Summary
Over the last ten years, various screens of small molecules have been conducted to find long-sought interventions in aging. Most of these studies were performed in invertebrates but the demonstration of pharmacological lifespan extension in the mouse has created considerable excitement. Since aging is a common risk factor for several chronic diseases, there is a reasonable expectation that some compounds capable of extending lifespan will be useful for preventing a range of age-related diseases. One of the potential targets is protein aggregation which is associated with several age-related diseases. Genetic studies have long indicated that protein homeostasis is a critical component of longevity but recently a series of chemicals have been identified in the nematode Caenorhabditis elegans that lead to the maintenance of the homeostatic network and extend lifespan. Here we review these interventions in C. elegans and consider the potential of improving health by enhancing protein homeostasis.
Introduction
The demographic shift toward an older population is a global issue that affects or will soon affect virtually every region around the world. Aging is the single largest risk factor for chronic disease in developed countries and is consequently responsible for tremendous social and economic burden. The development of preventative therapies aimed at reducing or delaying age-related disease must be a priority for the biomedical community but the traditional models of drug discovery are failing when it comes to the major chronic diseases of the elderly. The disappointing outcomes of dozens of phase III clinical trials in Alzheimer’s disease, Parkinson’s disease and others suggests that preclinical studies in animal models are less relevant that we would hope. This has led to ask whether targeting aging mechanisms would lead to better outcomes. The rationale for this is based on the observation that aging is a major risk factor for many human diseases and the mechanistic similarities between aging and disease pathology. The large number of genes that influence the aging of model organisms such as D. melanogaster, S. cerevisiae and C. elegans could provide some insight into this issue. These genes encode a wide variety of intracellular signaling processes and many of them are orthologous to human genes known to influence disease progression through endocrine signaling, cell cycle checkpoint functions and protein turnover. This at least is consistent with a mechanistic relationship between aging and disease. Furthermore, some pathological features of certain diseases are now being seen as a more general feature of aging. Perhaps the clearest example of this is the failure of protein homeostasis, associated with age-related neurological disease, which leads to the formation of intra- or extracellular protein aggregates. Aggregate formation is a long-studied common feature of many diverse human diseases, especially neurodegenerative conditions where aberrant forms of proteins such as α-synuclein (Parkinson’s), β-amyloid (Alzheimer’s) and huntingtin (Huntington’s) may contribute to disease progression (Selkoe 2003) but also in non-neurological systemic diseases like type II diabetes and several myopathies. For a while, the protein aggregates themselves were considered to be the toxic insult leading to cell death but it now seems likely that soluble aggregate precursors such as soluble oligomers or fibrils create problems by influencing cell function (Kopito & Ron 2000).
It is now becoming clear that loss of protein homeostasis is a general feature of aging. Even in the pre-genetic era of aging studies the accumulation of conformationally altered proteins was observed during aging often in the form of a complex mixture of lipids, carbohydrates, fluorescent pigments and aggregates of oxidized proteins within lysosomes (Ames et al. 1993; Porta 2002). More recently, the biochemical composition of age-related protein alterations has been probed with techniques commonly applied to neurological disease proteins. Hundreds of proteins with diverse functions were found in detergent-insoluble extracts from old but not young C. elegans worms. (David et al. 2010; Reis-Rodrigues et al. 2011). Moreover, reduction of the expression of many genes encoding proteins that become insoluble during aging results in extended lifespan consistent with a connection between the aggregation process and aging (David et al. 2010; Reis-Rodrigues et al. 2011).
These studies have demonstrated that a process long associated with age-related neurodegenerative disease is a feature of general aging. This is consistent with the fact that a number of genes modulate both longevity and the onset of age-related aggregation of neurotoxic proteins and suggests that the loss of protein homeostasis provides a common mechanism of aging and disease. Although it is not clear why protein aggregation occurs, alterations in the balance of protein synthesis, protein folding and protein degradation all likely play important roles in this process. Numerous studies of longevity in C. elegans provide ample evidence that these processes are critical longevity determinates. For example, genes encoding translational machinery, molecular chaperones, autophagy, the ER unfolded protein response and proteosomal functions all either directly affect lifespan or are required for lifespan extension in some long-lived mutants. It follows that pharmacologically targeting age-related loss in protein homeostasis could reduce and/or postpone age-related disease pathology and extend lifespan.
