Abstract
Long-distance migration is a strategy some animals use to survive a seasonally changing environment. To reach favorable grounds, migratory animals have evolved sophisticated navigational mechanisms that rely on a map and compasses. In migratory insects, the existence of a map sense (sense of position) remains poorly understood, but recent work has provided new insights into the mechanisms some compasses use for maintaining a constant bearing during long-distance navigation. The best-studied directional strategy relies on a time-compensated sun compass, used by diurnal insects, for which neural circuits have begun to be delineated. Yet, a growing body of evidence suggests that migratory insects may also rely on other compasses that use night sky cues or the Earth's magnetic field. Those mechanisms are ripe for exploration.
Introduction
To match their ecological needs, insects from diverse taxa, such as butterflies, moths, locusts, and dragonflies, undertake impressive seasonal mass migrations traveling up to thousands of kilometers to their ultimate destination [1-4] (Figure 1a). The yearly migration south of the eastern North American monarch butterfly (Danaus plexippus) to central Mexico is the most striking long-distance migration documented in insects [3, reviewed in 5 and 6].
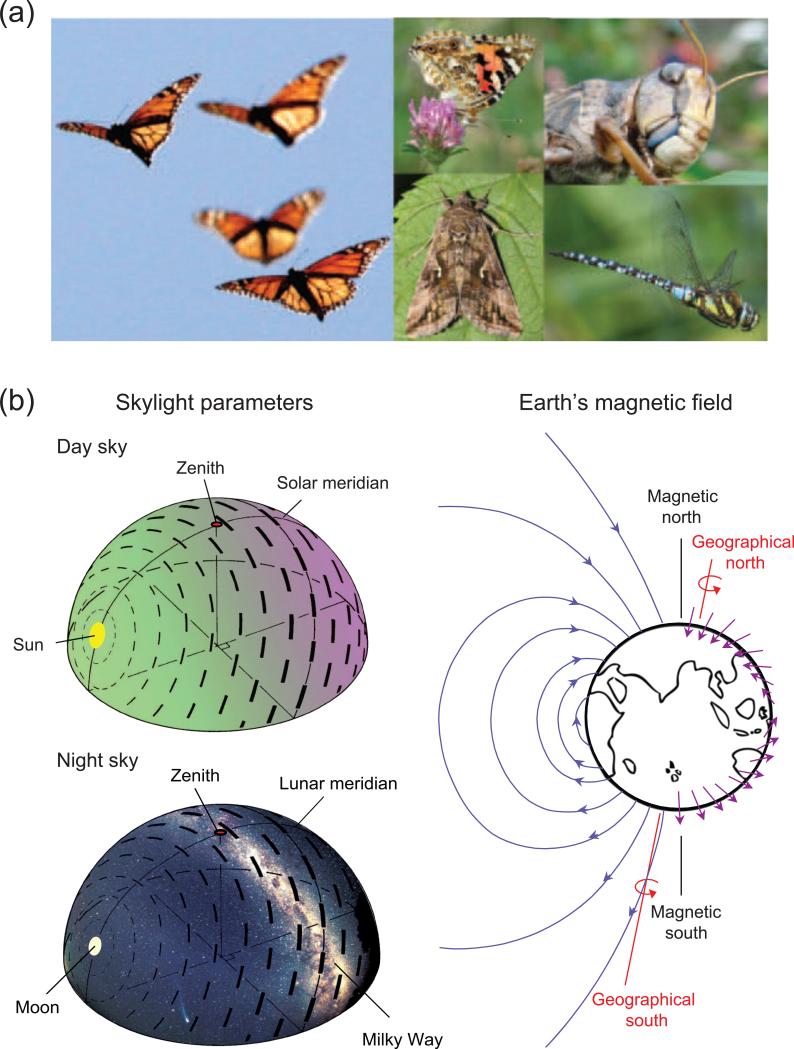
Figure 1. Sensory cues used for long-distance orientation by migratory insects.
(a) Several insect species undergo seasonal long-distance migration, including (from left to right) the monarch butterfly, other day-active butterflies, nocturnal moths, the desert locust, and dragonflies. Monarch picture: courtesy of Monarch Watch (www.monarchwatch.org). (b) Migratory insects exploit environmental cues for long-range navigation. (Top left) Migrants flying during the day can extract directional information from the sun position in the sky (yellow oval), as well as the derived patterns of polarized skylight (E-vector; dashed lines) and the spectral gradient, which ranges from longer wavelengths of light (green) dominating in the solar hemisphere to shorter wavelengths (violet) dominating in the antisolar hemisphere. The bar thickness of dashed lines represents the degree of polarization, while the bar orientation represents the angle of polarization relative to solar position as viewed from the center of the celestial sphere. Modified from [20••]. (Bottom left) The moon and its polarization pattern, stars and the Milky Way are celestial cues available to migratory insects flying at night. (Right) The Earth's magnetic field emerges from the southern hemisphere, wraps around the globe and re-enters at the northern hemisphere (blue lines). Migrating animals can extract directional information (compass sense) and positional information (map sense) from the polarity, inclination and intensity of the Earth's magnetic field, which are gradually changing across the Earth's surface (represented by the angle and length of the violet arrows). Modified from [41].
