Abstract
Objective
It is unclear whether high fructose corn syrup (HFCS), which contains a higher amount of fructose and provides an immediate source of free fructose, induces greater systemic concentrations of fructose as compared to sucrose. It is also unclear whether exposure to higher levels of fructose leads to increased fructose-induced adverse effects. The objective was to prospectively compare the effects of HFCS- versus sucrose-sweetened soft drinks on acute metabolic and hemodynamic effects.
Materials/Methods
Forty men and women consumed 24 oz of HFCS- or sucrose-sweetened beverages in a randomized crossover design study. Blood and urine samples were collected over 6 hr. Blood pressure, heart rate, fructose, and a variety of other metabolic biomarkers were measured.
Results
Fructose area under the curve and maximum concentration, dose normalized glucose area under the curve and maximum concentration, relative bioavailability of glucose, changes in postprandial concentrations of serum uric acid, and systolic blood pressure maximum levels were higher when HFCS-sweetened beverages were consumed as compared to sucrose-sweetened beverages.
Conclusions
Compared to sucrose, HFCS leads to greater fructose systemic exposure and significantly different acute metabolic effects.
Keywords: soft drinks, sweetened beverages, adverse metabolic effects, carbohydrate metabolism
INTRODUCTION
Over the past four decades, the prevalence of health disorders, including hypertension, obesity, metabolic syndrome, diabetes, and kidney disease, has drastically increased. In the United States, one-third of the population has hypertension, one-third of adults and one-sixth of children are obese, 7% have diabetes, and about 20 million have kidney disease [1–5]. In parallel to the dramatic rise in the prevalence of these cardio-renal diseases, a similar increase in the consumption of fructose has occurred. Recent studies have implicated excessive fructose intake as one of the factors driving the increases in these health disorders [3, 6–8].
Rapidly metabolized by the body, fructose has been shown to cause a variety of metabolic effects, such as lactic acidosis, lipogenesis, hypertriglyceridemia, liver injury, high blood pressure, insulin resistance, and increased weight gain [7, 9–15]. Fructose is also the only natural sugar capable of causing a rise in uric acid levels [16]. Thus, there is a growing concern that fructose may pose a great health risk and several studies have suggested that the excessive consumption of fructose-containing sweeteners, regardless of its composition, may be a contributing factor in the pathogenesis of cardio-renal diseases [6, 12, 13, 17–23].
While fructose is a simple sugar that exists naturally in fruits and vegetables, the majority of dietary fructose comes from two sweeteners, sucrose and high fructose corn syrup (HFCS), which are commonly used in manufactured foods and beverages. Specifically, the increase in fructose consumption is primarily due to the increased use of HFCS in the Western diet. Based upon disappearance data, the annual per capita intake of HFCS from 1967 to 2006 increased from 0.03 lbs to 58.2 lbs, while sucrose decreased from 98.5 lbs to 62.3 lbs [6, 24].
Sucrose is a disaccharide and consists of 50% fructose and 50% glucose. The HFCS grade used in soft drinks consists of 55% fructose, 42% glucose, and 3% oligosaccharides [25]. Because of the higher fructose dose, soft drinks sweetened with HFCS would provide more fructose into the systemic circulation than soft drinks sweetened with sucrose. Furthermore, HFCS provides an immediate source of free fructose and glucose, while sucrose must first be broken down by sucrase. The expression and function of sucrase has been shown to be negatively influenced by such factors as genetic polymorphisms and regulatory inhibition by glucose [26–29].
Due to the potential inefficiency of sucrase, we hypothesized that the amount of fructose available for absorption is reduced, resulting in a lower relative fructose bioavailability from sucrose. Therefore, we speculated that higher fructose systemic concentrations, either through the higher fructose dose or also from increased fructose bioavailability from HFCS, would lead to increased fructose-induced adverse metabolic effects. Thus, the aim of the present study was to conduct a prospective randomized crossover study comparing the effects of HFCS- versus sucrose-sweetened beverages on the pharmacokinetics of fructose and acute metabolic and hemodynamic changes.
SUBJECTS AND METHODS
Subjects
Sixty-nine healthy subjects, aged 18 years or older, of either gender, and of any ethnicity were recruited to participate in the study through advertisements or from the study participant database. During a screening visit, a participant’s eligibility was ascertained through a health information questionnaire and limited laboratory analyses. Specifically, individuals with a history of hypoglycemia, gout, hepatic or renal disease, diabetes mellitus, or who had a fasting blood glucose level ≥ 126 mg/dl or random blood glucose ≥ 200 mg/dl at the screening visit were excluded from the study. Subjects who consumed more than 7 alcoholic drinks per week, who took medication (except for oral contraceptives), who were pregnant or lactating, or who donated blood within 8 weeks prior to the screening visit were also excluded. Blood glucose levels were determined using the OneTouch Ultra Test Strips and OneTouch Ultra 2 Blood Glucose Meter (LifeScan, Inc., Milpitas, CA). The study was approved by the University of Florida IRB and all study participants signed informed, written consent.
Study Design
The study was a prospective, randomized, single-blinded, crossover trial. Acute changes in metabolic and hemodynamic parameters, such as fructose, glucose, and uric acid concentration, were measured in participants over a 6-hr period on 2 separate study visits. Qualified participants were randomized in blocks of four, using Proc Plan in SAS 9.1.3. Subjects were randomized to two different sequences. Subjects randomized to the first sequence received HFCS-sweetened soft drinks at study visit 1 and sucrose-sweetened soft drinks at study visit 2. Subjects randomized to the second sequence received sucrose-sweetened soft drinks at study visit 1 and HFCS-sweetened soft drinks at study visit 2. The two 6-hr study visits were separated by a minimum of 2 days and were conducted at the Clinical Translational Science Institute (CTSI) at the University of Florida, Gainesville, FL. Both the subjects and CTSI nurses were blinded to the sweetener contained in the soft drinks.