Identifying compounds that slow aging
A small number of compounds have been tested for their ability to increase lifespan and improve healthspan in mammals. A few compounds appear to conditionally extend lifespan in mice. For example, nordihydroguaiaretic acid and aspirin (2-acetoxybenzoic acid) increase lifespan of male but not female mice (Strong et al. 2008). The difference may be due to sex-specific pharmacokinetics; perhaps different doses would reveal lifespan extension for both sexes. Some studies have been prompted by genetically defined targets such as SIRT1 and mTOR signaling. For example, the immunosuppressant rapamycin inhibits mTOR signaling and is able to increase lifespan in both male and female mice when administered late in life (Harrison et al. 2009; Miller et al. 2011). In addition, the polyphenol resveratrol (3,5,4'-trihydroxy-trans-stilbene), a natural stilbene found in some fruits and originally suggested to be an activator of NAD+-dependent protein deacetylases of the sirtuin family, improves some health indicators and increases the lifespan of mice on a high-calorie diet, but fails to increase lifespan in mice on a standard diet (Baur et al. 2006; Miller et al. 2011). These mouse studies of resveratrol and rapamycin in mice were prompted by earlier observations of lifespan extension in model organisms.
Despite these efforts to uncover compounds with anti-aging properties in mammals, the number of compounds identified so far is rather small and the results are not exempt from some controversy. High throughput screens for enhanced longevity in mice would be extremely expensive and time consuming, therefore; there is much interest in refining our search of chemical structures by exploiting the invertebrate aging models to accelerate the discovery of compounds with potential activity in mammals.
An important role for C. elegans
Our rich knowledge of aging through gene regulation in C. elegans often overshadows the utility of the worm for small molecule screening. Several screens have been performed on these worms to undercover molecules with a broad range of activities like anti-helminthic, compounds with neuronal activity and antifungals, among others (Novak & Vanek 1992; Davies & McIntire 2004; Kwok et al. 2006; Tampakakis et al. 2008; Moy et al. 2009; Lemieux et al. 2011). Additionally, a number of pharmacological interventions on the aging process have been demonstrated in this system (Harrington & Harley 1988; Adachi & Ishii 2000; Melov et al. 2000; Fei et al. 2003; Evason et al. 2005; Benedetti et al. 2008; McColl et al. 2008; Srivastava et al. 2008; Pietsch et al. 2009; Saul et al. 2009; Honda et al. 2010; Onken & Driscoll 2010; Alavez et al. 2011; Powolny et al. 2011). When hits are discovered there is considerable value in combining compound treatments with genetic manipulation (pharmacogenetics), transcript profiling (pharmacogenomics) and metabolomics. In so doing, the mechanism of compound action can be elucidated and its potential as antiaging or therapeutic drug further evaluated in a mammal model.
Many of the C. elegans small molecule studies to date have focused on the effects of natural products. These compounds are of particular interest thanks to the notion that natural products are more likely to exhibit significant biological effects than synthetic compounds. There are anecdotal accounts suggesting that hits derived from screens of diverse chemical libraries are enriched for natural products. This may be due to the fact that organisms may have evolved in the presence of such compounds and consequently evolved specific metabolic responses. However, they also tend to be non-drug like in their structure and chemical properties.