To successfully reach their goal, migratory animals likely use a map for a sense of position and compasses for directional information [7]. Migratory insects do seem to have some map sense as they ‘know’ what direction they need to travel to reach their goal. However, it is unknown whether they have a complete map sense; that is, know where they are at any point along the migration route relative to their destination. Having a complete map sense would be important for course correction, if, for example, a migrant is displaced from its normal heading by strong winds. Once a displaced migrant has a sense of its position, it can then use directional information provided by compasses to course correct.
Most is known about the compasses and the environmental cues migrants exploit to maintain a directional bearing. A well-studied compass used by insects is a time-compensated sun compass, which uses sensory cues from the daylight sky. Other directional systems that could be used in insects include compasses that may use night sky cues (e.g., moon and stars) and a magnetic compass, which uses aspects of the Earth's magnetic field (Figure 1b). The relative importance of these compasses likely depends on the environment and lifestyle. Here, we focus on recent findings concerning the mechanisms underlying the different strategies for long-distance navigation in migratory insects, with a focus on the time-compensated sun compass.
The insect sun compass
Many day-active migratory insects use a time-compensated sun compass. Skylight features containing information about the solar azimuth (the horizontal position of the sun) are detected, adjusted for time of day, and used to generate motor commands that ensure a constant directional bearing [6]. In addition to the sun, the solar azimuth can also be inferred from scattered sunlight, e.g. the skylight polarization pattern and the skylight spectral gradient (Figure 1b; top left panel).
Time-compensated sun compass navigation has been demonstrated behaviorally in the migratory monarch butterfly and other butterfly species [8-12]. Monarchs use the sun as the primary orientation cue [13], but also have the capacity to utilize the skylight polarization pattern [14,15]. Behavioral responses to polarized light have also been shown in the migratory desert locust (Schistocerca gregaria) [16]. Both species detect polarized light with a specialized dorsal rim area of the compound eye and process this information in an elaborate neuronal network in the brain [17-21]. The anatomical organization and the physiological responses of the involved neurons are surprisingly similar between species (Figure 2). The degree of conservation is remarkable given the large evolutionary distance between the two [20••]. Polarization sensitive (POL) neurons have been found across many brain regions, most prominently in the central complex (CX) [20••,22-24], the presumed site of the sun compass.
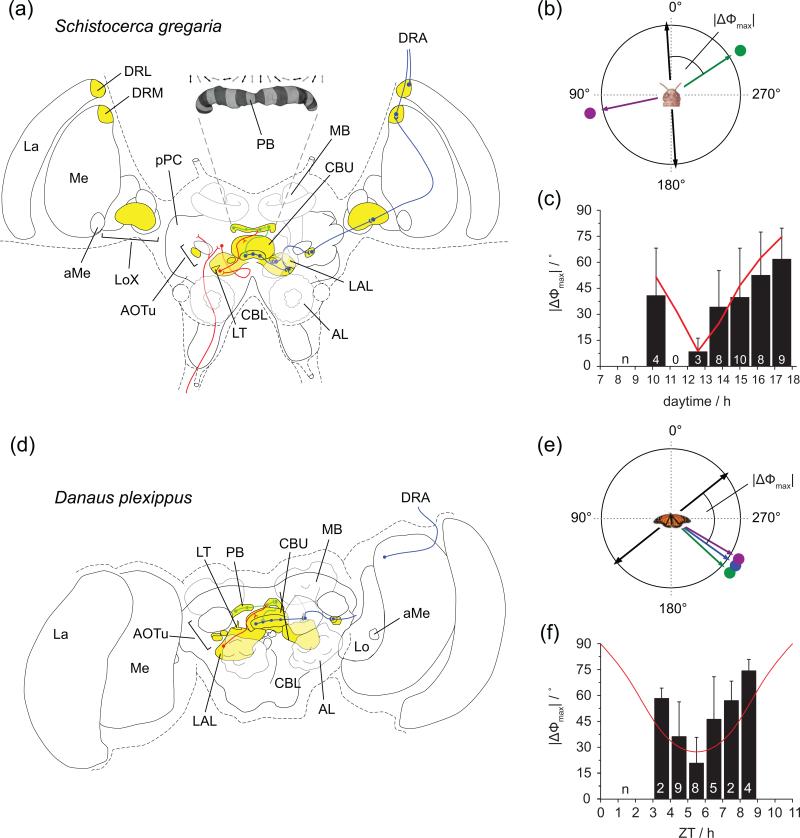
Figure 2. Neuronal organization of the insect sun compass.
