Summary
Understanding the factors that contribute to age-related cognitive decline is imperative, particularly as age is the major risk factor for several neurodegenerative disorders. Levels of several cytokines increase in the brain during aging, including IL-1β, whose levels positively correlate with cognitive deficits. Previous reports show that reducing the activity of the mammalian target of rapamycin (mTOR) extends lifespan in yeast, nematodes, Drosophila, and mice. It remains to be established, however, whether extending lifespan with rapamycin is accompanied by an improvement in cognitive function. In this study, we show that 18-month-old mice treated with rapamycin starting at two months of age perform significantly better on a task measuring spatial learning and memory compared to age-matched mice on the control diet. In contrast, rapamycin does not improve cognition when given to 15-month-old mice with pre-existing, age-dependent learning and memory deficits. We further show that the rapamycin-mediated improvement in learning and memory is associated with a decrease in IL-1β levels and an increase in NMDA signaling. This is the first evidence to show that a small molecule known to increase lifespan also ameliorates age-dependent learning and memory deficits.
Keywords: learning and memory, mTOR, NMDA, aging, IL-1β, cytokines
Introduction
Life expectancy at birth is steadily increasing, and the elderly population is the fastest growing component of our society; the percentage of the U.S. population age 65 and older has increased from 8.3% in 1950 to 12.3% in 2000, and is forecast to reach 21.1% by 2050. Similar growth for aging population is also present in Europe (8.2% in 1950, 14.7% in 2000, and an estimated 29.2% by 2050 (Demeny & McNicoll 2003). Brain aging is associated with structural and functional changes that invariably lead to a decrease in cognitive functions even in healthy individuals, as well as changes that increase the brain's susceptibility to neurodegenerative disorders (Mattson & Magnus 2006). Therefore, understanding the molecular basis of age-related learning and memory deficits and thus increasing health span is a critical step toward increasing the quality of life among the elderly population (Warner 2007).
The mammalian target of rapamycin (mTOR) is a conserved and ubiquitously expressed protein kinase that plays a key role in controlling energy metabolism (Wullschleger et al. 2006). mTOR binds to raptor, PRAS40 and mLT8 to form the mTOR complex 1, which controls protein homeostasis by facilitating protein translation and inhibiting protein degradation. Additionally, mTOR can bind to rictor, mLST8 and hSIN to form the mTOR complex 2, which is mainly involved in regulating cellular shape (Wullschleger et al. 2006). The role of TOR in aging has been clearly and extensively demonstrated (Sharp & Strong 2010; Ingram & Roth 2011). Decreasing TOR signaling in lower organisms such as yeast, C. elegans and Drosophila significantly and consistently increases lifespan (Vellai et al. 2003; Jia et al. 2004; Kapahi et al. 2004; Kaeberlein et al. 2005; Powers et al. 2006). Similar results have been obtained in mammals where it has been shown that pharmacologically reducing mTOR signaling with rapamycin increases median and maximal lifespan by 9% and 13%, respectively (Harrison et al. 2009). Furthermore, genetic reduction of mTOR signaling was shown to increase lifespan and age-related motor dysfunction (Selman et al. 2009). It remains to be determined whether increasing lifespan by decreasing mTOR signaling has beneficial effects on age-related learning and memory deficits.
Rapamycin is an immuno-modulator first isolated from Streptomyces hygroscopicus and has been approved by the U.S. Food and Drug Administration for clinical use as an immunosuppressant. Most of the rapamycin effects on the immune system are thought to be mediated by its ability to inhibit mTOR (Saemann et al. 2009), although recent reports also show that rapamycin may also affect the immune system independently of mTOR (Janes & Fruman 2009). Even though the mechanism of action of rapamycin is not completely understood, rapamycin inhibits lymphocytes pool expansion and modulates innate immune cells (reviewed in (Janes & Fruman 2009).
The immune system plays a critical role in regulating brain aging and an age-related increase in brain inflammation has been well documented (Lucin & Wyss-Coray 2009; Finch et al. 2010). Microglia are the primary immune cells of the brain and their activation increases as a function of age, which can be either neuroprotective or neurotoxic. Toward this end, microglia may secrete anti-inflammatory cytokines, such as interleukin (IL) 4 and IL-10, or pro-inflammatory cytokines, such as IL-1β or TNFα (Lucin & Wyss-Coray 2009). A growing body of evidence shows that cytokines mediate several functions in the brain including long-term potentiation and long-term depression (Yirmiya & Goshen 2011), two cellular mechanisms thought to be related to learning and memory. Specifically, IL-1β has been directly linked to memory impairment in the elderly (Allan et al. 2005; Viviani et al. 2006). Whereas physiological IL-1β levels are required for normal learning and memory, high levels of IL-1β are detrimental and impair cognitive functions (Ross et al. 2003). Here we show that rapamycin, a small molecule known to increase lifespan, ameliorates age-dependent cognitive decline by an IL-1β-mediated mechanism.