Sugar Load from Soft Drinks
As mentioned, the majority of dietary fructose is currently ingested as HFCS and sucrose. Since soft drinks are a major source of added sugar, we elected to treat our participants with a beverage that was manufactured with either HFCS or sucrose [30, 31]. Dr Pepper sweetened with HFCS was purchased locally (Lot# NOV 24 08 12:33 to 12:58RS02218X). Dr Pepper sweetened with cane sugar (sucrose) was purchased from the Dr Pepper Bottling Company (Lot# 800807:11 TBC, http://www.dublindrpepper.com/, Dublin, TX). Except for the sweetener, the compositions of the two Dr Pepper products were similar. Sugar profiles of the two types of Dr Pepper were analyzed before and after the end of the study by Silliker, Inc. (Illinois Laboratory, Chicago Heights, IL). From 24 oz of the soft drinks, the total sugar load from the HFCS-sweetened beverage was 68.0 g and from the sucrose-sweetened beverage was 69.4 g (Table 1). The HFCS-sweetened beverage contained 39.2 g of fructose and 28.8 g of glucose. Meanwhile, the sucrose-sweetened beverage contained 34.6 g of fructose and 34.8 g of glucose. Thus, there were about 5 more grams of fructose in the 24 oz of HFCS-sweetened soft drink, resulting in about a 13% higher dose.
Table 1.
Carbohydrate amounts in HFCS- and sucrose-sweetened soft drinks.
| Sweetener | Carbohydrate | Amount (g in 24 oz)
|
|
|---|---|---|---|
| Before Study | After Study | ||
| HFCS | Fructose | 41.6 ± 0.1 | 36.8 ± 0.3 |
| Glucose | 30.2 ± 0.2 | 27.4 ± 0.1 | |
| Sucrose | BLD | BLD | |
| Sucrose | Fructose | 27.1 ± 0.4 | 32.2 ± 0.1 |
| Glucose | 27.4 ± 0.4 | 32.2 ± 1.9 | |
| Sucrose | 20.0 ± 0.3 | BLD | |
BLD below level of detection; HFCS high fructose corn syrup. For each sugar analysis, three 12 oz cans were used. For each Data given as mean ± standard deviation.
Study Protocol
Subjects were instructed to abstain from consuming alcohol for three days prior to each study visit. Following a minimum of an 8-hr overnight fast and no exercising, the participants reported to the CTSI in the morning. They were then assigned to a hospital room and allowed to rest for 15 min before measurement of blood pressure (BP) and heart rate (HR). Afterwards, an intravenous catheter was inserted by a CTSI nurse. The participants were then randomly challenged with 24 oz of cold, carbonated soft drinks sweetened with either HFCS or sucrose. The soft drinks were poured into 4 cups (~6 oz/cup) and participants were given approximately 5 minutes to consume the sugar load (~75 sec/cup). Subjects were not given any additional caloric intake during the 6-hr study period.
Study Measurements
Body weight and height were measured at each study visit. Body mass index (BMI) was calculated and averaged. BP, HR, and blood samples were obtained at the following time points: 0 min (fasting), 15 min, 30 min, 60 min, 90 min, 2 hr, 3 hr, 4.5 hr, and 6 hr. BP and HR were measured using a Microlife Model #3AC1-AP blood pressure monitor (Microlife USA, Inc., Clearwater, FL), which has been approved by the British Society of Hypertension [32]. Plasma from blood collected in BD Vacutainer® tubes (BD, Franklin Lakes, NJ) containing sodium heparin were used to quantify fructose. Plasma from blood collected in tubes containing sodium fluoride and potassium oxalate were used to measure glucose and lactate. Serum triglycerides, uric acid, creatinine, and insulin were assayed from blood collected in serum separation tubes. Since the study is similar to a 75-g oral glucose tolerance test, which usually lasts for 2 hr, we measured insulin only at 0, 30 60, and 120 min [33]. Samples were immediately centrifuged and separated by CTSI technicians. Urine fructose, uric acid, and creatinine were measured from samples collected prior to treatment and a 6 hr pooled urine collection after the consumption of the soft drinks. All samples were stored at −80°C until analyses.
Laboratory Analysis
Plasma fructose concentrations were measured by an assay developed on liquid chromatography-tandem mass spectrometry (Le MT, Galloway CD, Frye RF. “Simplified method for quantifying fructose in human plasma using liquid chromatography-tandem mass spectrometry.” Unpublished data, 2009.) The fractional excretion of fructose (FE_fructose) was calculated from the following equation:
where SCreat was serum creatinine, SFr was serum fructose, UCreat was urine creatinine, and UFr was urine fructose.
Plasma glucose and lactate concentrations were measured by CTSI with the YSI 2300 STAT Plus analyzer (YSI Inc, Yellow Springs, OH). Triglcyerides (Tg), uric acid, and creatinine concentrations were analyzed with the VetACE system (Alfa Wassermann Inc., West Caldwell, NJ). Insulin concentrations were measured with an ELISA immunoassay (ALPCO Diagnostics, Salem, NH). Fractional excretion of uric acid (FEUA) was calculated from the following equation:
where SUA was serum uric acid and UUA was urine uric acid [34].
Statistical Analysis
Pharmacokinetic parameters
WinNonlin™ Professional Edition Version 2.1 (Pharsight Corporation, Mountain View, CA) was used to calculate the following pharmacokinetic parameters: area under the curve (AUC) of plasma concentration versus time, maximum observed concentration (Cmax), elimination half-life (HL), mean residence time (MRT), and time of Cmax (Tmax). Due to differences in doses, fructose and glucose AUC/D and Cmax/D were calculated by normalizing by the average doses of the respective sugars from each treatment. Noncompartmental analysis was conducted using linear/log trapezoidal as the calculation method.