(a) Simplified schematic of the polarization vision pathway in a frontal view of the desert locust brain. Highlighted in yellow are brain regions involved in processing of compass information. Key neural projections of the polarization vision pathway are shown as solid lines. Filled circles indicate proposed output and open semicircles indicate proposed input sites. Blue: input stage neurons; green: intermediate stage neurons; red: output stage neurons. Note that for clarity inputs are represented in the left hemisphere, while outputs are shown in the right hemisphere. The inset illustrates the mapping of zenithal E-vectors in the columns of the protocerebral bridge (PB). CBU, upper division of the central body; CBL, lower division of the central body; MB, mushroom body; La, lamina; Me, medulla; aMe, accessory medulla; Lo, lobula; LoX, lobula complex; AOTu, anterior optic tubercle; LAL, lateral accessory lobe; LT, lateral triangle; pPC, posterior protocerebrum; AL, antennal lobe. (b) E-vector tuning (black bidirectional arrow) and azimuth tuning (colored arrows) of an input stage compass neuron from the locust (green stimulation and ultraviolet stimulation). E-vector tuning was determined by presenting a rotating polarizer from the zenith (blue light), and azimuth tuning was determined by presenting an unpolarized light-spot that moved around the animal at constant elevation. The tuning angle was defined as the maximal activity during stimulus presentation (mean angle of Rayleigh-test). The angle between E-vector tuning and azimuth tuning (response to green light spot) is defined as |ΔΦmax|-value. (c) Time dependent adjustment of E-vector tunings in neurons of the input stage of the compass network of the locust. Plotted is the |ΔΦmax|-value against the recording time of neuron. The red line represents a celestial ΔΦ function fitted to the data, describing the angular difference between solar azimuth and E-vector orientation for individual points of an ideal natural sky, calculated for the season from which the locusts had been reared. The fit line was calculated for 60° elevation above the horizon according to the visual axis of the locust DRA and for the Tropic of Cancer (23.4°N), its natural habitat. Number of recordings is indicated in bars (mean ± SD). Modified from [27•]. (d) Polarization vision pathway in the monarch butterfly brain. Colors and symbols as in (a). (e and f) E-vector tuning and azimuth tuning (e), and adjustment of E-vector tuning over the course of the day (f) for the monarch butterfly. Symbols as in (b and c). The angle between E-vector tuning and mean azimuth tuning is defined as |ΔΦmax|-value. Polarized light was presented in the ultraviolet range. The red line in (f) represents the prediction of the angular difference between the mean perceived E-vector for the region of sky viewed by the monarch DRA and the solar azimuth over the course of the day. The values were calculated for the latitude and season from which migratory monarchs had been captured. ZT, Zeitgeber time of the 11-hr light:13-hr dark lighting cycle in which the monarchs have been maintained; 0 represents lights on. Bars, mean ± SD. Modified from [20••].
The POL-network can be divided into three processing stages. At the input stage, neurons characterized by a high signal to noise ratio, reliable E-vector (electric field vector) tuning, and low spontaneous activity [25] relay compass information sensed by the eyes to downstream areas (Figure 2a and 2d; blue lines). Importantly, most of these neurons also respond to unpolarized compass stimuli. When presented with light spots that move around the animal, these cells show maximal activity at a particular azimuthal position [20••,26,27•]. While locust neurons from the anterior optic tubercle show spectral and spatial opponency (green light versus ultraviolet light), corresponding monarch neurons respond independent of stimulus color (Figure 2b and 2e). This supports behavioral studies showing that the monarch uses the sun itself as the main compass cue [10,11,13,14]. Polarized-light information may be used to improve the encoded directional signal and thus enable reliable navigation on days with fluctuating visibility of the sun. In contrast, the locust appears to obtain the major directional signal from polarized light and may use the skylight spectral gradient to resolve the inherent directional ambiguity of E-vectors (Figure 1b) [27•]. In both species, a coherent signal of integrated compass cues is passed on to the second processing stage of the POL-network.
This second stage resides in the CX and has been studied extensively in the locust [21,25,28••,29] (Figure 2a and 2d; green lines). Here, information from both eyes is integrated and wide receptive fields ensure position-invariant neuronal responses. Additionally, neurons in the protocerebral bridge (a CX-component, consisting of a linear array of 16 columns) possess E-vector tunings that vary with the innervated column, creating a systematic representation of zenithal E-vectors [28••]. Because each cell is tuned to particular E-vectors, their population indirectly encodes the solar azimuth (Figure 2a, inset). This arrangement is thus suited to act as an internal compass. Whether this representation is a general feature of insects remains an open question. Considering the high degree of conservation of the involved pathways and overall CX-anatomy across insects, central encoding of skylight cues for navigation might be an ancient feature of the insect brain. Alternatively, this function could have been adopted for migration through re-dedication of existing CX-circuits. In the light of other reported roles of the CX, e.g. in learning and memory [30], it is unlikely that CX function in migratory insects is restricted to migratory behavior. A broader role in controlling motor output that integrates many behaviorally relevant features and motivational states seems likely.
The CX-output neurons, the third processing stage of the POL-network (Figure 2a and 2d; red lines), have indeed been proposed to integrate diverse sensory features of the environment with the body orientation of the animal [21,25]. These cells converge in the lateral accessory lobes, from where neurons might connect to descending pathways [20••,29,31,32•]. However, a coherent hypothesis of how the POL network generates motor commands to ensure a constant compass bearing remains to be determined.
Time compensation by the circadian clock
To maintain a fixed flight bearing throughout the day, migrating insects have to continuously adjust their direction relative to moving skylight parameters, as the solar azimuth constantly changes over the course of the day. This time compensation is provided by an endogenous timekeeping mechanism, the circadian clock, whose involvement has been demonstrated behaviorally in disappearance bearing and flight simulator experiments in monarch butterflies and neotropical butterflies [8-11].
Even though the site of the circadian clocks necessary for time compensation has previously been proposed to be located in the brains of monarchs and locusts [11,24,33], a recent study in monarch butterflies has shown that the compensation clocks are in fact located in the antennae [34••] (Figure 3). This surprising discovery raises the questions of how antennal clocks communicate timing information to the sun compass or its output structures and how the integrated information is represented at the motor output stage.