Results
Spatial learning and memory decreases as a function of age
The hippocampus is a brain region critical for learning and memory. The spatial version of the Morris water maze (MWM) is a routinely used task to assess hippocampal function in mice. To determine how spatial learning and memory change as a function of age in mice, we tested 2-, 6-, 15-, and 18-month-old C57BL6/129svj mice (n=10/age group) using the spatial version of the MWM. Specifically, mice were trained to find a hidden platform using extra maze cues. The mice received 4 training trials per day for 5 consecutive days and their time to find the platform was recorded and analyzed using a mixed-model, repeated-measures ANOVA with age group as the categorically fixed effects, days as the numeric covariate, animals as the random effect, and escape latency as the dependent variable. We found a significant effect for days (F = 151.4; p < 0.0001), indicating that the mice significantly learned the task across sessions (Fig. 1A). More important, we found a significant age group-day interaction (F = 33.95; p < 0.0001), indicating that one or more of the groups was different from the others (Fig. 1A). We then conducted a Bonferroni's post hoc analysis to identify changes among the groups. We found that at day 2, the 18-month-old mice performed significantly worse (longer escape latency) than 2- and 6-month-old mice (p < 0.05). No other statistically significant differences were found for this day (Fig. 1A). At day 3, the 18-month-old mice performed significantly worse than 2-month-old mice (p < 0.001), 6-month-old mice (p < 0.001) and 15-month-old mice (p < 0.01). No other statistically significant differences were found for this day. At day 4 and 5, the 18-month-old mice were still significantly different than 2- and 6-month-old mice (p < 0.01); however, their performance was similar to that of 15-month-old mice (p > 0.05). Additionally, while at day 4 the escape latency of the 15-month-old mice was not significantly different than 2- and 6-month-old mice, after 5 days of training the 15-month-old mice performed significantly worse than 2- and 6-month-old mice (p < 0.05; Fig. 1A). Overall, these data show that under the conditions used here, age-dependent spatial learning deficits can first be detected at 15 months of age.
Figure 1. Age-dependent learning and memory is first apparent in 15-month-old mice.
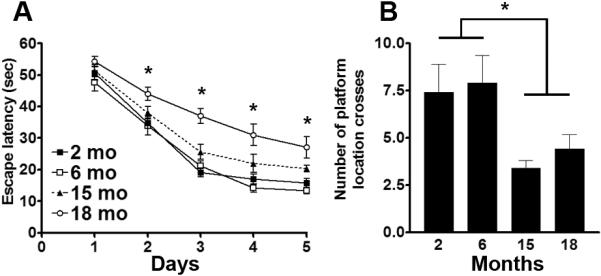
C57BL6/129svj mice of different ages (n = 10/age group) were trained in the spatial version of the Morris water maze (MWM) and received 4 training trials per day for 5 days. (A) Learning curves indicating the escape latency of each age-group across the 5 days of training. Each time-point represents the average escape latency for the 4 training trials of that specific day. Mice significantly learned the task as indicated by a reduced time to find the hidden platform (F = 151.4; p < 0.0001). Bonferroni's post hoc analysis showed that the learning deficits were first detected at 15 months of age. Indeed, at day 5, the 15-month-old mice performed significantly worse than 2- and 6-month-old mice (p < 0.05). The learning deficits in the 18-month-old mice were detected as early as after two days of training. (B) Twenty-four hours after the last training trial, the spatial memory of the mice was tested by assessing how many times mice crossed over the platform location during the 60-second probe trial. One-way ANOVA, followed by Bonferroni's post hoc test, indicated that the 15- and 18-month-old mice performed significantly worse (lower number of platform location crosses) than 2- and 6-month-old mice (one-way ANOVA: F = 3.881; p = 0.01. Post hoc: p < 0.05). Data are presented as means ± SEM.
To assess spatial memory, 24 hours after the last training trial, probe trials were conducted during which mice were placed into the maze for 60 seconds without any platform being present. Using a dedicated tracking system, we measured how many times mice crossed the former platform location. One-way ANOVA indicated a significant age effect (F = 3.881; p = 0.01; Fig. 1B). A Bonferroni's multiple comparison test indicated that the 15- and 18-month-old mice performed significantly worse than 2- and 6-month old mice (p < 0.05; Fig. 1B), further confirming that age-dependent spatial learning deficits can be detected by 15 months of age.
Rapamycin administration improves learning and memory
Rapamycin was previously shown to increase lifespan in genetically heterogeneous mice (Harrison et al. 2009); however, the effects of rapamycin (or other genetic and pharmacological interventions aimed at decreasing mTOR signaling) on age-dependent cognitive decline have not been elucidated. To determine whether rapamycin can also ameliorate or reverse age-dependent cognitive deficits, 60 C57BL6/129svj mice were randomly assigned to one of the following three groups (n=20 mice/group; Fig. 2A): (1) mice treated with encapsulated rapamycin (14mg per kg food) starting at 2 months of age for 16 months (herein referred to as Rapa2–18 mice); (2) mice treated with rapamycin between 15 and 18 months of age (herein referred to as Rapa15–18 mice); (3) mice kept on the control diet (food containing empty microcapsule) throughout life (herein referred to as CTL mice). The 15-month time-point was chosen because at this age, these mice already show age-dependent cognitive deficits (Fig. 1); therefore, this group will allow us to determine whether rapamycin can reverse cognitive deficits. Note that when the mice were not on rapamycin (e.g., from 0 to 2 months of age or from 0 to 15 months of age for the Rapa15–18 mice), they were kept on the control diet. Importantly, we used the same formulation of rapamycin that has been shown to increase lifespan in mice (Harrison et al. 2009).