Relative fructose bioavailability between sucrose and HFCS was calculated using the following equation (AUCH = AUC from HFCS, AUCS = AUC from sucrose, ClH= clearance from HFCS, ClS = clearance from sucrose, DH = dose from HFCS, DS = dose from sucrose, FH = bioavailability from HFCS, and FS = bioavailability from sucrose):
Relative glucose bioavailability was also calculated. Paired t-test was used to compare relative bioavailability.
Statistical methods
For 40 completed subjects, the study had at least 80% power at α = 0.05 two-sided to detect a paired difference in means of 0.455σ (σ = 1.00 μmol/L for fructose and σ = 66 μmol/L for serum uric acid) [35]. Data for non-fasting participants were excluded from analyses for each study visit. Non-fasting state was determined by elevated glucose, insulin, or fructose levels measured at time 0.
Linear mixed effect models for a crossover design were used to compare the effects of HFCS versus sucrose treatments on AUC, Cmax, HL, MRT, and Tmax of the various response parameters [36]. The treatment, sequence, and visit effects were assessed in the models as fixed effects with subjects within sequence as random effect. In addition, fasting values of the metabolic and hemodynamic parameters during study visits 1 and 2 were included in the model as covariates.
Linear mixed effect models were also used to compare the effects of HFCS versus sucrose treatments on the repeated measures data collected over the 6 hr study period of each of the response parameters. Treatment, time, interaction of treatment and time, and sequence were included in the models as fixed effects with fasting values as a covariate and subjects within sequence as random effect. Autoregessive (1) covariance structure was used for the repeated measures over time within each treatment and unstructured covariance structure was used for the repeated measures over two treatments within same subjects. Pre-planned contrasts were utilized to compare between the treatments at each time point. Based on the Shapiro-Wilk test, fructose, glucose, insulin, lactate, triglyceride, FE_fructose, and FEUA were not normally distributed. Analyses for these variables were based on log10 transformed data. Results were reported by back transforming the least square means and 95% confidence interval (CI). Fructose and glucose concentrations were also normalized by their respective doses from each treatment. Dose adjusted concentrations from 15 min to 6 hr time period were analyzed. All analyses were conducted using SAS 9.2. To adjust for multiple comparisons, statistical significance was defined as p < 0.005.
RESULTS
Baseline Characteristics
Fifty-one subjects, from ages 18 to 52, met the inclusion criteria and were randomized to participate in the study (Figure 1). Four subjects were withdrawn for reasons unrelated to the study. An additional seven subjects (3 after consuming HFCS-sweetened soft drinks and 4 from sucrose-sweetened soft drinks) were withdrawn after developing asymptomatic reactive hypoglycemia (mean blood glucose 52.3 ± 4.0 mg/dl) from the sugar load. Hypoglycemia was defined as blood glucose ≤ 60 mg/dl that was confirmed by two separate measurements. Overall, 40 individuals completed both study visits and their baseline characteristics are shown in Table 2.
Figure 1.
Study population. Sixty-nine subjects were recruited. Forty participants completed both treatment arms.
Table 2.
Baseline characteristics of study participants.
| Variable | Completed Subjects (n = 40) |
|---|---|
| Age | 27.1 ± 8.6 |
| Female | 24 (60.0) |
| Race | |
| White, European American | 23 (57.5) |
| Black, African American | 4 (10.0) |
| Asian | 7 (17.5) |
| Other/Multiracial | 6 (15.0) |
| BMI | 25.9 ± 4.9 |
| Glucose (mg/dL) | 81.0 ± 4.8 |
| Insulin (μIU/mL) | 9.8 ± 12.7 |
| Tg (mg/dL) | 86.5 ± 39.1 |
| SBP (mmHg) | 118.4 ± 9.7 |
| DBP (mmHg) | 75.0 ± 6.4 |
| HR (bpm) | 66.2 ± 8.3 |
| Fructose (μM) | 5.4 ± 4.5 |
| FE_fructose (%) | 55.0 ± 57.7 |
| SUA (mg/dL) | 4.9 ± 1.0 |
| FEUA (%) | 5.5 ± 2.0 |
| Lactate (mg/dL) | 0.7 ± 0.2 |
BMI body mass index; DBP diastolic blood pressure; FE_fructose fractional excretion of fructose; FEUA fractional excretion of uric acid; HR heart rate; SBP systolic blood pressure; SUA serum uric acid; Tg triglycerides. Data given as either mean ± standard deviation or n (%). For completed subjects, data for response parameters represent fasting levels at study visit 1.
Effects of HFCS versus Sucrose on Acute Metabolic and Hemodynamic Parameters
Table 3 lists the fasting levels of the various response parameters measured at the two study visits for both of the treatment sequences. The sequence and visit effects were insignificant for all of the response parameters.
Table 3.
Fasting levels of response parameters at each study visit of completed subjects.
| Parameter | Treatment Sequence
|
|||
|---|---|---|---|---|
| ALL
| ||||
| HFCS → Sucrose
|
Sucrose → HFCS
|
|||
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| Fructose (μM) | 5.4 ± 3.9 | 4.4 ± 1.4 | 5.4 ± 5.0 | 4.6 ± 2.0 |
| FE_fructose (%) | 45.9 ± 28.3 | 42.0 ± 23.2 | 62.5 ± 73.5 | 48.0 ± 35.9 |
| SUA (mg/dL) | 4.9 ± 0.9 | 4.8 ± 0.8 | 4.9 ± 1.0 | 5.0 ± 1.0 |
| FEUA (%) | 5.5 ± 1.5 | 5.1 ± 2.2 | 5.5 ± 2.3 | 5.0 ± 2.1 |
| Glucose (mg/dL) | 79.7 ± 5.3 | 81.4 ± 6.5 | 82.1 ± 4.1 | 80.3 ± 6.7 |
| Insulin (μIU/mL) | 10.4 ± 15.7 | 9.0 ± 13.1 | 9.2 ± 9.8 | 9.6 ± 10.7 |
| Lactate (mg/dL) | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.3 |
| Tg (mg/dL) | 92.2 ± 46.5 | 79.9 ± 45.7 | 81.9 ± 32.2 | 94.6 ± 44.0 |
| SBP (mmHg) | 118.1 ± 9.5 | 119.8 ± 9.5 | 118.7 ± 10.1 | 119.1 ± 10.2 |
| DBP (mmHg) | 74.7 ± 6.2 | 74.4 ± 8.5 | 75.3 ± 6.8 | 74.9 ± 6.8 |
| HR (bpm) | 66.5 ± 7.3 | 66.4 ± 7.4 | 65.9 ± 9.2 | 66.5 ± 9.3 |
DBP diastolic blood pressure; FE_fructose fractional excretion of fructose; FEUA fractional excretion of uric acid; HR heart rate; SBP systolic blood pressure; SUA serum uric acid; Tg triglycerides. Data given as mean ± standard deviation.