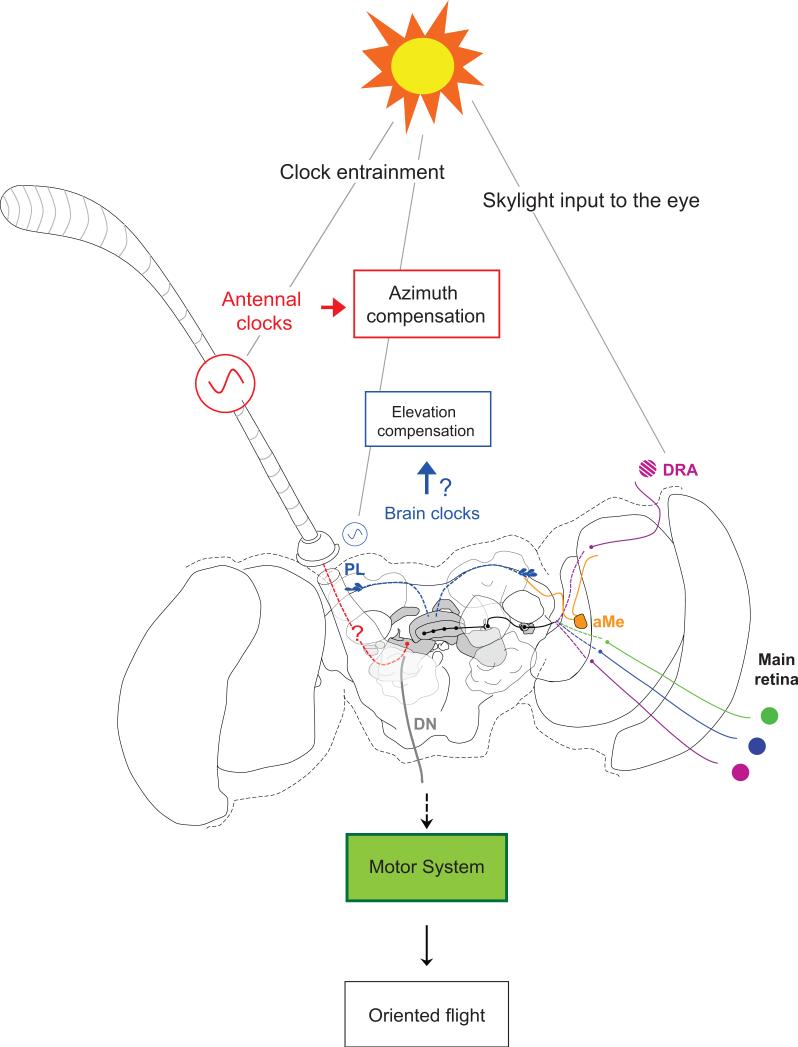
Figure 3. Integrated model of the components of the time-compensated sun compass in the monarch butterfly.
Skylight input to the eye: the dorsal rim area (DRA) of the eye senses the angle of ultraviolet polarized light (crosshatched violet circle) and the main retina senses color (violet, blue and green circles) or the sun itself (without discrimination of colors). Both skylight cues are integrated and transmitted to the central complex (CX; in grey), through neural pathways that are not yet completely defined (dashed colored lines and black solid lines). Clock entrainment: Blue light entrains (synchronizes) circadian clocks in the antennae and the brain to the 24-hr day. The antennal clocks, involved in solar azimuth compensation, provide the major timing information to sun compass orientation behavior. The neural pathway connecting the antennal clocks to the CX is not yet determined (dashed red line with question mark). A minor influence of the brain clock on output neurons of the CX could be mediated through a neural pathway connecting the clock cells in the pars lateralis (PL) to the CX (blue dashed lines). In addition, the timing component of the solar elevation compensation may originate from the brain clocks in the PL (blue spots), as a CRYPTOCHROME1 positive fiber pathway (orange lines) connects the PL to the accessory medulla (aMe) and terminates in the proximity of proposed projections from the DRA [15]. The integrated signal in the central complex or its output structures is finally transmitted via descending neurons (DN) to motor circuits that generate oriented flight behavior via yet unknown pathways. Modified from [6].
Recent work in the locust has shown a linear correlation of E-vector tunings to the time of day in descending brain neurons, which mediate behaviorally relevant signals from the brain to the motor circuits in the thoracic ganglia [32•]. This finding provides a potential time-compensation mechanism via adjustment of direction tunings at the output side of the internal sun compass. This integration of time and direction occurs either at the dendritic arborizations of descending neurons in the brain or upstream. The lateral accessory lobes appear to be promising candidate structures for future studies, as they receive most of the output from the central complex and are connected to multiple other brain regions [35] (Figure 3).
Besides changes in solar azimuth, the solar elevation also changes over the course of the day, which results in a constantly changing relation between the skylight polarization pattern and the solar azimuth. To provide consistent azimuth information from all skylight cues to the migrating animal, the E-vector tuning of POL-neurons has to be continuously adjusted. Such daytime changes in E-vector tuning of input stage POL-neurons have been shown in locusts and monarchs [20••,27•] (Figure 2c and 2f). In monarch brains, CRYPTOCHROME-1 positive fibers have been shown to connect the circadian clocks in the pars lateralis to the accessory medulla [15], which is in close proximity to the proposed projection areas of monarch DRA photoreceptors [15], the input to all POL-neurons. It is thus tempting to hypothesize that the brain clocks in the pars lateralis in monarchs are providing the timing component of solar “elevation compensation” (Figure 3). Defining whether monarch input stage POL-neurons are innervating the accessory medulla, as shown for POL-neurons of the optic lobe in locusts [24,36], will be a critical step to understand which clocks contribute to this mechanism.