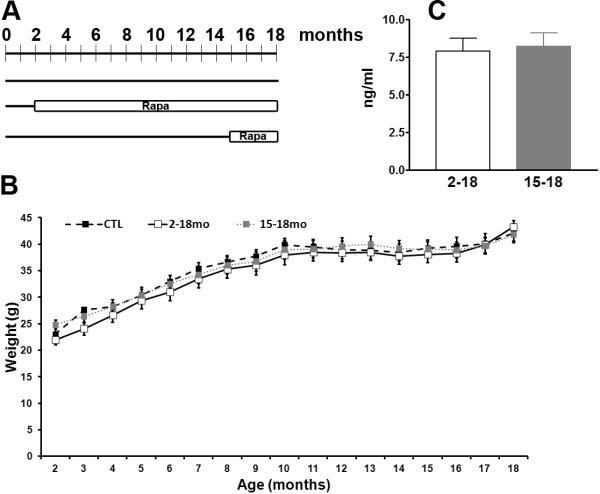
Figure 2. Life-long rapamycin treatment results in no gross health problems.
(A) Diagram depicting the experimental design. Twenty mice were used for each group, all mice were 18 months old at the end of the treatment. (B) Body weight of the mice was taken at the beginning of the treatment and monthly thereafter. The weight gained over the 16 month long experiment was similar across the three different groups (p > 0.05). (C) Before the mice were terminated, blood was collected from the retro-orbital vein and analyzed for rapamycin levels by HPLC. Rapamycin was not detectable in the CTL mice and the levels were similar between the Rapa2–18 and the Rapa15–18 mice. Data are presented as means ± SEM.
Mice were weighed before starting the rapamycin treatment and monthly thereafter. Notably, all three groups of mice gained weight throughout the experiments and no statistically significant differences were found (Fig. 2B; p > 0.05 as determined by two-way ANOVA). We could not accurately assess food intake because 4–5 mice were housed in each cage. However, considering that all mice significantly gained weight throughout the duration of the experiment and the body weight gain was similar across the different groups, it is tempting to speculate that food intake was similar among all mice. At the end of the treatment, all of the mice from the three groups were 18 months of age, and blood was collected to assess rapamycin levels. We found that the rapamycin levels were similar between the Rapa2–18 and the Rapa15–18 mice (p < 0.05 as determined by student t-test; Fig. 2C) and were below detection levels in CTL mice.
To assess the effect of rapamycin on learning and memory, at the end of the rapamycin treatment, mice were trained in the MWM to find a hidden platform using extra maze cues. Escape latency data were analyzed using a mixed-model, repeated-measures ANOVA, with treatment as the categorically fixed effect, days as the numeric covariate, animals as the random effect, and escape latency as the dependent variable. We found a significant effect for days (F = 33.26; p < 0.0001), indicating that the mice significantly learned the task across sessions (Fig. 3A). More important, we found a significant treatment-day interaction (F = 7.104; p = 0.001), indicating that one or more of the groups was different from the others (Fig. 3A). We therefore performed a post hoc test with Bonferroni corrections and compared each of the individual groups to each other. We found that the Rapa2–18 mice performed significantly better than the other two groups (p < 0.05). In contrast, we found that the escape latency of the Rapa15–18 mice was not significantly different than the CTL mice (Fig. 3A). It is important to note that the escape latency of the Rapa2–18 mice was significantly better only after 5 days of training and not during the first 4 days. These results are internally consistent if one considers the behavioral paradigm used. Specifically, during the first few days of training, mice find the platform by chance and thus all groups must perform similarly. If one group of mice performed significantly different from the others in the first or second day of training, such difference may not actually be due to learning deficits but to other confounding biases (e.g., swimming abilities or motivation). As mice in the Rapa2–18 group received more training, they started to learn the spatial cues in the room and found the platform faster and faster with each subsequent trial. Because some learning is also present in the CTL and Rapa15–18 mice, it is not surprising that learning differences among the three groups were detected during the later days of training. These results indicate that short-term rapamycin administration to 15-month-old mice, an age at which mice already have spatial learning deficits, has no impact on learning. In contrast, life-long rapamycin administration ameliorates age-dependent spatial learning.
Figure 3. Rapamycin prevents age-dependent cognitive deficits.
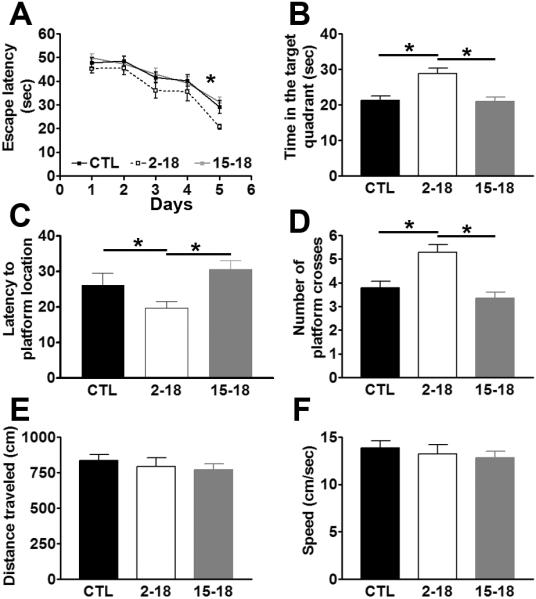
18-month-old CTL, Rapa2–18 and Rapa15–18 mice were trained in the spatial version of the Morris water maze (MWM) and received 4 training trials per day for 5 days. (A) Learning curves indicating the escape latency of each age-group across the 5 days of training. Each time-point represents the average escape latency for the 4 training trials of that specific day. Mice significantly learned the task as indicated by a reduced time to find the hidden platform (F = 33.26; p < 0.0001). Bonferroni's post hoc analysis showed that at day 5, the Rapa2–18 mice performed significantly better than the other two groups (p < 0.05). (B–D) Reference memory, measured 24 hours after the last training trial, was significantly improved in the Rapa2–18 mice compared to the CTL and Rapa15–18 mice, respectively. Three months of rapamycin administration, however, did not have any effect on reference memory. (E–F) Distance traveled and swimming speed during the probe trials were not significantly different among the 3 groups of mice. Data are presented as means ± SEM.