Fructose, FE_fructose, and Relative Bioavailabilty of Fructose
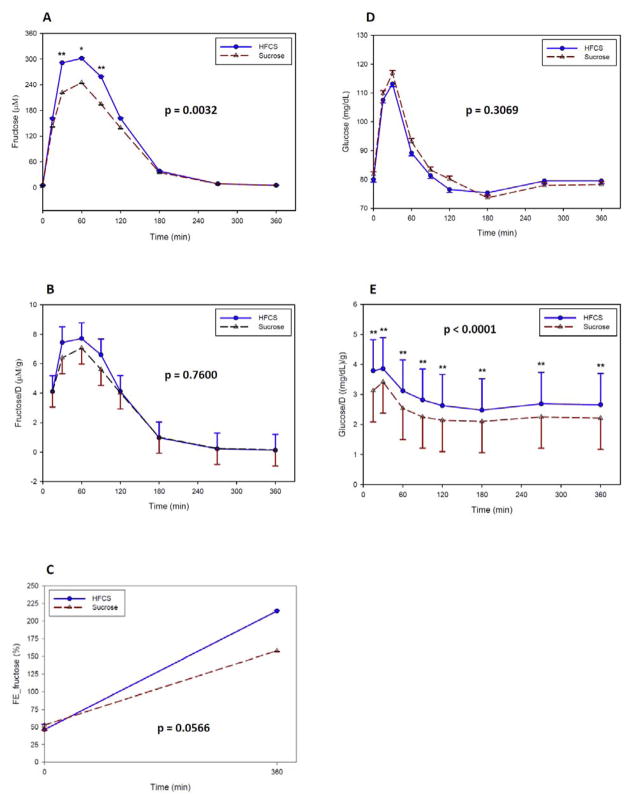
Fructose AUC was about 20% greater and Cmax was about 15% greater from the HFCS-sweetened beverages than from the sucrose-sweetened beverages (Table 4). From the repeated measures data, there was also a significant treatment effect of HFCS (p = 0.0032) versus sucrose on changes in postprandial fructose concentrations over the 6 hr study period (Figure 2A). Fructose levels were higher from the HFCS treatment at 30 and 90 min.
Table 4.
Effects of consuming HFCS- versus sucrose-sweetened beverages on fructose, FE_fructose, and glucose.
| Variable | Parameter | Treatment
|
||||
|---|---|---|---|---|---|---|
| HFCS
|
Sucrose
|
P-value | ||||
| Mean | 95% CI | Mean | 95% CI | |||
| AUC (min*μM)* | Fructose | 38791 ± 1624 | 35533 – 42049 | 32327 ± 1614 | 29087 – 35567 | <.0001 |
| AUC/D ((min*μM)/g) | Fructose | 989.5 ± 43.3 | 902.6 – 1076.3 | 934.4 ± 43.0 | 848.0 – 1020.8 | 0.1076 |
| Cmax (μM)* | Fructose | 363.4 ± 17.6 | 328.1 – 398.8 | 317.0 ± 17.5 | 281.9 – 352.2 | 0.0043 |
| Cmax/D (μM/g) | Fructose | 9.3 ± 0.5 | 8.3 – 10.2 | 9.2 ± 0.5 | 8.2 – 10.1 | 0.8039 |
| Tmax (min) | Fructose | 57.4 ± 4.2 | 49.1 – 65.8 | 59.7 ± 4.1 | 51.4 – 68.0 | 0.6172 |
| MRT (min) | Fructose | 87.8 ± 2.0 | 83.8 – 91.8 | 89.6 ± 2.0 | 85.6 – 93.5 | 0.3063 |
| Half-life (min) | Fructose | 35.4 ± 2.2 | 31.0 – 39.8 | 39.3 ± 2.2 | 34.9 – 43.7 | 0.2079 |
| Relative bioavailabilityR | Fructose | 1.07 ± 0.24 | 0.1219 | |||
| ΔFE_fructose (%) | FE_fructose | 311.8 ± 66.7 | 178.8 – 444.8 | 302.9 ± 66.0 | 171.4 – 434.4 | 0.9249 |
| AUC (min* mg/dL) | Glucose | 29911 ± 323 | 29266 – 30557 | 30053 ± 320 | 29413 – 30694 | 0.6492 |
| AUC/D ((min* mg/dL)/g)* | Glucose | 1038.2 ± 9.9 | 1018.4 – 1058.0 | 863.1 ± 9.8 | 843.5 – 882.7 | <.0001 |
| Cmax (mg/dL) | Glucose | 120.3 ± 2.6 | 115.2 – 125.4 | 123.5 ± 2.5 | 118.4 – 128.6 | 0.1778 |
| Cmax/D ((mg/dL)/g)* | Glucose | 4.2 ± 0.1 | 4.0 – 4.3 | 3.5 ± 0.1 | 3.4 – 3.7 | <.0001 |
| MRT (min) | Glucose | 172.5 ± 0.9 | 170.8 – 174.3 | 170.4 ± 0.9 | 168.6 – 172.1 | 0.0292 |
| Tmax (min) | Glucose | 30.1 ± 2.7 | 24.8 – 35.4 | 30.2 ± 2.6 | 24.9 – 35.4 | 0.9842 |
| Relative bioavailability*R | Glucose | 1.20 ± 0.07 | <.0001 | |||
AUC area under the curve; AUC/D AUC divided by the respective sugar dose of the treatment; Cmax maximum observed concentration; Cmax/D Cmax divided by the respective sugar dose of the treatment; MRT mean residence time; Tmax = time of Cmax. FE_fructose fractional excretion of fructose. CI confidence interval. Data given as least square mean ± standard error. Linear mixed effect models were used to analyze the parameters.