In addition, a seasonal reversal of compass-mediated directional preference coincidental to changes in daylength has been documented in several lepidopteran migratory species [37•,38••], including the monarch butterfly [3,5]; all of them migrate southward during the fall and northward during the spring (in the northern hemisphere). The challenge is to understand how changes in temporal information are encoded at the neural level for bidirectional behavioral responses (south vs. north).
A magnetic compass for insect migration?
The earth's magnetic field is another source of directional information used by animals for long-distance migration [39-41]. The polarity, inclination angle and intensity of the field provide information enabling animals to maintain a constant heading (compass sense) and/or to locate their geographical position relative to their destination (map sense) (Figure 1b; right) [39]. Unlike the sun compass, a magnetic compass does not need to be continuously time compensated. Sensitivity to the magnetic field has been proposed to occur mainly through two distinct mechanisms, a ferromagnetic particle (magnetite)-based mechanism [42], and a light-dependent chemical-based mechanism [43]. Using genetic and behavioral approaches in Drosophila, the UV/blue-light photoreceptor CRYPTOCROME (CRY), proposed previously as a candidate magnetoreceptor [43], has recently been shown to mediate light-dependent magnetosensitivity in flies [44••].
While there is behavioral evidence for magnetoreception in a wide-range of organisms, including migratory and non-migratory vertebrate and invertebrate species [40,45], the use of a magnetic sense for navigation in migratory insects remains unclear. The monarch butterflies’ ability to navigate in the southwesterly direction on completely overcast days [46], when skylight cues are not available, suggests that a magnetic compass could be used as a backup compass. Behavioral orientation responses of migratory butterflies to alteration of the surrounding magnetic field have been inconclusive, because of contradictory results ([10,13,47,48]; reviewed in [6]). However, the presence of cellular and molecular substrates for sensing magnetic fields suggests the existence of a magnetic compass in monarchs. In addition to magnetite particles [49,50], monarchs have recently been shown to possess the molecular capacity to transduce magnetic information in a light-dependent manner through their CRY proteins (see below). These two mechanisms could co-exist, but, in migratory birds [51], it appears that the light-dependent mechanism predominates. Monarch butterflies possess two distinct CRYs, a Drosophila-like CRY which functions in the light input pathway of the circadian clock and a vertebrate-like CRY which functions as the main transcriptional repressor of the circadian clock [52]. Both monarch CRYs can in fact rescue magnetosensitivity in CRY-deficient transgenic adult flies in a light-dependent manner [53•]. This finding prompts the search of a light-dependent behavioral correlate in migrating monarch butterflies. To ultimately understand the neural basis of the magnetic sense mediated by light-dependent mechanisms in migratory insects, it will be critical to define the location of the magnetoreceptor and determine how directional information is communicated to relevant brain areas.
Wind selection and drift compensation
Another way by which migratory insects optimize their migratory route is through the selection of favorable winds and active compensation from wind drift. These phenomena have been documented in almost all high-flying species , including butterflies [54,55], moths [38••,56] and locusts [2,57]. The migratory direction has evolved to occur primarily downwind and most frequently at an altitude reached by riding the thermals (warm air currents) preceding fast moving cold fronts. Wind selection is beneficial in that it allows the conservation of energy essential for long-distance migration, and is of particular relevance for moths for which the migration window is reduced to a few nights [58]. Although it is not yet known how wind detection is achieved in migratory insects, based on studies in Drosophila[59], wind detection is likely mediated by antennal mechanoreceptors.
Because even favorable wind directions do not always match the preferred headings of migratory insects, compensating for crosswind drift is necessary to maintain the appropriate course. Wind drift compensation has been observed in many migratory species, including the monarch [46,60] and other butterflies [38••,61], high-flying moths [38••,56], locusts [2], and dragonflies [62], and relies on a compass sense. If diurnal migrants are likely to use a sun compass for this course correction, this orientation cue seems unlikely to be used by nocturnal migrants; the geomagnetic field has been proposed to be the most likely cue used by these animals [56].
The other possible cues on which migrants could rely at night are the moon and its associated polarization pattern, as well as the stars, especially the Milky Way (Figure 1b; bottom left panel). Although in disfavor previously [56], the existence of a lunar or stellar compass in nocturnal migratory insects is possible, and precedent for the use of stars to derive compass information exists in night-migratory birds [63]. In addition, arthropods have been shown to use the moon for precise navigational tasks other than long distance migration (e.g., local foraging and homing) [64,65]. Deciphering the compass mechanism used by nocturnal migrant insects is an exciting, wide-open area for exploration.
Conclusions and outlook
In recent years, there has been an increased understanding of how migratory insects solve the navigational problems critical for a successful migration. A comparative approach across a few diverse species has revealed a surprising similarity in the way in which the neural circuit integrates complex skylight information, and such an approach may be critical for defining common principles underlying long-distance migrations in many animals. Yet, critical aspects of the integration of time and space remain to be determined. By contrast, understanding how night sky and magnetic cues are used for compass information in migratory insects is at an early stage. In the future, the development of genomic resources [66••] along with genetic approaches in migratory species, for example to label neurons and knockout genes, will allow the manipulation of specific neuronal types and their functions to further our understanding of the molecular and neural basis of long-distance migration.