To assess spatial memory, 24 hours after the last training trial, probe trials were conducted during which mice were placed into the maze for 60 seconds without any platform being present. Using a dedicated tracking system, we measured the time mice spent in the target quadrant, the time to cross the former platform location and the number of crossings. Over the 60-second probe trial, the CTL mice spent 21.30 ± 1.24 seconds in the target quadrant, while the Rapa2–18 and the Rapa15–18 mice spent 28.73 ± 1.65 and 20.97 ± 1.18 seconds, respectively (Fig. 3B). One-way ANOVA showed that there was a significant difference among the three groups (F = 10.16; p = 0.0002). Bonferroni's post hoc analysis showed that the Rapa2–18 mice performed significantly better than CTL and Rapa15–18 mice (p < 0.01 and p < 0.001, respectively). Similar results were obtained when we measured the latency to cross the former platform location and number of platform location crossing (Fig. 3C–D). For the latency to cross the platform location F = 4.15; p < 0.02; for the number of platform location crossing, F = 11.51; p < 0.0001. To determine whether mouse physical performance may account for the changes in spatial learning and memory, we measured the distance mice traveled during the probe trials and the swim speed. One-way ANOVA indicated that both parameters were not significantly different across all 3 groups of mice (Fig. 3E–F; p = 0.65 for both measurements). Taken together, these findings clearly indicate that when given to 2-month-old mice throughout their life, rapamycin slows the development of age-associated memory deficits. In contrast, when given to 15-month-old mice, rapamycin does not reverse the already existing age-dependent cognitive deficits. However, we cannot exclude that a longer treatment paradigm or different concentrations of rapamycin may have other effects on learning and memory in 15-month-old mice.
Rapamycin decreases mTOR signaling and brain cytokine levels
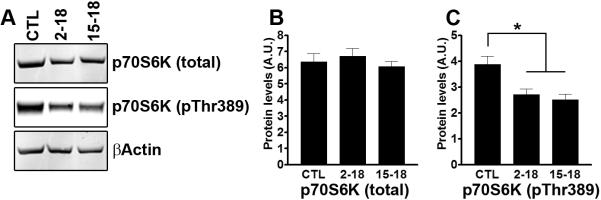
We have previously shown that 10 weeks of rapamycin treatment given to young 6-month-old mice reduces mTOR signaling in the brain (Caccamo et al. 2010a). mTOR activity is routinely assessed by measuring the steady-state levels of p70S6K, a protein kinase directly phosphorylated by mTOR at threonine 389 (Hay & Sonenberg 2004). Western blots analyses of proteins extracted from CTL, Rapa2–18 and Rapa15–18 mice showed that the levels of total p70S6K were similar across the three different groups (Fig. 4A–B; F = 0.48; p = 0.62 as calculated by one-way ANOVA). In contrast, we found that the levels of p70S6K phosphorylated at threonine 389 were significantly changed among the three groups as indicated by one-way ANOVA calculations (F = 8.62; p = 0.01; Fig. 4A, C). Bonferroni's multiple comparison test indicated that the Rapa2–18 and the Rapa15–18 mice were not significantly different from each other but that they were both significantly different from the CTL mice (p< 0.05 and p < 0.01, respectively). Together these data suggest that the kinase activity of mTOR is reduced in the rapamycin fed mice compared to the age-matched mice fed the control diet.
Figure 4. Rapamycin decreases mTOR signaling.
(A) Representative Western blots from proteins isolated from CTL, Rapa2–18 and Rapa15–18 mice and probed with the indicated antibodies. (B–C) Quantitative analysis of the blots shows that the total levels of p70S6K levels were similar across the 3 different groups. In contrast, the levels of p70S6K phosphorylated at Thr389 were significantly reduced by rapamycin (n=8). Data were analyzed by one-way ANOVA and Bonferroni's post hoc analysis, and presented as means ± SEM.
