymph node lesion than in the primary lesion. This suggests that the metastatic lymph node lesions were probably composed of populations that acquired the propensity to strongly express VEGF-D in primary lesions and moved to lymph nodes. In contrast to sarcomas metastasizing to lymph nodes, soft tissue sarcomas that metastasized to non-lymph node sites showed VEGF-D expression in only one of seven cases. VEGF-C, the expression of which was positive in only two of seven cases, did not seem to be an essential factor for lymph node metastasis of soft tissue sarcomas. Lahat et al. have also described that no difference in VEGF-C expression was observed between metastatic lymph node-negative and -positive soft tissue sarcomas [21].
It is well known that VEGF-C and -D up-regulate the growth of lymphatic endothelial cells via VEGFR-2 and -3 and enhance lymphatic metastasis [13, 14]; however, tumor cells need to accomplish several steps for lymphatic metastasis, not only lymphangiogenesis but also other factors, such as invasion to the lymphatic system, survival in the circulation, and extravasation [24]. Vascular permeability is one of the important factors that make the vascular endothelium susceptible to tumor invasion into vascular systems [25, 26]. Several molecules involved in cell motility, such as PGI/AMF and RhoA, were reported to increase the permeability of vascular endothelium [23, 27], and therefore we investigated the effect of angiogenic and lymphangiogenic factors and PGI/AMF on the motility of lymphatic endothelial cells. PGI/AMF is a molecule secreted from tumor cells, including fibrosarcoma and osteosarcoma cell lines, and stimulates the motility of tumor cells in an autocrine or paracrine fashion [28, 29]. We expected that this molecule also affects lymphatic endothelial cell motility and affects the stability of endothelium; however, the current study revealed that only VEGF-C and -D, but not PGI/AMF, up-regulated the random motility of lymphatic endothelial cells. In addition, there was a report that aortic endothelial cells transfected with VEGFR-2 showed enhanced migration compared to control cells [30], which prompted us to perform a Transwell directed motility assay of lymphatic endothelial cells using VEGFs. None of VEGF-C, -D and PGI/AMF, however, affected directed motility, suggesting that these molecules were not chemoattractants for lymphatic endothelial cells.
After confirming that VEGF-C and -D, not PGI/AMF, are key factors affecting the random motility of lymphatic endothelial cells, we performed a transendothelial migration assay using VEGFs. The numbers of HT-1080 and HuO9 cells that migrated through the monolayer of lymphatic endothelial cells were increased by both VEGF-C and -D and by only VEGF-D, respectively. In particular, VEGF-D increased the number of sarcoma cells migrating through lymphatic endothelial monolayers with a lower concentration than VEGF-C. This result means that VEGF-D facilitates sarcoma cell entry into lymphatic systems by affecting the endothelium. He et al. also described that blocking the signals of VEGFR-3, a receptor of VEGF-C and -D, can inhibit the entry of lung cancer cells into lymphatic vessels [31]. Meanwhile, other authors have reported that VEGF-C but not VEGF-D induced vascular permeability [16, 32, 33]. This discrepancy can probably be explained by the difference in characteristics between vascular and lymphatic endothelial cells. Interestingly, VEGF-C and -D did not stimulate the migration of HT1080 and HuO9 cells through the membranes without HLEC, although the two cell lines expressed VEGFR-3. The signal pathways of VEGF-D-VEGFR3 are probably dependent on cell types.
We speculated that the fact VEGF-D induced the migration of HT1080 fibrosarcomas through the lymphatic endothelial monolayer at a lower concentration than VEGF-C can explain the strong relationship between VEGF-D expression and lymph node metastasis in soft tissue sarcomas. Soft tissue sarcomas rarely metastasize to lymph nodes compared with carcinomas [5]. Resolving why soft tissue sarcomas hardly metastasize to lymph nodes and to identify molecules similar to VEGF-D that induce soft tissue sarcomas to metastasize to lymph nodes will contribute to the regulation of lymph node metastasis of carcinomas.
There are some limitations of this study. First, the number of cases is relatively small, which prevented us from performing exact histological matching between the lymph node and non-lymph node metastasis groups, due to the rarity of lymph node metastasis in soft tissue sarcomas [5]. In addition, to avoid any modification of the specimens we omitted patients who had received radiation or chemotherapy in this study. However, patients with lymph node metastasis usually undergo radiation therapy or systemic chemotherapy before lymphadenectomy [34], which makes it difficult to collect unmodified specimens of lymph nodes. Greater accumulation of cases or a multi-institutional study may be needed, although our institute treats 40–50 patients with sarcoma every year. Second, no human sarcoma cell lines steadily metastasize to lymph nodes. It would be interesting to establish human sarcoma cell lines that metastasize to lymph nodes, for example, by transfection with genes including vegfs.
Conclusions
We showed that VEGF-D expression was strongly related to lymph node metastasis of soft tissue sarcomas. The important propensity of this molecule to increase lymph node metastases is not only lymphangiogenesis but also down-regulation of the barrier function of lymphatic endothelial monolayers, which facilitates sarcoma cell entry into the lymphatic circulation. Investigating VEGF-D will help to elucidate the mechanisms of lymph node metastasis.