Highlights.
Migratory insects rely on compass mechanisms for direction during navigation
The neural basis and circuit of the time-compensated sun compass are being elucidated
Favorable winds and compensation of crosswind drift augment compass mechanisms
Night sky and magnetic compasses remain ill defined for long-distance migration
Acknowledgements
Some of the work discussed in this review was supported by AFOSR FA9550-10-1- 0480 and NIH grant R01 GM086794. C.M. is supported by a Charles A. King Trust Postdoctoral Fellowship, Bank of America, N.A., Co-Trustee and S.H. by a long-term fellowship from the Human Frontier Science Program (LT000379/2009-L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of particular interest
- 1.Dingle H. Migration: The biology of life on the move. University O; New York: 1996. [Google Scholar]
- 2.Kennedy JS. The migration of the desert locust (Schistocerca gregaria Forsk.). I. The behaviour of swarms. II. A theory of long-range migrations. Philos Trans R Soc Lond B Biol Sci. 1951;235:163–290. doi: 10.1098/rstb.1951.0003. [DOI] [PubMed] [Google Scholar]
- 3.Urquhart FA. The monarch butterfly : international traveler. Nelson-Hall; Chicago, Ill.: 1987. [Google Scholar]
- 4.Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML. Simple rules guide dragonfly migration. Biol Lett. 2006;2:325–329. doi: 10.1098/rsbl.2006.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brower LP. Monarch butterfly orientation: Missing pieces of a magnificent puzzle. Journal of Experimental Biology. 1996;199:93–103. doi: 10.1242/jeb.199.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Reppert SM, Gegear RJ, Merlin C. Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 2010;33:399–406. doi: 10.1016/j.tins.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould JL. Sensory bases of navigation. Curr Biol. 1998;8:R731–738. doi: 10.1016/s0960-9822(98)70461-0. [DOI] [PubMed] [Google Scholar]
- 8.Perez SM, Taylor OR, Jander R. A sun compass in monarch butterflies. Nature. 1997;387:29. [Google Scholar]
- 9.Oliveira EG, Srygley RB, Dudley R. Do neotropical migrant butterflies navigate using a solar compass? J Exp Biol. 1998;201:3317–3331. doi: 10.1242/jeb.201.24.3317. [DOI] [PubMed] [Google Scholar]
- 10.Mouritsen H, Frost BJ. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc Natl Acad Sci U S A. 2002;99:10162–10166. doi: 10.1073/pnas.152137299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froy O, Gotter AL, Casselman AL, Reppert SM. Illuminating the circadian clock in monarch butterfly migration. Science. 2003;300:1303–1305. doi: 10.1126/science.1084874. [DOI] [PubMed] [Google Scholar]
- 12.Nesbit RL, Hill JK, Woiwod IP, Sivell D, Bensusan KJ, Chapman JW. Seasonally adaptative migratory headings mediated by a sun compass in the painted lady butterfly, Vanessa cardui. Animal Behaviour. 2009;78:1119–1125. [Google Scholar]
- 13.Stalleicken J, Mukhida M, Labhart T, Wehner R, Frost B, Mouritsen H. Do monarch butterflies use polarized skylight for migratory orientation? J Exp Biol. 2005;208:2399–2408. doi: 10.1242/jeb.01613. [DOI] [PubMed] [Google Scholar]
- 14.Reppert SM, Zhu H, White RH. Polarized light helps monarch butterflies navigate. Curr Biol. 2004;14:155–158. doi: 10.1016/j.cub.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, Stalleicken J, Yuan Q, Casselman A, Reppert SM. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron. 2005;46:457–467. doi: 10.1016/j.neuron.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Mappes M, Homberg U. Behavioral analysis of polarization vision in tethered flying locusts. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:61–68. doi: 10.1007/s00359-003-0473-4. [DOI] [PubMed] [Google Scholar]
- 17.Homberg U, Paech A. Ultrastructure and orientation of ommatidia in the dorsal rim area of the locust compound eye. Arthropod Struct Dev. 2002;30:271–280. doi: 10.1016/s1467-8039(02)00010-5. [DOI] [PubMed] [Google Scholar]
- 18.Stalleicken J, Labhart T, Mouritsen H. Physiological characterization of the compound eye in monarch butterflies with focus on the dorsal rim area. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:321–331. doi: 10.1007/s00359-005-0073-6. [DOI] [PubMed] [Google Scholar]
- 19.Labhart T, Baumann F, Bernard GD. Specialized ommatidia of the polarization-sensitive dorsal rim area in the eye of monarch butterflies have non-functional reflecting tapeta. Cell Tissue Res. 2009;338:391–400. doi: 10.1007/s00441-009-0886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron. 2011;69:345–358. doi: 10.1016/j.neuron.2010.12.025. [This study provides data on the anatomical and physiological basis of sun compass navigation in the monarch butterfly. It highlights a surprising degree of similarity between the sun compass network in the monarch and the locust brains with respect to single cell morphologies and responses to polarized and unpolarized light. It also suggests that time-dependent adjustment of E-vector tuning is a fundamental feature of sun compass coding.] [DOI] [PubMed] [Google Scholar]
- 21.Homberg U, Heinze S, Pfeiffer K, Kinoshita M, Jundi B. Central neural coding of sky polarization in insects. Philos Trans R Soc Lond B Biol Sci. 2011;366:680–687. doi: 10.1098/rstb.2010.0199. el. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitzthum H, Muller M, Homberg U. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci. 2002;22:1114–1125. doi: 10.1523/JNEUROSCI.22-03-01114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer K, Kinoshita M, Homberg U. Polarization-sensitive and light-sensitive neurons in two parallel pathways passing through the anterior optic tubercle in the locust brain. J Neurophysiol. 2005;94:3903–3915. doi: 10.1152/jn.00276.2005. [DOI] [PubMed] [Google Scholar]