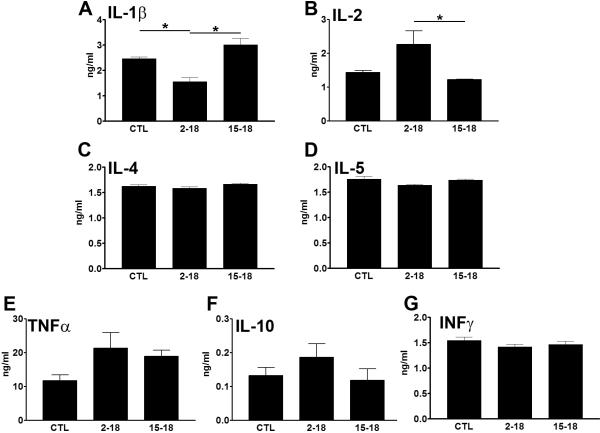
Rapamycin forms a complex with FK506-binding protein 12 (FKBP12), a peptidyl propyl cis/trans isomerase that mediates conformational changes in target proteins (Neuhaus et al. 2001). The rapamycin/FKBP12 complex inhibits mTORC1 by blocking its interaction with raptor (Neuhaus et al. 2001). mTOR plays a key role in controlling the innate immune system and regulates the levels of several cytokines (Saemann et al. 2009). Notably, the age-dependent increase in pro-inflammatory cytokines in the brain has been associated with learning and memory deficits (Sparkman & Johnson 2008; Tarr et al. 2011). To determine how brain cytokine profiles change following rapamycin administrations, protein extracted from the whole brain of CTL, Rapa2–18 and Rapa15–18 mice were used in a multiplex system to quantitatively assess selective cytokine levels. Specifically, we measured the steady-state levels of IL-1β, IL-2, IL-4, IL-5, TNF-α, and INF-γ (Fig. 5A–G). We found only IL-1β and IL-2 levels to be significantly different among the three groups of mice (F = 16.98; p < 0.001; and F = 5.73; p = 0.01, respectively, as indicated by one-way ANOVA). Bonferroni's multiple comparison test indicated that the levels of IL-1β were significantly lower in the Rapa2–18 mice compared to the CTL and Rapa15–18 mice (p < 0.01 and p < 0.001, respectively; Fig. 5A). The levels of IL-1β in the Rapa15–18 mice were not significantly different from those in the CTL mice. The post-hoc test also showed that IL-2 levels in the Rapa2–18 mice were significantly higher than those in Rapa15–18 mice (p < 0.05; Fig. 5B). No significant differences, however, were found between the CTL and the Rapa2–18 mice and the CTL and Rapa15–18 mice (Fig. 5B), suggesting that the improvement in learning and memory in the Rapa2–18 mice was not linked to IL-2 levels as they did not change in this group of mice. In contrast, these data clearly indicate a strong correlation between IL-1β levels and changes in learning and memory. Indeed, IL-1β levels were significantly decreased in the only group of mice that showed a rapamycin-mediated improvement in learning and memory. This finding is consistent with the data showing that high levels of IL-1β impair hippocampal-dependent learning and memory (Barrientos et al. 2009; Tarr et al. 2011).
Figure 5. Rapamycin decreases whole brain IL-1β levels.
Quantitative measurements of several cytokine levels using a multiplex system (n=6/group). (A) IL-1β levels were significantly lower in the Rapa2–18 mice compared to the CTL and Rapa15–18 mice. (B) In contrast, IL-2 levels were significantly lower in the Rapa15–18 mice compared to the CTL and Rapa2–18 mice. (C–G) No other statistically significant changes were found for the other cytokines. Proteins were isolated from whole brain. Data were analyzed by one-way ANOVA and Bonferroni's post hoc analysis, and presented as means ± SEM.
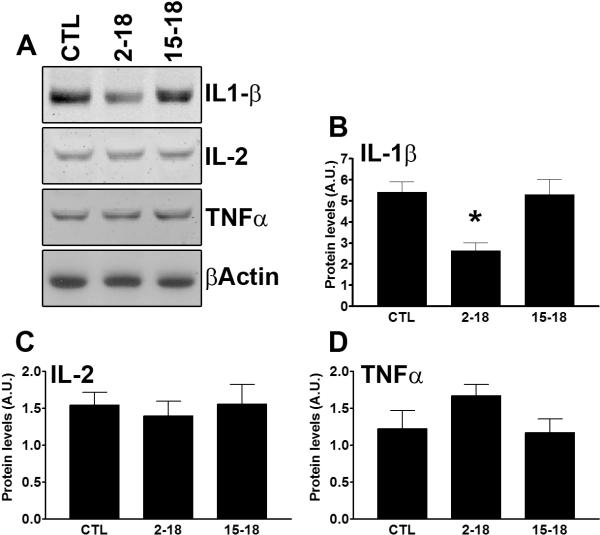
To confirm the data obtained with the multiplex system, we subsequently measured the levels of selective cytokine by Western blot. Specifically, considering that the changes in learning and memory were measured with an hippocampal-dependent task, we measured the levels of IL-1β, IL-2 and TNFα in protein extracted from the hippocampi of treated and untreated mice (n=6/genotype). Consistent with the multiplex data, we found that rapamycin significantly changed IL-1β levels in the hippocampus (Fig. 6A–B; p = 0.004 calculated by one way ANOVA). Post hoc analysis showed that IL-1β levels in the Rapa2–18 mice were significantly lower than the other two groups (Fig. 6A–B; p < 0.01). In contrast, we found that the levels of IL-2 and TNFα were not statistically different among the three groups (Fig. 6A, C–D; data analyzed by one way ANOVA). Together these data further confirm a strong correlation between hippocampal IL-1β levels and learning and memory.
Figure 6. IL-1β levels were reduced in the hippocampus of the Rapa2–18 mice.
Hippocampi from frozen hemi-brains were removed and processed for protein extraction. (A) Representative Western blots from proteins isolated from the hippocampi of CTL, Rapa2–18 and Rapa15–18 mice and probed with the indicated antibodies. (B–D) Quantitative analysis of the blots shows that hippocampal IL-1β levels were significantly reduced in the Rapa2–18 mice compared to the other two groups. In contrast, the levels of IL-2 and TNFα were similar among the three groups (n=6/genotype). Data were analyzed by one-way ANOVA and Bonferroni's post hoc analysis, and presented as means ± SEM.