Examples of natural products that extend lifespan include extracts from Ginkgo biloba (Wu et al. 2002) and blueberry phenols, both of which also increase stress resistance (Wilson et al. 2006). Resveratrol has been reported to increase lifespan in Drosophila and C. elegans in some laboratories but not in others (Bass et al. 2007). Curcumin, the main component of the Indian spice turmeric, is also able to increase lifespan through a mechanism that involves the regulation of protein homeostasis (Alavez et al. 2011). Recently, the disaccharide trehalose has also been reported to increase lifespan in C. elegans through a mechanism that involves daf-2 suppression (Honda et al. 2010). Finally, the garlic constituent diallyl trisulfide increases the lifespan of C. elegans by enhancing the activity of the pro-longevity transcription factor, SKN-1 (Powolny et al. 2011).
The increase in C. elegans lifespan elicited by the treatment with scavengers such as vitamin E (Harrington & Harley 1988), flavonoid component tamarixetin (Wu et al. 2002), lipoic acid (Benedetti et al. 2008) or synthetic superoxide dismutase/catalase mimetics (Melov et al. 2000) suggest that free radicals could play a role during the aging process. However, the increase in lifespan elicited by antioxidants tends to be variable and not free of controversies (Keaney & Gems 2003; Benedetti et al. 2008). Additionally, it has not been demonstrated that the lifespan extension observed with such compounds results from antioxidant activity; these compounds could modulate alternative signaling pathways that may be responsible for the increased lifespan.
Genetic evidence showing the relevance of the nervous system in controlling lifespan has been found in C. elegans. Mutants that show alterations in the ciliated sensory neurons, the cells specialized to sense environmental signals in worms, or in sensory signal transduction have an increased lifespan (Apfeld & Kenyon 1999; Alcedo & Kenyon 2004). In line with this idea, an interesting study showing that the nervous system could have a preponderant role in controlling aging has been reported (Evason et al. 2005). A screen of 19 FDA-approved drugs showed that the anticonvulsant ethosuximide and several derivatives were able to increase lifespan in worms. Additional evidence for this notion came from a study where more than 80 000 compounds were tested for their ability to increase lifespan (Petrascheck et al. 2007). This study revealed that lifespan can be extended by compounds that interfere with serotoninergic neurotransmission involved in food sensing.
Taken together, more compounds are known to extend the lifespan of C. elegans than any other species. Considering the large number of genes known to modulate worm lifespan, it is not too surprising that multiple compounds have been discovered using this model. Whether such compounds have similar activities in mammals remains to be seen but meanwhile we can ask whether pharmacological interventions that extend lifespan also affect other aspects of aging including age-related disease pathology.
Relevance of worm models of age-related diseases
In addition to the multiple benefits and resources for exploitation of the worm system as a platform for drug discovery, worms have also been genetically engineered to express human disease-associated proteins such as β-amyloid (Link 1995; Link et al. 2003), polyglutamine (Gidalevitz et al. 2006), α-synuclein (Hamamichi et al. 2008) or aggregating forms of SOD-1 (Gidalevitz et al. 2009). For example, Christopher Link’s laboratory has provided the worm community with a robust model of protein aggregation in which human Aβ peptide3–42 is expressed under the control of the unc-54 promoter in muscle tissue (Link et al. 2003). Models expressing tau protein in neurons (Kraemer et al. 2003; Miyasaka et al. 2005), α-synuclein in different tissues (Lakso et al. 2003; Cao et al. 2005; Hamamichi et al. 2008; van Ham et al. 2008) and mouse prion protein (Park & Li 2008), among others, have also been generated in C. elegans. Elegant work form the laboratory of Richard Morimoto has yielded a series of transgenic worms expressing chains of polyQ of different lengths tagged to YFP driven by the myosin promoter (unc-54) that show aggregation rate dependent polyQ repeat length and a paralysis phenotype (Gidalevitz et al. 2006). Morimoto’s lab has also generated transgenic worms expressing mutated forms of human SOD-1, associated with human ALS, under control of the myo-3 promoter that also produce a similar paralysis phenotype associated with a dramatic pattern of SOD-1 aggregation (Gidalevitz et al. 2009).