- 24.El Jundi B, Homberg U. Evidence for the possible existence of a second polarization-vision pathway in the locust brain. J Insect Physiol. 2010;56:971–979. doi: 10.1016/j.jinsphys.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. J Neurosci. 2009;29:11783–11793. doi: 10.1523/JNEUROSCI.1870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinoshita M, Pfeiffer K, Homberg U. Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J Exp Biol. 2007;210:1350–1361. doi: 10.1242/jeb.02744. [DOI] [PubMed] [Google Scholar]
- 27•.Pfeiffer K, Homberg U. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr Biol. 2007;17:960–965. doi: 10.1016/j.cub.2007.04.059. [This study reveals that unpolarized and polarized light stimuli are integrated in compass neurons of the locust anterior optic tubercle. The authors show that polarization sensitive neurons are also tuned to the azimuth position of unpolarized light, and provide evidence that these cells likely use the skylight spectral gradient to disambiguate the E-vector signal. Importantly, they present the first evidence of a daytime dependent adjustment of E-vector tunings ensuring that skylight cues provide consistent directional information despite changing solar elevation, a process termed “elevation compensation”.] [DOI] [PubMed] [Google Scholar]
- 28••.Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [This study shows that the locust central complex, which is shown to contain a complete representation of azimuthal space, is the likely neural substrate of the insect sun compass. The authors show that neurons innervating individual segments of the array of columns in the central complex are each tuned to specific E-vector angles of polarized light, and that the defined neuronal population maps this compass cue systematically onto the width of the central complex.] [DOI] [PubMed] [Google Scholar]
- 29.Heinze S, Homberg U. Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J Neurosci. 2009;29:4911–4921. doi: 10.1523/JNEUROSCI.0332-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 31.Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: Intrinsic and columnar neurons. J Comp Neurol. 2008;511:454–478. doi: 10.1002/cne.21842. [DOI] [PubMed] [Google Scholar]
- 32•.Träger U, Homberg U. Polarization-sensitive descending neurons in the locust: connecting the brain to thoracic ganglia. J Neurosci. 2011;31:2238–2247. doi: 10.1523/JNEUROSCI.3624-10.2011. [This paper reports for the polarized-light responsive neurons connecting the brain to the thoracic ganglia of the locust that change E-vector tuning over the course of the day. The authors suggest that adjusting E-vector tuning in descending neuronal pathways might be the neural basis of azimuth compensation required for long-distance migration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homberg U. In search of the sky compass in the insect brain. Naturwissenschaften. 2004;91:199–208. doi: 10.1007/s00114-004-0525-9. [DOI] [PubMed] [Google Scholar]
- 34••.Merlin C, Gegear RJ, Reppert SM. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science. 2009;325:1700–1704. doi: 10.1126/science.1176221. [This study reveals the unexpected location of the circadian clocks used for time-compensated sun compass navigation. Comprehensive data show that the clocks are located in the antennae, and not in the brain. This study more generally provides evidence that antennal clocks can directly regulate brain function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homberg U. Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A. 1994;175:597–610. [Google Scholar]
- 36.Homberg U, Wurden S. Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol. 1997;386:329–346. [PubMed] [Google Scholar]
- 37•.Chapman JW, Reynolds DR, Hill JK, Sivell D, Smith AD, Woiwod IP. A seasonal switch in compass orientation in a high-flying migrant moth. Curr Biol. 2008;18:R908–909. doi: 10.1016/j.cub.2008.08.014. [This study shows that high-flying migratory moths reverse their compass orientation according to season, and use similar compass mechanisms for flight orientation in both directions.] [DOI] [PubMed] [Google Scholar]
- 38••.Chapman JW, Nesbit RL, Burgin LE, Reynolds DR, Smith AD, Middleton DR, Hill JK. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science. 2010;327:682–685. doi: 10.1126/science.1182990. [A study using entomological radars to show that the selection of seasonally favorable high-altitude winds and crosswind drift compensation are common strategies used by high-flying migratory insects. Model simulations of trajectory show these behaviors maximize the distances traveled and decrease the drift from optimal directions.] [DOI] [PubMed] [Google Scholar]
- 39.Lohmann KJ, Lohmann CM, Putman NF. Magnetic maps in animals: nature's GPS. J Exp Biol. 2007;210:3697–3705. doi: 10.1242/jeb.001313. [DOI] [PubMed] [Google Scholar]
- 40.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 41.Lohmann KJ. Q&A: Animal behaviour: Magnetic-field perception. Nature. 2010;464:1140–1142. doi: 10.1038/4641140a. [DOI] [PubMed] [Google Scholar]