Rapamycin rescues NMDA signaling
Spatial learning and memory is highly dependent on hippocampal NMDA signaling, which decreases during aging and is altered in the 3×Tg-AD mice (Caccamo et al. 2010b). To determine whether the effects of rapamycin on learning and memory may also be mediated by changes in NMDA activity, we measured NMDA signaling in the hippocampi of CTL, Rapa2–18 and Rapa15–18 mice.
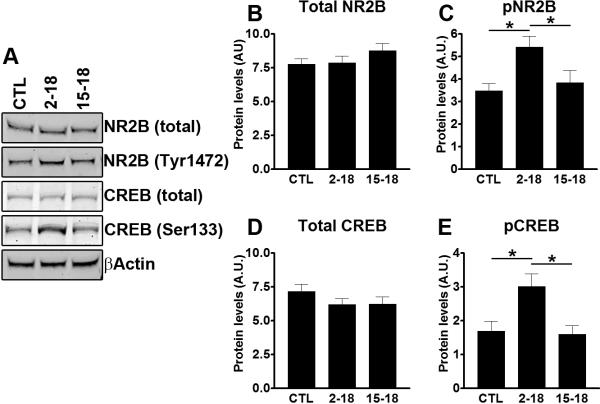
The NMDA receptors are heteromeric complexes containing both NR1 and NR2 subunits and each subunit has different subtypes (Cull-Candy et al. 2001). The NR2B subunit plays a key role in facilitating memory consolidation (Sakimura et al. 1995). We thus measured hippocampal steady-state levels of total NR2B and NR2B phosphorylated at Tyr1472. Although we found that the total levels of NR2B were not statistically different across the three groups of mice (Fig. 7A–B; p = 0.29), the levels of NR2B phosphorylated at Tyr1472 were significantly increased in the Rapa2–18 mice compared to CTL and Rapa15–18 mice (Fig. 7A, C; one way ANOVA: p = 0.015; post hoc analysis: p < 0.05 when compared the Rapa2–18 mice to the CTL mice, and to the Rapa15–18 mice). This finding is remarkable, as the reduced phosphorylation of NR2B at Thr1472 correlates with receptor endocytosis, which leads to reduced NMDA receptor signaling (Snyder et al. 2005; Caccamo et al. 2010b). We next examined whether the rapamycin-mediated increase in surface expression of NR2B receptors would facilitate the NMDA receptor-mediated biochemical signaling. Specifically, one way by which NMDA receptors facilitates learning and memory is by phosphorylating and activating CREB, a transcription factor that plays a key role in learning and memory (Shaywitz & Greenberg 1999). We found that the hippocampal levels of total CREB were similar among the three groups of mice (Fig. 7A, D; p = 0.32). In contrast, one-way ANOVA showed a significant change in the levels of phosphorylated CREB among the three groups (Fig. 7A, E; p = 0.006). Bonferroni's multiple comparison test showed that the levels of phosphorylated CREB were significantly higher in the Rapa2–18 mice compared to the CTL and Rapa15–18 mice (p < 0.05 for both comparisons). Taken together, these data suggest that rapamycin ameliorates age-dependent cognitive deficits by decreasing hippocampal IL-1β levels and by facilitating NR2B surface expression and overall NMDA signaling.
Figure 7. Increased NMDA signaling in the Rapa2–18 mice.
(A) Representative Western blots from proteins isolated from CTL, Rapa2–18 and Rapa15–18 mice (n=8/group) and probed with the indicated antibodies. (B–E) Quantitative analysis of the blots shows that that the levels of total NR2B and CREB were similar across the three different groups. In contrast, the levels of NR2B phosphorylated at Tyr1472 were significantly increased in the Rapa2–18 mice compared to the other two groups. Similarly, phosphor-CREB levels were increased in the Rapa2–18 mice compared to CTL and Rapa15–18 mice. Data were analyzed by one-way ANOVA and Bonferroni's post hoc analysis, and presented as means ± SEM.
Discussion
Data from the National Institute on Aging Interventions Testing Program have unambiguously shown that rapamycin increases median and maximal lifespan in genetically heterogeneous mice (Harrison et al. 2009). Here we used the same formulation used by Harrison and colleagues and show, for the first time, that rapamycin ameliorates age-dependent cognitive deficits. This is a major finding as a meaningful lifespan extension must be complemented by an improvement in health span. Only doing so, lifespan extension will lead to a better long-term health outcome for the aging population. Although its mechanism of action is not fully understood, the findings that rapamycin increases lifespan while also positively impacting age-dependent disorders such as Alzheimer's, Huntington's diseases (Berger et al. 2006; Caccamo et al. 2010a), makes this small molecule a key compound in aging research.
We showed that rapamycin alters brain mTOR signaling, suggesting that it crosses the blood brain barrier. Indeed, there is a large body of evidence in animal models and in human studies showing that rapamycin crosses the blood brain barrier (e.g., (Cloughesy et al. 2008). mTOR signaling has been shown to modulate learning and memory (Casadio et al. 1999; Tischmeyer et al. 2003; Ehninger et al. 2008; Puighermanal et al. 2009; Caccamo et al. 2010a). Thus, there is clear evidence that mTOR is necessary for learning and memory. For example, inhibiting mTOR with rapamycin has detrimental effects on long-term memory facilitation and consolidation in gerbils and Aplysia californica (Casadio et al. 1999; Tischmeyer et al. 2003). In contrast, there is also strong evidence showing that hyperactive mTOR signaling is detrimental to learning and memory (Bolduc et al. 2008). Toward this end, it has been shown that cognitive deficits in a mouse model of tuberous sclerosis are associated with hyperactive mTOR signaling (Ehninger et al. 2008). Indeed, rapamycin was shown to rescue learning and memory deficits in the mutant mice (Ehninger et al. 2008). Similarly, cognitive impairments due to cannabis consumption are linked to hyperactive mTOR and can be reversed by rapamycin (Puighermanal et al. 2009). Along these lines, we have previously shown that mTOR is hyperactive in a mouse model of Alzheimer's disease (Caccamo et al. 2010a; Caccamo et al. 2011), and more important, we showed that rapamycin can improve the cognitive deficits associated with the AD-like pathology (Caccamo et al. 2010a). Overall these data, together with the data presented here showing that a small decrease in mTOR signaling in aged mice has beneficial effects on learning and memory, suggest that there may be a window of mTOR activity that is necessary for learning and memory, whereas complete blockage of mTOR or hyperactive mTOR are detrimental to learning and memory.