Paired t-test was used.
p-value < 0.005.
Figure 2.
Effect of consuming HFCS- versus sucrose-sweetened beverages during a 6 hr period on (A) fructose, (B) normalized fructose by dose of each treatment, (C) FE_fructose, (D) glucose, and (E) normalized glucose by dose of each treatment. Values are least square means ± standard errors. P-value shown represents overall treatment effect. P-value: * = < 0.05; ** = < 0.005. FE_fructose fractional excretion of fructose; HFCS high fructose corn syrup.
Although the values were higher from HFCS, treatment effects were no longer significant when normalized for the differences in dose of fructose between HFCS and sucrose (Table 4, Figure 2B). Thus, a gram of fructose from either HFCS or sucrose is absorbed in a similar manner, which is indicated by a lack of difference in relative fructose bioavailability between the two sweeteners (1.07 ± 0.24, p = 0.1219). Although there was a greater fractional excretion of fructose from HFCS-sweetened soft drinks, the effect was not significant (Figure 2C).
Glucose
Glucose AUC, Cmax, and changes in postprandial glucose concentrations were very similar between HFCS and sucrose (Table 4, Figure 2D). However, dose normalized glucose AUC and Cmax were significantly higher from the HFCS treatment compared to sucrose. In addition, dose normalized glucose concentrations were higher at all time points (Figure 2E). The relative bioavailability of glucose indicates that a gram of glucose from HFCS reaches the systemic circulation more efficiently than from sucrose (1.20 ± 0.07, p < 0.0001).
BP and HR
The observed maximum SBP was significantly different between the two treatments (Table 5). In contrast, DBP did not differ between the treatment groups and neither did heart rate (Table 5).
Table 5.
Effects of consuming HFCS- versus sucrose-sweetened beverages on SBP, DBP, and HR.
| Variable | Parameter | Treatment
|
P-value | |||
|---|---|---|---|---|---|---|
| HFCS
|
Sucrose
|
|||||
| Mean | 95% CI | Mean | 95% CI | |||
| AUC (min* mmHg) | SBP | 43832 ± 309 | 43214 – 44449 | 43588 ± 306 | 42977 – 44200 | 0.4802 |
| Observed max (mmHg)* | SBP | 133.5 ± 1.0 | 131.4 – 135.5 | 130.2 ± 1.0 | 128.1 – 132.2 | 0.0047 |
| AUC (min* mmHg) | DBP | 27460 ± 263 | 26935 – 27985 | 27363 ± 260 | 26842 – 27883 | 0.7425 |
| Observed max (mmHg) | DBP | 84.1 ± 0.8 | 82.4 – 85.7 | 84.0 ± 0.8 | 82.4 – 85.6 | 0.9470 |
| AUC (min* bpm) | HR | 23856 ± 263 | 23328 – 24384 | 23791 ± 262 | 23266 – 24316 | 0.7745 |
| Observed max (bpm) | HR | 75.3 ± 0.9 | 73.5 – 77.1 | 75.0 ± 0.9 | 73.2 – 76.8 | 0.7488 |
AUC area under the curve; DBP diastolic blood pressure; HR heart rate; SBP systolic blood pressure. CI confidence interval. Data given as least square mean ± standard error. Linear mixed effect models were used to analyze the parameters.
Paired t-test was used.
p-value < 0.005.
SUA and FEUA
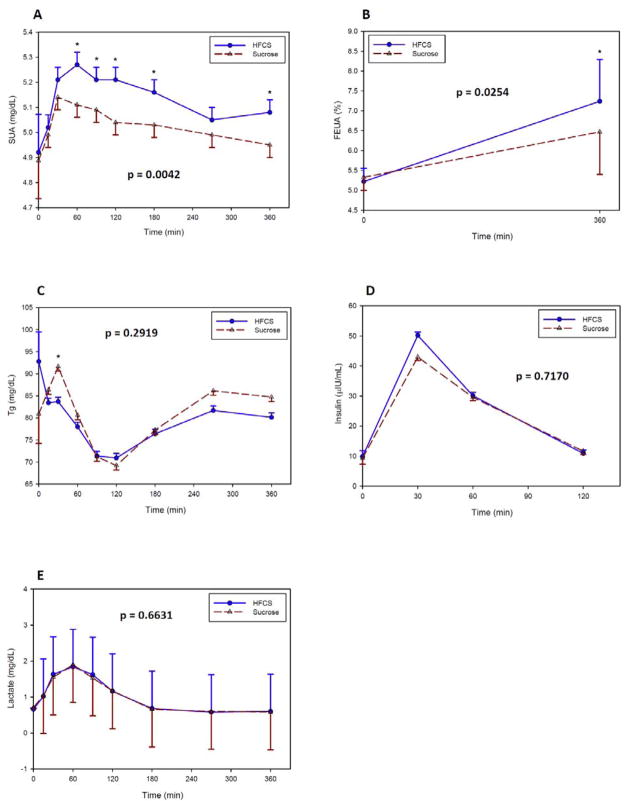
There were no treatment differences in AUC and Cmax of serum uric acid or fractional excretion of uric acid (Table 6). However, there was a significantly higher effect from HFCS than from sucrose on postprandial changes in levels of SUA (p = 0.0042, Figure 3A). Although, FEUA was higher at the end of the 6 hr study visit from HFCS, the treatment effect did not meet our definition of statistical significance (p = 0.0254, Figure 3B).