These models have been used to shed light on the mechanisms modulated by several drugs used in the clinic. For example, the mechanism of action of tetracyclines, a group of compounds used clinically to treat central nervous system damage, has been advanced by using an unc-54/human Aβ3–42 minigene strain (Diomede et al. 2010). The ability of a stress response mimetic derived from hydroxylamine (NG-094) to decrease protein aggregation in a worm model of Huntington’s disease has been also reported (Haldimann et al. 2011). Additionally, trehalose reduces polyglutamine aggregation (Punc-54::q35::yfp) with no evident side effects (Honda et al. 2010). However, coffee extract improves the life conditions of the unc-54/human Aβ3–42 minigene strain in a skn-1 dependent fashion without apparent reduction on Aβ3–42 aggregation (Dostal et al. 2010). This suggests the participation of detoxification, antioxidant or other alternative activities in the protection mediated by this extract. Recently, we exploited some of these models as well as others expressing mutated metastable endogenous worm proteins previously used as indicators of protein homeostasis (Zengel & Epstein 1980; Anderson & Brenner 1984) to test several compounds that we identified with pro-longevity properties (Alavez et al. 2011). We found that these compounds significantly decreased the paralysis phenotype associated with protein aggregation in these models through a mechanism that requires components of the protein homeostasis network. In line with this observation, we found that the effect on lifespan, at least for the compound with a major impact on longevity, Thioflavin T, depends on two transcription factors that have long been associated with the control of stress resistance and longevity, Heat Shock Factor 1 (HSF-1) (Hsu et al. 2003) and SKN-1 (Tullet et al. 2008). These results support the idea that the modulation of protein homeostasis could play a relevant role in controlling aging. In line with this hypothesis several compounds that increase lifespan in C. elegans are also shown able to ameliorate the toxicity of several of these protein aggregation models (Table 1).
Table 1.
Compounds that increase lifespan and modulate protein aggregation in C. elegans
| Compound | Increase in LS | Model of aggregation | Potential Mechanism | Reference |
|---|---|---|---|---|
| EGb761 | 8% | Aβ toxicity | Stress resistance | Wu et al. 2006 |
| Reserpine | 31% | Aβ toxicity | Stress resistance, requires Ser | Srivastava et al. 2008 |
| Trehalose | 30% | PolyQ toxicity, lipofuscin | Stress resistance, DAF-16 dependent | Honda et al. 2010 |
| Metformin | 40% | AGE pigments | DR mimetic, SKN-1 dependent | Onken et al. 2010 |
| Thioflavin T | 45% | Aβ and PolyQ toxicity | Protein homeostasis HSF-1 and SKN-1 | Alavez et al. 2011 |
| Curcumin | 30% | Aβ and PolyQ toxicity | Protein homeostasis | Alavez et al. 2011 |
| Rifampicin | 30% | Aβ and PolyQ toxicity | Protein homeostasis | |
| Celecoxib | 10–20% | PolyQ toxicity | COX-2 inhibition, DAF-16 dependent | Ching et al. 2011 |
| Ferulsinaic acid | 18–22% | AGE pigments | Stress resistance | Sayed 2011 |
| NG-094 | 20% (polyQ) | PolyQ toxicity | HSF-1 dependent | Haldimann et al. 2011 |
There are of course many limitations of these models and we should not underestimate the complexity of human disease by comparison to the events modeled in the worm. Indeed most of the enzymatic machinery that processes neurotoxic proteins is not present in the worm and in most cases the worms lack critical modulators of disease progress such as complex inflammatory responses.
If this turns out that compounds that slow aging also slow disease progression, it will strongly suggest that aging and age-related disease pathology are closely related and therefore interventions that slow aging should be investigated in a range of age-related disease models. The worm models of age-related disease provide a useful starting point, where we can refine approaches for mouse disease models (Teschendorf & Link 2009).