- 42.Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 43.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–1018. doi: 10.1038/nature07183. [This paper demonstrates the necessity of CRYPTOCHROME (CRY) for light-dependent magnetosensitivity in Drosophila. By testing magnetosensitive responses in a binary-choice behavioral assay, the authors show that wild-type flies perceive the magnetic field under UV/blue light and that Cry-deficient flies lose their ability to perceive the magnetic field, independent of Cry function in the circadian clock.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liedvogel M, Mouritsen H. Cryptochromes--a potential magnetoreceptor: what do we know and what do we want to know? J R Soc Interface. 2010;7(Suppl 2):S147–162. doi: 10.1098/rsif.2009.0411.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt-Koenig K. Orientation of autumn migration in the monarch butterfly. In: Malcolm SB, Zalucki MP, editors. Biology and conservation of the monarch butterfly. Natural History Museum of Los Angeles County; 1993. pp. 275–283. [Google Scholar]
- 47.Perez SM, Taylor OR, Jander R. The effect of a strong magnetic field on monarch butterfly (Danaus plexippus) migratory behavior. Naturwissenschaften. 1999;86:140–143. [Google Scholar]
- 48.Srygley RB, Dudley R, Oliveira EG, Riveros AJ. Experimental evidence for a magnetic sense in Neotropical migrating butterflies (Lepidoptera: Pieridae). Animal Behaviour. 2006;71:183–191. [Google Scholar]
- 49.Jones DS, MacFadden BJ. Induced magnetization in the monarch butterfly, Danaus plexippus(Insecta, Lepidoptera). J Exp Biol. 1982;96:1–9. [Google Scholar]
- 50.Larue A, Naber S, Talnagi J. Geomagnetic navigation in monarchs and black swallowtails. Ohio J Sci. 2006;106:117–123. [Google Scholar]
- 51.Stapput K, Thalau P, Wiltschko R, Wiltschko W. Orientation of birds in total darkness. Curr Biol. 2008;18:602–606. doi: 10.1016/j.cub.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 52.Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463:804–807. doi: 10.1038/nature08719. [A transgenic study showing that both types of CRY in the monarch butterfly function in the magnetoreception system of Drosophila at wavelengths in the UV/blue range, and thus have the ability to sense the magnetic field. Importantly, this paper provides evidence of a novel photochemical mechanism of CRY-mediated magnetoreception that does not utilize tryptophan triad-generated radical pairs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibo DL. Altitudes attained by migrating monarch butterflies, Danaus plexippus (Lepidoptera, Danaidae), as reported by glider pilots. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1981;59:571–572. [Google Scholar]
- 55.Gibo DL, Pallett MJ. Soaring flight of monarch butterflies, Danaus plexippus (Lepidoptera, Danaidae), during the late summer migration in southern Ontario. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1979;57:1393–1401. [Google Scholar]
- 56.Chapman JW, Reynolds DR, Mouritsen H, Hill JK, Riley JR, Sivell D, Smith AD, Woiwod IP. Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Curr Biol. 2008;18:514–518. doi: 10.1016/j.cub.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 57.Draper J. The direction of locust migration. Journal of Animal Ecology. 1980;49:959–974. [Google Scholar]
- 58.Hill JK, Gatehouse AG. Phenotypic plasticity and geographical variation in the pre-reproductive period of Autographa gramma (Lepidoptera:Noctuidae) and its implications for migration in this species. Ecol entomol. 1993;18:39–46. [Google Scholar]
- 59.Budick SA, Reiser MB, Dickinson MH. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol. 2007;210:4092–4103. doi: 10.1242/jeb.006502. [DOI] [PubMed] [Google Scholar]
- 60.Gibo DL. Flight strategies of migrating monarch butterflies (Danaus plexippus L.) in southern Ontario. In: Danthanarayana W, editor. Insect Flight, Dispersal and Migration. Springer-Verlag; 1986. pp. 172–184. [Google Scholar]
- 61.Srygley RB. Compensation for fluctuations in crosswind drift without stationary landmarks in butterflies migrating over seas. Animal Behaviour. 2001;61:191–203. doi: 10.1006/anbe.2000.1551. [DOI] [PubMed] [Google Scholar]
- 62.Srygley RB. Wind drift compensation in migrating dragonflies Pantala (Odonata: Libellulidae). J Insect Behav. 2003;16:217–232. [Google Scholar]
- 63.Emlen ST. The stellar-orientation system of a migratory bird. Sci Am. 1975;233:102–111. doi: 10.1038/scientificamerican0875-102. [DOI] [PubMed] [Google Scholar]
- 64.Dacke M, Byrne MJ, Baird E, Scholtz CH, Warrant EJ. How dim is dim? Precision of the celestial compass in moonlight and sunlight. Philos Trans R Soc Lond B Biol Sci. 2011;366:697–702. doi: 10.1098/rstb.2010.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ugolini A, Fantini T, Innocenti R. Orientation at night: an innate moon compass in sandhoppers (Amphipoda: Talitridae). Proc Biol Sci. 2003;270:279–281. doi: 10.1098/rspb.2002.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Zhan S, Merlin C, Boore JL, Reppert SM. The monarch genome yields insights into long-distance migration. Cell. 1011147:xxx–xxx. doi: 10.1016/j.cell.2011.09.052. [This study reports the draft genome of the monarch butterfly and a full set of protein-coding genes. This is the first characterized genome of a butterfly and of a long-distance migratory species. Gene annotation focused on gene families involved in the major aspects of the long-distance migration.] [DOI] [PMC free article] [PubMed] [Google Scholar]