Table 6.
Effects of consuming HFCS- versus sucrose-sweetened beverages on SUA, FEUA, Tg, Insulin, and Lactate.
| Variable | Parameter | Treatment
|
P-value | |||
|---|---|---|---|---|---|---|
| HFCS
|
Sucrose
|
|||||
| Mean | 95% CI | Mean | 95% CI | |||
| AUC (min* mg/dL) | SUA | 1848.6 ± 15.1 | 1818.5 – 1878.7 | 1811.3 ± 14.9 | 1781.5 – 1841.0 | 0.0827 |
| Cmax (mg/dL) | SUA | 5.4 ± 0.1 | 5.3 – 5.5 | 5.4 ± 0.1 | 5.2 – 5.5 | 0.4947 |
| ΔFEUA (%) | FEUA | 7.6 ± 0.4 | 6.8 – 8.3 | 7.0 ± 0.4 | 6.2 – 7.7 | 0.2671 |
| AUC (min* mg/dL) | Tg | 30661 ± 798 | 29069 – 32253 | 31964 ± 790 | 30387 – 33540 | 0.1949 |
| Cmax (mg/dL) | Tg | 101.7 ± 2.4 | 97.0 – 106.5 | 108.4 ± 2.4 | 103.7 – 113.1 | 0.0108 |
| AUC (min*μUI/mL) | Insulin | 3717.2 ± 304.4 | 3106.7 – 4327.8 | 3824.3 ± 302.2 | 3217.8 – 4430.7 | 0.6846 |
| Cmax (μUI/mL) | Insulin | 58.1 ± 5.8 | 46.4 – 69.8 | 61.2 ± 5.8 | 49.6 – 72.8 | 0.5905 |
| AUC (min* mg/dL) | Lactate | 355.4 ± 11.1 | 333.1 – 377.8 | 354.2 ± 11.0 | 332.1 – 376.4 | 0.8907 |
| Cmax (mg/dL) | Lactate | 2.0 ± 0.1 | 1.9 – 2.2 | 2.1 ± 0.1 | 1.9 – 2.2 | 0.4401 |
AUC area under the curve; Cmax maximum observed concentration. FEUA fractional excretion of uric acid; SUA serum uric acid; Tg triglycerides. CI confidence interval. Data given as least square mean ± standard error. Linear mixed effect models were used to analyze the parameters.
Paired t-test was used.
p-value < 0.005.
Figure 3.
Effect of consuming HFCS- versus sucrose-sweetened beverages during a 6 hr period on (A) SUA, (B) FEUA, (C) Tg, (D) insulin, and (E) lactate. Values are least square means ± standard errors. P-value shown represents overall treatment effect. * p-value < 0.05. FEUA fractional excretion of uric acid; HFCS high fructose corn syrup; SUA serum uric acid; Tg triglycerides.
Tg, Insulin, and Lactate
There were no treatment differences in AUC and Cmax of Tg, insulin and lactate (Table 6). There were also no contrast differences in postprandial concentrations at any time points between HFCS versus sucrose for Tg (Figure 3C), insulin (Figure 3D), and lactate (Figure 3E).
DISCUSSION
In this study, we compared the acute metabolic and hemodynamic effects of HFCS and sucrose in 40 healthy subjects. We found treatment differences in fructose, glucose, SUA, and SBP. The following metabolic parameters were higher from the HFCS-sweetened beverages than from the sucrose-sweetened beverages: fructose AUC and Cmax, dose normalized glucose AUC and Cmax, relative bioavailability of glucose, changes in postprandial concentrations of SUA, and observed maximum of SBP. There were no differences in relative fructose bioavailability, FE_fructose, FEUA, DBP, HR, Tg, insulin, and lactate. To our knowledge this is the first study to show HFCS is more likely to cause acute adverse effects than sucrose.
We hypothesized that the formulation of HFCS would result in greater systemic fructose exposure than from sucrose. First, HFCS contains more fructose than sucrose. Second, HFCS consists of free fructose and glucose, thus, allowing for the immediate transport of these simple sugars in the intestine. Meanwhile, sucrose must first be metabolized by sucrase before fructose and glucose are available for uptake. Studies have shown that the expression of sucrase can be negatively affected by genetic polymorphisms [26, 27]. Its activity has also been shown to be inhibited by glucose [28, 29]. Thus, we hypothesize that sucrase may potentially be a bottleneck, preventing complete metabolism of sucrose in the gut. Therefore, less fructose would be available for transport. In our study, we found that fructose AUC was about 20% greater and Cmax was about 15% greater from the HFCS-sweetened beverages than from sucrose-sweetened beverages. However, the relative bioavailability was not different. Thus, the difference in fructose plasma concentrations between the sweeteners is most likely due to the higher fructose dose from HFCS, which was about 13% greater than from sucrose. Interestingly, we also detected a significant difference in dose normalized glucose AUC and Cmax. This was surprising since the glucose dose from sucrose was 6 g or about 21% higher than from HFCS. This finding suggests that glucose is more efficiently absorbed into the body from HFCS than from sucrose. The mechanism for this enhanced bioavailability of glucose needs to be further elucidated.
Our study found a significant increase in SBP, about 3 mmHg, from HFCS compared to sucrose. However, the increase was very acute. The impact of chronic exposure of higher fructose bioavailability on affecting sustained elevated blood pressure needs to be investigated. Nevertheless, our finding potentially supports the postulated link between high fructose intake and increased SBP. Jalal et al recently reported an association between high fructose intake from added sugars and increased risk of elevated SBP in the National Health and Nutrition Examination Survey [8]. In a randomized study consisting of 74 men, Perez-Pozo showed that the ingestion of fructose was associated with an increase in BP [37]. Others have also found a relationship of sugar-sweetened soft drink intake with BP [38, 39], although this was not observed in a study in which much of the fructose intake originated from fruits [40].