Taken together, these results suggest that the protein homeostatic network can be manipulated by using small molecules to suppress age-related disease pathologies and highlight some important concepts for a novel approach to the discovery of new drugs with the potential to modulate aging and improve the conditions of conformational diseases (Fig. 1). However, it is important to consider that compounds that affect the dynamics and patterns of protein aggregation could also generate some soluble oligomeric species potentially toxic to the cell. Additionally, at the concentration required to affect protein aggregation, some compounds could have off-target effects acting in parallel to produce additional deleterious effects on the cell physiology. Therefore, caution ought to be taken when considering compounds identified through this experimental approach for use as potential therapeutic drugs.
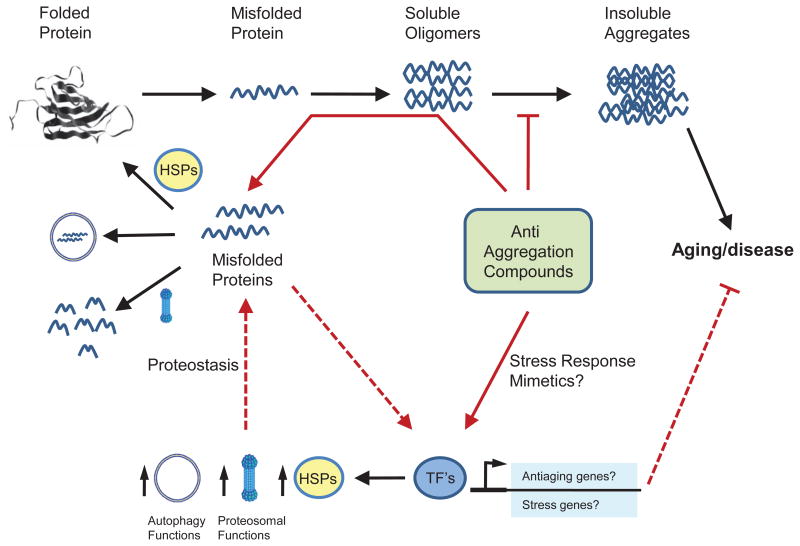
Figure 1. Model depicting the potential mechanisms influenced by compounds with anti-aggregation properties in C. elegans.
These compounds may act as a stress response mimetic that induces some components of the protein homeostasis network. This induction may involve the participation of diverse transcription factors (TF’s), including HSF-1, SKN-1 and DAF-16, that could both activate the protein homeostasis response or favor the expression of anti-aging or stress responsive genes. Alternatively, they may act directly on the misfolded cascade to affect the spatial and temporal distribution as well as the size of protein aggregates. All the potential actions of these compounds are marked with red arrows. Symbols representing autophagy, proteasomal functions and heat shock proteins (HSP’s) exemplify the requirement of protein homeostasis for suppression of protein aggregation by some compounds. Dashed red arrows represent potential indirect actions of these compounds.
Is protein homeostasis necessary for chemically induced lifespan extension?
The relevance of protein homeostasis with respect to aging and disease has been eloquently discussed elsewhere. However, it is not yet clear whether enhancing protein homeostasis is sufficient or necessary for lifespan extension. Genetic exploration of this issue is underway but small molecules may also prove useful. We previously noted that lithium, a drug frequently used in the treatment of bipolar disorder, extends lifespan in C. elegans and suggested that an epigenetic mechanism was at play (McColl et al. 2008). Lithium also appears to suppress protein aggregation in a cell line derived from human neurons; in this case the aggregation of α-synuclein, a protein associated with Parkinson’s disease (Kim et al. 2011). Moreover, lithium decreases the aggregation of polyQ in Drosophila (Berger et al. 2005) and rescues from polyglutamine induced cell death in neuronal and nonneuronal cell lines (Carmichael et al. 2002). We have recently tested lithium in additional aggregation models including the strain expressing human Aβ peptide3–42 under the control of a muscle specific (myo-3) promoter in the muscle tissue and find that it effectively suppresses paralysis associated with Aβ aggregation (unpublished data).