Acknowledgements
We thank Ms Hiroe Miyahata from Gunma University Graduate School of Medicine for her technical assistance with IHC analyses.
This paper was supported in part by NIH grant CA-51714 (AR).
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- HLEC
human lymphatic endothelial cells
- PDGF
platelet-derived growth factor
- FGF
fibroblast growth factor-basic
- ANG-1
angiopoietin-1
- PGI
phosphoglucose isomerase
- ABC
avidin-biotin-peroxidase complex
- FBS
fetal bovine serum
- ANOVA
analysis of variance
- MFH
malignant fibrous histiocytoma
- AMF
autocrine motility factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest and no financial conflicts.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg RD, Martin RG, Romsdahl MM, Barkley HT., Jr Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.D'Adamo DR, Anderson SE, Albritton K, Yamada J, Riedel E, Scheu K, Schwartz GK, Chen H, Maki RG. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J. Clin. Oncol. 2005;23:7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 4.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, Nelson MD, Krailo MD, Ingle AM, Blaney SM, Kandel JJ, Yamashiro DJ. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J. Clin. Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 5.Behranwala KA, A'Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Ann. Surg. Oncol. 2004;11:714–719. doi: 10.1245/ASO.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann. Surg. 1993;217:72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruka W, Emrich LJ, Driscoll DL, Karakousis CP. Prognostic significance of lymph node metastasis and bone, high-grade soft tissue sarcomas major vessel, or nerve involvement in adults with high-grade soft tissue sarcomas. Cancer. 1988;62:999–1006. doi: 10.1002/1097-0142(19880901)62:5<999::aid-cncr2820620527>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97:2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 9.Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann. Surg. 1975;182:597–602. doi: 10.1097/00000658-197511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. Faseb J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 11.Achen MG, Mann GB, Stacker SA. Targeting lymphangiogenesis to prevent tumour metastasis. Br. J. Cancer. 2006;94:1355–1360. doi: 10.1038/sj.bjc.6603120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim. Biophys. Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 14.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 15.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc. Natl. Acad. Sci. U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 17.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat. Rev. Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 19.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32–39. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin. Cancer Res. 2003;9:716–721. [PubMed] [Google Scholar]
- 21.Lahat G, Lazar A, Wang X, Wang WL, Zhu QS, Hunt KK, Pollock RE, Lev D. Increased vascular endothelial growth factor-C expression is insufficient to induce lymphatic metastasis in human soft-tissue sarcomas. Clin. Cancer Res. 2009;15:2637–2646. doi: 10.1158/1078-0432.CCR-08-2442. [DOI] [PubMed] [Google Scholar]
- 22.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. Embo J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funasaka T, Haga A, Raz A, Nagase H. Tumor autocrine motility factor induces hyperpermeability of endothelial and mesothelial cells leading to accumulation of ascites fluid. Biochem. Biophys. Res. Commun. 2002;293:192–200. doi: 10.1016/S0006-291X(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 24.Gershenwald JE, Fidler IJ. Cancer. Targeting lymphatic metastasis. Science. 2002;296:1811–1812. doi: 10.1126/science.10731318. [DOI] [PubMed] [Google Scholar]
- 25.Prager GW, Lackner EM, Krauth MT, Unseld M, Poettler M, Laffer S, Cerny-Reiterer S, Lamm W, Kornek GV, Binder BR, Zielinski CC, Valent P. Targeting of VEGF-dependent transendothelial migration of cancer cells by bevacizumab. Mol. Oncol. 2010;4:150–160. doi: 10.1016/j.molonc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahni A, Arevalo MT, Sahni SK, Simpson-Haidaris PJ. The VE-cadherin binding domain of fibrinogen induces endothelial barrier permeability and enhances transendothelial migration of malignant breast epithelial cells. Int. J. Cancer. 2009;125:577–584. doi: 10.1002/ijc.24340. [DOI] [PubMed] [Google Scholar]
- 27.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D'Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe H, Kanbe K, Chigira M. Differential purification of autocrine motility factor derived from a murine protein-free fibrosarcoma. Clin. Exp. Metastasis. 1994;12:155–163. doi: 10.1007/BF01753982. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa T, Funasaka T, Tsutsumi S, Raz T, Tanaka N, Raz A. Differential regulation of phosphoglucose isomerase/autocrine motility factor activities by protein kinase CK2 phosphorylation. J. Biol. Chem. 2005;280:10419–10426. doi: 10.1074/jbc.M409457200. [DOI] [PubMed] [Google Scholar]
- 30.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 31.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 32.Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J. Biol. Chem. 1998;273:6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- 33.Stacker SA, Vitali A, Caesar C, Domagala T, Groenen LC, Nice E, Achen MG, Wilks AF. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J. Biol. Chem. 1999;274:34884–34892. doi: 10.1074/jbc.274.49.34884. [DOI] [PubMed] [Google Scholar]
- 34.Al-Refaie WB, Andtbacka RH, Ensor J, Pisters PW, Ellis TL, Shrout A, Hunt KK, Cormier JN, Pollock RE, Feig BW. Lymphadenectomy for isolated lymph node metastasis from extremity soft-tissue sarcomas. Cancer. 2008;112:1821–1826. doi: 10.1002/cncr.23363. [DOI] [PubMed] [Google Scholar]