Several mechanisms have been proposed for fructose-induced high blood pressure, including fructose-induced hyperuricemia [38]. This is an appealing mechanism since previous studies have shown that fructose can increase uric acid levels [16, 41]. Fructose increases uric acid both by acute effects related to ATP consumption and purine degradation, but also via chronic effects to stimulate uric acid synthesis [16, 41, 42]. Importantly, Feig et al showed that by lowering uric acid levels there was a decrease in BP in hypertensive adolescents with newly diagnosed hypertension [43]. Futhermore, Perez-Pozo et al showed that by lowering uric acid with allopurinol, the effect of fructose (200 g/d for two weeks) on elevated BP was prevented in healthy adults [37]. Finally, an epidemiological study has linked uric acid with soft drink ingestion and hypertension in adolescents [44]. In our study, we detected a treatment difference in SUA levels, which was higher from HFCS than sucrose. Although the difference was small, about 0.2 mg/dL, our findings highlight that SUA levels can increase when fructose levels increase in the body. Thus, our data potentially support the link between higher fructose levels, elevated uric acid levels, and higher SBP levels, although other mechanisms by which fructose could raise blood pressure remains possible [45].
Because of the similarity in composition between HFCS and sucrose, it has been speculated that the metabolic effects of these sweeteners are also similar. Studies directly comparing the effects of HFCS versus sucrose are limited. Nevertheless, Melanson et al, Akhaven et al, Soenen et al, and Stanhope et al conducted short-term studies comparing the two sweeteners. These studies found no significant differences on glucose, ghrelin, leptin, insulin, Tg, uric acid, glucagon-like peptide 1, appetite, and food intake [14, 46–48]. While their findings seemingly conflict with our results, these studies did not assess fructose bioavailability and did not account for fructose levels. If fructose is an important factor driving the development of various adverse metabolic effects, we hypothesizes that higher fructose exposure would lead to greater effects. If in these studies, there were no differences in exposure to fructose between their study groups, it would not be surprising that HFCS and sucrose resulted in similar effects. Importantly, fructose bioavailability can vary greatly due to various factors, such as individual differences in fructose absorption and metabolism, the effects of glucose on impacting fructose uptake, and liquid versus solid versus mixed sources of fructose-containing sweeteners [49–54]. In our study, we were able to detect a higher exposure to fructose from HFCS than from sucrose. Thus, this may explain why we were able to detect a difference in metabolic and hemodynamic effects between the two sweeteners whereas other studies have not.
Our study has several limitations. First, it was determined from the sugar profile analyses that the sucrose in the soft drinks was being hydrolyzed. At the start of the study, about 60% of the sucrose had already been hydrolyzed and by the end of the study, all of the sucrose had been broken down. As a result, the potential important role of sucrase was marginalized and may have reduced our ability to detect a difference in fructose relative bioavailability between HFCS and sucrose, which may have resulted in greater differences in fructose AUC and Cmax. However, the external validity of the study is high since soft drinks are a major source of sucrose and HFCS. For future studies, a more controlled environment can be obtained by having the sugar mixtures made immediately prior to the study visits. Second, the study population consisted of young and healthy individuals. Their responses may have been less dramatic than older individuals who are metabolically at risk, such as those with abdominal obesity or those with metabolic syndrome.
In conclusion, our findings suggest there are differences on various acute metabolic and hemodynamic responses between HFCS and sucrose. A major strength of our study was the fructose measurements. This allowed us to determine that the consumption of HFCS resulted in higher systemic fructose exposure, which may have driven the significant treatment differences detected on glucose, SUA, and, SBP. Although the treatment effects on acute metabolic responses were small, the effects may increase with continued chronic exposure to these sweeteners. Furthermore, it still needs to be determined if there are differences in fructose exposure and metabolic effects from HFCS and sucrose if the sweeteners were consumed over a longer period of time versus the acute bolus that was given in our study. Importantly, further studies are needed to evaluate the impact of variable fructose absorption and/or metabolism on higher fructose exposure and how that may affect long-term metabolic responses and disease risks. Although we did find differences between HFCS and sucrose, both sweeteners are currently consumed in excessive amounts, which may play an important role in driving the prevalence of cardio-renal diseases.
Acknowledgments
We thank all the volunteers who participated in the study and the staff at the CTSI at the University of Florida. The authors’ responsibilities were as follows: MTL, RFF, MSS, RJJ, and JAJ designed research; MTL, RFF, and CJR conducted research; MTL, JC, and KKM analyzed data; MTL wrote the paper; JAJ had primary responsibility for final content. All authors read and approved the final manuscript.
List of Abbreviations
- AUC
area under the curve
- AUCH
AUC from HFCS
- AUCS
AUC from sucrose
- AUC/D
dose normalized area under the curve
- BMI
body mass index
- BP
blood pressure
- ClH
clearance from HFCS
- ClS
– clearance from sucrose
- Cmax
maximum observed concentration
- CTSI
Clinical Translational Science Institute
- DH
dose from HFCS
- DS
dose from sucrose
- FH
bioavailability from HFCS
- FS
bioavailability from sucrose
- FE_fructose
fractional excretion of fructose
- FEUA
fractional excretion of uric acid
- HFCS
high fructose corn syrup
- HL
elimination half-life
- HR
heart rate
- MRT
mean residence time
- SCreat
serum creatinine
- SFr
serum fructose
- SUA
serum uric acid
- Tg
triglycerides
- Tmax
time of maximum observed concentration
- UCreat
urine creatinine
- UFr
urine fructose
- UUA
urine uric acid
Footnotes
Clinical Trial Registration Number: NCT00661947
Conflict of Interest: RJJ has a patent application on inhibition of fructokinase as a mechanism to treat sugar craving. RJJ also has a lay book, The Sugar Fix: The High-Fructose fallout That Is Making You Fat and Sick (Rodale and Simon and Schuster, 2008). None of the authors declared a conflict of interest.