The fact that such diverse agents as lithium and ThT both extend lifespan and suppress protein aggregation is leading us to test additional structurally and functionally diverse compounds. Preliminary data suggest that many compounds that extend lifespan also suppress paralysis associated with protein aggregation. This raises the possibility that maintenance of protein homeostasis is necessary for lifespan extension. It will be difficult to prove that protein homeostasis is sufficient for lifespan extension because of the challenge of identifying “off target” effects of compounds. However, further research on the mechanisms of individual compounds should reveal the extent of influence that protein homeostasis has on aging pathology and rates.
Prospects
It seems possible that prevention and cures of age-related diseases will emerge from chemical screens that initially target aging. A remarkable effort to uncover this kind of compounds for use in mammals is currently going on through the National Institute of Aging’s “Aging Intervention Testing Program” (AITP). This multi-institutional program is devoted to the investigation of treatments with the potential to extend lifespan and delay disease and dysfunction in mice (Harrison et al. 2009; Miller et al. 2011). What the AITP and other mammalian researchers need are good candidate compounds with a mechanistic understanding of how these compounds can slow aging before undertaking the more expensive and lengthy mouse longevity studies. The worm and other invertebrate models can provide these. There are strong indications that approaches that target the maintenance protein homeostasis could be an excellent way forward. In the meantime the use of model systems, particularly C. elegans, could be of great help to undercover new drugable pathways and to unravel the complex connections between aging and disease.
Acknowledgments
We would like to thank Aric N. Rogers and David J. S. Zucker for helpful discussion. G.J.L. is supported by the NIH AG21069, AG22868, AG029631-01A1, ES016655, the Larry L. Hillblom Foundation and UL1 RR024917. S.A. was supported by the U19AGO231222 from the Longevity Consortium.
References
- Adachi H, Ishii N. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2000;55:B280–285. doi: 10.1093/gerona/55.6.b280. [DOI] [PubMed] [Google Scholar]
- Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Brenner S. A selection for myosin heavy chain mutants in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1984;81:4470–4474. doi: 10.1073/pnas.81.14.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti MG, Foster AL, Vantipalli MC, White MP, Sampayo JN, Gill MS, Olsen A, Lithgow GJ. Compounds that confer thermal stress resistance and extended lifespan. Experimental gerontology. 2008;43:882–891. doi: 10.1016/j.exger.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ttofi EK, Michel CH, Pasco MY, Tenant S, Rubinsztein DC, O'Kane CJ. Lithium rescues toxicity of aggregate-prone proteins in Drosophila by perturbing Wnt pathway. Hum Mol Genet. 2005;14:3003–3011. doi: 10.1093/hmg/ddi331. [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem. 2002;277:33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, McIntire SL. Using C. elegans to screen for targets of ethanol and behavior-altering drugs. Biol Proced Online. 2004;6:113–119. doi: 10.1251/bpo79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A, Doglia SM, De Luigi A, Salmona M. Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol Dis. 2010;40:424–431. doi: 10.1016/j.nbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Dostal V, Roberts CM, Link CD. Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of beta-amyloid peptide toxicity. Genetics. 2010;186:857–866. doi: 10.1534/genetics.110.120436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–262. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Inoue K, Ganapathy V. Structural and functional characteristics of two sodium-coupled dicarboxylate transporters (ceNaDC1 and ceNaDC2) from Caenorhabditis elegans and their relevance to life span. J Biol Chem. 2003;278:6136–6144. doi: 10.1074/jbc.M208763200. [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Krupinski T, Garcia S, Morimoto RI. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet. 2009;5:e1000399. doi: 10.1371/journal.pgen.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann P, Muriset M, Vigh L, Goloubinoff P. The Novel Hydroxylamine Derivative NG-094 Suppresses Polyglutamine Protein Toxicity in Caenorhabditis elegans. J Biol Chem. 2011;286:18784–18794. doi: 10.1074/jbc.M111.234773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc Natl Acad Sci U S A. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Tanaka M, Honda S. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging cell. 2010;9:558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Keaney M, Gems D. No increase in lifespan in Caenorhabditis elegans upon treatment with the superoxide dismutase mimetic EUK-8. Free Radic Biol Med. 2003;34:277–282. doi: 10.1016/s0891-5849(02)01290-x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Rane A, Lussier S, Andersen JK. Lithium protects against oxidative stress-mediated cell death in alpha-synuclein-overexpressing in vitro and in vivo models of Parkinson's disease. J Neurosci Res. 2011;89:1666–1675. doi: 10.1002/jnr.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci U S A. 2003;100:9980–9985. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok TC, Ricker N, Fraser R, Chan AW, Burns A, Stanley EF, McCourt P, Cutler SR, Roy PJ. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Liu J, Mayer N, Bainton RJ, Ashrafi K, Werb Z. A whole-organism screen identifies new regulators of fat storage. Nat Chem Biol. 2011;7:206–213. doi: 10.1038/nchembio.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, Wood DE, Sahagan BG. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer's disease model. Neurobiology of aging. 2003;24:397–413. doi: 10.1016/s0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J Biol Chem. 2008;283:350–357. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T, Ding Z, Gengyo-Ando K, Oue M, Yamaguchi H, Mitani S, Ihara Y. Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis. 2005;20:372–383. doi: 10.1016/j.nbd.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Moy TI, Conery AL, Larkins-Ford J, Wu G, Mazitschek R, Casadei G, Lewis K, Carpenter AE, Ausubel FM. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem Biol. 2009;4:527–533. doi: 10.1021/cb900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Vanek Z. Screening for a new generation of anthelminthic compounds. In vitro selection of the nematode Caenorhabditis elegans for ivermectin resistance. Folia Microbiol (Praha) 1992;37:237–238. doi: 10.1007/BF02933156. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Li L. Cytoplasmic expression of mouse prion protein causes severe toxicity in Caenorhabditis elegans. Biochem Biophys Res Commun. 2008;372:697–702. doi: 10.1016/j.bbrc.2008.05.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–556. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- Pietsch K, Saul N, Menzel R, Sturzenbaum SR, Steinberg CE. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology. 2009;10:565–578. doi: 10.1007/s10522-008-9199-6. [DOI] [PubMed] [Google Scholar]
- Porta EA. Pigments in aging: an overview. Annals of the New York Academy of Sciences. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Experimental gerontology. 2011;46:441–452. doi: 10.1016/j.exger.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, Hughes RE. Proteomic Analysis of Age-dependent Changes in Protein Solubility Identifies Genes that Modulate Lifespan. Aging cell. 2011 doi: 10.1111/j.1474-9726.2011.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul N, Pietsch K, Menzel R, Sturzenbaum SR, Steinberg CE. Catechin induced longevity in C. elegans: from key regulator genes to disposable soma. Mech Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Sayed AA. Ferulsinaic acid attenuation of advanced glycation end products extends the lifespan of Caenorhabditis elegans. J Pharm Pharmacol. 2011;63:423–428. doi: 10.1111/j.2042-7158.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Arya U, SoundaraRajan T, Dwivedi H, Kumar S, Subramaniam JR. Reserpine can confer stress tolerance and lifespan extension in the nematode C. elegans. Biogerontology. 2008;9:309–316. doi: 10.1007/s10522-008-9139-5. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampakakis E, Okoli I, Mylonakis E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protoc. 2008;3:1925–1931. doi: 10.1038/nprot.2008.193. [DOI] [PubMed] [Google Scholar]
- Teschendorf D, Link CD. What have worm models told us about the mechanisms of neuronal dysfunction in human neurodegenerative diseases? Mol Neurodegener. 2009;4:38. doi: 10.1186/1750-1326-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Smith JV, Paramasivam V, Butko P, Khan I, Cypser JR, Luo Y. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell Mol Biol (Noisy-le-grand) 2002;48:725–731. [PubMed] [Google Scholar]
- Zengel JM, Epstein HF. Identification of genetic elements associated with muscle structure in the nematode Caenorhabditis elegans. Cell motility. 1980;1:73–97. doi: 10.1002/cm.970010107. [DOI] [PubMed] [Google Scholar]