It is very well established that the production of pro-inflammatory cytokines increases in the brain as a function of age while the levels of anti-inflammatory cytokines decrease (Sparkman & Johnson 2008). Hence, it has been proposed that the age-dependent shift in the balance between pro- and anti-inflammatory cytokines may prime the brain to brain disorders (Viviani & Boraso 2011). This view is supported by a large body of work conducted in several laboratories showing that the activation of several cytokines can lead to deficits in several learning and memory tasks, including hippocampal-dependent tasks (Barrientos et al. 2009; Tarr et al. 2011). IL-1β levels increase as a function of age in the hippocampus (O'Donnell et al. 2000) and its involvement in memory has been clearly established using complementary approaches (Allan et al. 2005). Specifically, there is a growing appreciation for a dual role of IL-1β in learning and memory; whereas IL-1β is required for memory, a chronic increase in IL-1β has negative effects on learning and memory (Ross et al. 2003). Notably, a genetic variation in the IL-1β converting enzyme, which regulates IL-1β, is associated with lower IL-1β production levels and better performance in cognitive functions in elderly people, suggesting that low levels of IL-1β are protective against age-associated learning and memory deficits (Trompet et al. 2008). The data presented here are consistent with these reports as we have shown that the improvement in behavior is strictly linked to a reduction of central IL-1β levels. Indeed, we show that rapamycin improved learning and memory only in the Rapa2–18 mice, which have significantly lower IL-1β levels compared to the other two groups. In contrast, the Rapa15–18 mice, in which IL-1β levels were not decreased, performed the same as the CTL mice. Further studies are needed to determine why IL-1β levels were only decreased in the Rapa2–18 mice and not in the Rapa15–18 mice. It is tempting to speculate that longer period of rapamycin administration or higher rapamycin concentrations may lead to a decrease in brain IL-1β levels and thereby to an improvement in learning and memory.
The NMDA receptors play a key role in synaptic plasticity and long-term potentiation, which is believed to be the cellular mechanism underlying learning and memory (Lee & Silva 2009). For example, during memory formation, CREB is phosphorylated at Ser133 via activation of NMDA receptors (Lee & Silva 2009). Notably, CREB is phosphorylated and activated following neuronal stimulation; these events are necessary to facilitate the transcription of proteins required for learning and memory (Lee & Silva 2009). NMDA function can be modulated by endocytosis (Snyder et al. 2001). Specifically, reduced phosphorylation of NR2B at Thr1472 correlates with receptor endocytosis, which leads to reduced NMDA receptor signaling (Snyder et al. 2001).
Taken together the data presented here suggest that rapamycin-mediated changes in IL-1β and NMDA signaling contribute to the learning and memory improvements in the Rapa2–18 mice. While there is evidence showing that the IL-1β effects on learning and memory could be mediated by its interaction with several ion channels, including the NMDA receptors (Viviani et al. 2006; Viviani & Boraso 2011), further studies are needed to assess whether the rapamycin-mediated changes in IL-1β levels and NMDA signaling contribute to the improvements in learning and memory via two independent pathways or via a single and interrelated pathway.
Experimental Procedures
Rapamycin administration
The rapamycin diet consisted of food containing microencapsulated rapamycin, which was prepared as described previously (Harrison et al. 2009). Food containing empty microcapsules was used as the control diet. During the entire treatment period mice were given ad libitum access to water and the rapamycin or control diet.
Behavioral testing
Mice were analyzed in the spatial reference of the Morris water maze, which was conducted in a 1.5 meter in diameter circular pool. Mice were give 4 training trials per day for 5 days and were trained to locate a hidden platform using extra maze cues that were distributed around the room. The water was made opaque by the addition of non-toxic paint and was maintained at 25°C. Each trial was terminated after 60 seconds or when a mouse found the escape latency. Sixty-second probe trials were conducted 24 hours after the last training trial, during which the platform was removed and mice were free to swim. The training and probe trials were recorded by a video camera mounted on the ceiling, and data were analyzed using the EthoVisioXT tracking system.
Protein extraction
Proteins were extracted as previously described (Caccamo et al. 2010b). Briefly, mice were sacrificed by CO2 asphyxiation and their brains extracted and frozen in dry ice for biochemical analysis. Frozen hemi-brains were homogenized in a solution of tissue protein extraction reagent (T-PER, Pierce, Rockford, IL) containing 0.7 mg/ml Pepstatin A supplemented with a complete Mini protease inhibitor tablet (Roche, Switzerland) and phosphatase inhibitors (Invitrogen, Carlsbad, CA). The homogenized mixes were centrifuged at 4°C for 1 hour at 100,000g. The supernatant was stored as the soluble fraction and used for Western blot and cytokine measurements (see below). For selective hippocampal extractions, hippocampi were dissected from frozen hemi-brains and homogenized in the same solution used for the whole brain using a manual pestle before centrifugation.