Financial Disclosure: The study was supported by the Center for Pharmacogenomics and the Clinical Translational Science Institute (UL1 RR029890) at University of Florida, Gainesville, FL 32610. MTL was supported by institutional NIH training grant (T32-DK007518-23) for the Division of Nephrology, University of Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiner DE, Tighiouart H, Elsayed EF, et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–11. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 4.Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. Health, United States, 2008 With Chartbook. Hyattsville, MD: 2009. [Google Scholar]
- 6.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Steinmann B, Gitzelman R, Berghe GVd. Disorders of fructose metabolism. In: Scriver C, Beaudet A, Sly W, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw Hill; 2001. pp. 1489–1520. [Google Scholar]
- 8.Jalal DI, Smits G, Johnson RJ, et al. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21:1543–9. doi: 10.1681/ASN.2009111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swarbrick MM, Stanhope KL, Elliott SS, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100:947–52. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig GM, Crane CW. Lactic acidosis complicating liver failure after intravenous fructose. Br Med J. 1971;4:211–2. doi: 10.1136/bmj.4.5781.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CM, Dulloo AG, Yepuri G, et al. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R730–7. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 12.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 13.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 14.Stanhope KL, Griffen SC, Bair BR, et al. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980;33:273–8. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- 16.Perheentupa J, Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967;2:528–31. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Lozada LG, Tapia E, Jimenez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–9. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 18.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–9. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montonen J, Jarvinen R, Knekt P, et al. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137:1447–54. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 20.Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–13. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 21.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 22.Aeberli I, Zimmermann MB, Molinari L, et al. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr. 2007;86:1174–8. doi: 10.1093/ajcn/86.4.1174. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 24.United States Department of Agriculture/Economic Research Service. [10 March 2009];US per capita food consumption: Sugars and Sweeteners. Assessed at http://www.ers.usda.gov/Data/FoodConsumption/FoodAvailQueriable.aspx.
- 25.Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58:724S–732S. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- 26.Ritz V, Alfalah M, Zimmer KP, et al. Congenital sucrase-isomaltase deficiency because of an accumulation of the mutant enzyme in the endoplasmic reticulum. Gastroenterology. 2003;125:1678–85. doi: 10.1053/j.gastro.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Sander P, Alfalah M, Keiser M, et al. Novel mutations in the human sucrase-isomaltase gene (SI) that cause congenital carbohydrate malabsorption. Hum Mutat. 2006;27:119–26. doi: 10.1002/humu.9392. [DOI] [PubMed] [Google Scholar]
- 28.Chantret I, Rodolosse A, Barbat A, et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107 (Pt 1):213–25. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 29.Gu N, Adachi T, Matsunaga T, et al. HNF-1alpha participates in glucose regulation of sucrase-isomaltase gene expression in epithelial intestinal cells. Biochem Biophys Res Commun. 2007;353:617–22. doi: 10.1016/j.bbrc.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 30.Bray GA. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol. 2010;21:51–7. doi: 10.1097/MOL.0b013e3283346ca2. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–10. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien E, Waeber B, Parati G, et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–6. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 34.Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93:2230–3. doi: 10.1210/jc.2007-2467. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002;25:353–7. doi: 10.2337/diacare.25.2.353. [DOI] [PubMed] [Google Scholar]
- 36.Shuster J. Design and analysis of experiments. In: Ambrosius W, editor. Topics in Biostatistics. Totowa, NJ: Humana Press; 2007. pp. 235–59. [Google Scholar]
- 37.Perez-Pozo SE, Schold J, Nakagawa T, et al. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Caballero B, Mitchell DC, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121:2398–406. doi: 10.1161/CIRCULATIONAHA.109.911164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown IJ, Stamler J, Van Horn L, et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension. 2011;57:695–701. doi: 10.1161/HYPERTENSIONAHA.110.165456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20:863–71. doi: 10.1681/ASN.2008050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972;21:713–21. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 42.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33:276–80. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen S, Choi HK, Lustig RH, et al. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–13. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madero M, Perez-Pozo SE, Jalal D, et al. Dietary fructose and hypertension. Curr Hypertens Rep. 2011;13:29–35. doi: 10.1007/s11906-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 46.Melanson KJ, Zukley L, Lowndes J, et al. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–12. doi: 10.1016/j.nut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Akhavan T, Anderson GH. Effects of glucose-to-fructose ratios in solutions on subjective satiety, food intake, and satiety hormones in young men. Am J Clin Nutr. 2007;86:1354–63. doi: 10.1093/ajcn/86.5.1354. [DOI] [PubMed] [Google Scholar]
- 48.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–94. doi: 10.1093/ajcn/86.5.1586. [DOI] [PubMed] [Google Scholar]
- 49.Kneepkens CM, Vonk RJ, Fernandes J. Incomplete intestinal absorption of fructose. Arch Dis Child. 1984;59:735–8. doi: 10.1136/adc.59.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beyer PL, Caviar EM, McCallum RW. Fructose intake at current levels in the United States may cause gastrointestinal distress in normal adults. J Am Diet Assoc. 2005;105:1559–66. doi: 10.1016/j.jada.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Born P. Carbohydrate malabsorption in patients with non-specific abdominal complaints. World J Gastroenterol. 2007;13:5687–91. doi: 10.3748/wjg.v13.i43.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truswell AS, Seach JM, Thorburn AW. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr. 1988;48:1424–30. doi: 10.1093/ajcn/48.6.1424. [DOI] [PubMed] [Google Scholar]
- 53.Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–8. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–62. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]