Western blot
For Western blot analysis proteins were resolved under reducing conditions in Bis-Tris SDS/PAGE precast gels (Invitrogen, Carlsbad, CA) of different concentrations. Proteins were then transferred to a nitrocellulose membrane, which was subsequently incubated in 5% solution of non-fat milk for 1 hour at 20°C. The proper primary antibody was then added and incubated overnight at 4°C. Blots were then washed in Tween 20-TBS (T-TBS) (0.02% Tween 20, 100 mM Tris pH 7.5; 150 nM NaCl) for 20 minutes and incubated at room temperature with the appropriate secondary antibody. The blots were washed in T-TBS for 20 minutes and incubated for 5 minutes with Super Signal (Pierce, Rockford, IL), washed again and exposed.
Measurement of Cytokine Production
The presence of cytokines in the brain lysates was assessed using a standard Luminex-based assay performed with the BioRad 8-plex panel, which included IL-1β, IL-2, IL-4, IL-5, IL-10, IFN-γ, GM-CSF, and TNF-α. The filter plates were prepared according to the manufacturer's protocol and 50 μl of each brain lysate was tested in duplicate. The cytokine standard provided with the 8-plex panel was subjected to serial 4-fold dilutions in the T-PER medium used for the brain lysates and tested in parallel. The filter plates were then read on a Bio-Plex 200 System and the signals were analyzed using the Bio-Plex Manager 5.0 software using standard protocols and settings. The fluorescence intensities and concentrations of the individual cytokines (picogram per milliliter) were determined for each sample.
Statistical analyses
Learning data were analysed using two-way analysis of variance (ANOVA), while the probe trials and the other biochemical data were analyzed using one-way ANOVA. These tests were followed by a post hoc Bonferroni test to determine individual differences among groups. Tests were performed using GraphPad Prism version 3.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
Acknowledgements
This work was supported by NIA grant awards: K99/R00AG29729-4 and R01AG037637 (Oddo, Principal Investigator); RC2AG036613 (Richardson, Principal Investigator, Strong and Oddo, Project Co-leaders), and by an award from the Glenn Foundation to S.O.
Footnotes
Author contribution S.M. performed most of the experiments, including the mouse treatment and the behavioral analyses. A.C. performed the protein extractions and assisted with the writing of the manuscript. D.X.M. helped performing some of the Western blots experiments. A.D.B. performed the cytokine measurements. M.A.J. measured the rapamycin levels in the blood. E.K. helped design and conducted the cytokine measurements, and edited the manuscript. R.S. provided the rapamycin and the control diets and edited the manuscript. A.R. contributed to the data analysis and the manuscript preparation. S.O. designed the experiments, analyzed and interpreted the data and wrote the manuscript.
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Human molecular genetics. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nature neuroscience. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. The Journal of biological chemistry. 2010a;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A, Oddo S. Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. The Journal of biological chemistry. 2011;286:8924–8932. doi: 10.1074/jbc.M110.180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Demeny PG, McNicoll G. Encyclopedia of population. Macmillan Reference USA; New York: 2003. [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature medicine. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE, Longo VD, de Magalhaes JP. Cell resilience in species life spans: a link to inflammation? Aging Cell. 2010;9:519–526. doi: 10.1111/j.1474-9726.2010.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes & development. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Roth GS. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp Gerontol. 2011;46:148–154. doi: 10.1016/j.exger.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Janes MR, Fruman DA. Immune regulation by rapamycin: moving beyond T cells. Sci Signal. 2009;2:pe25. doi: 10.1126/scisignal.267pe25. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development (Cambridge, England) 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science (New York, N.Y. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nature reviews. Neuroscience. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nature reviews. Neuroscience. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transpl. 2001;7:473–484. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- O'Donnell E, Vereker E, Lynch MA. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. The European journal of neuroscience. 2000;12:345–352. doi: 10.1046/j.1460-9568.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes & development. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature neuroscience. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science (New York, N.Y. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ZD, Strong R. The role of mTOR signaling in controlling mammalian life span: what a fungicide teaches us about longevity. J Gerontol A Biol Sci Med Sci. 2010;65:580–589. doi: 10.1093/gerona/glp212. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nature neuroscience. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature neuroscience. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, McLinden KA, Kranjac D, Kohman RA, Amaral W, Boehm GW. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1beta expression. Behavioural brain research. 2011;217:481–485. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, Gundelfinger ED. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. The European journal of neuroscience. 2003;18:942–950. doi: 10.1046/j.1460-9568.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- Trompet S, de Craen AJ, Slagboom P, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Ford I, Gaw A, Macfarlane PW, Packard CJ, Stott DJ, Jukema JW, Westendorp RG. Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain : a journal of neurology. 2008;131:1069–1077. doi: 10.1093/brain/awn023. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Viviani B, Boraso M. Cytokines and neuronal channels: a molecular basis for age-related decline of neuronal function? Experimental gerontology. 2011;46:199–206. doi: 10.1016/j.exger.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. The Journal of biological chemistry. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Warner HR. Time, damage, and aging: what really matters? Rejuvenation Res. 2007;10:373–375. doi: 10.1089/rej.2007.0595. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]