Abstract
Nickel (Ni) is a worldwide pollutant and contaminant that humans are exposed to through various avenues resulting in multiple toxic responses - most alarming is its clear carcinogenic nature. A variety of particulate Ni compounds persist in the environment and can be distinguished by characteristics such as solubility, structure, and surface charge. These characteristics influence cellular uptake and toxicity. Some particulate forms of Ni are carcinogenic and are directly and rapidly endocytized by cells. A series of studies conducted in the 1980’s observed this process, and we have reanalyzed the results of these studies to help elucidate the molecular mechanism of particulate Ni uptake. Originally the process of uptake observed was described as phagocytosis, however in the context of recent research we hypothesize that the process is macropinocytosis and/or clathrin mediated endocytosis. Primary considerations in determining the route of uptake here include calcium dependence, particle size, and inhibition through temperature and pharmacological approaches. Particle characteristics that influenced uptake include size, charge, surface characteristics, and structure. This discussion is relevant in the context of nanoparticle studies and the emerging interest in nano-nickel (nano-Ni), where toxicity assessments require a clear understanding of the parameters of particulate uptake and where establishment of such parameters is often obscured through inconsistencies across experimental systems. In this regard, this review aims to carefully document one system (particulate nickel compound uptake) and characterize its properties.
Keywords: macropinocytosis, phagocytosis, clathrin-mediated endocytosis, nickel, nickel sulfide, nickel subsulfide, nanoparticles, toxicity, endocytosis
Introduction
Nickel (Ni) is a metallic element that naturally occurs in the Earth’s crust. Due to its unique physical and chemical properties, its alloys are highly used in the manufacturing of jewelry, medical implants, stainless steel, Ni-Cd batteries and in the nickel-plating industry. With high industrial demand for nickel, its mining, extraction, and use continue to rise, leading to a 6% yearly increase of in-use stocks which are made from the conversion of ore to Ni (Rauch and Pacyna, 2009). This widespread extraction and use has resulted in increased levels of nickel in biogeochemical cycles and, as a result, increased human exposure through environmental contamination and occupational exposure.
The production, processing, and recycling of nickel products has resulted in a high level of pollution such that nickel contamination now occurs in water, soil and the ambient air, where levels of nickel can range, depending on the source, between 25 ng/m3 and 170 ng/m3 (Lippmann et al., 2006). The species of nickel in all cases varies and can include oxides, sulfides, silicates, and soluble compounds such as chlorides, and metallic nickel. Distinguishing between the different forms of nickel is critical in understanding the threat nickel poses to human health, because only certain forms of nickel penetrate the cell and alter the intracellular dose of Ni2+. In turn, only some forms of nickel pose a serious threat to human health.
It may seem that enacting public health measures in order to protect against nickel toxicity would be a simple task of identifying the toxic forms and taking the necessary measures. However, identifying toxic forms of nickel and ascertaining how they are toxic has been a complex journey for several reasons. First, nickel often exists as a group of compounds and rarely do pollution sources lead to a single form of nickel or come from a single source. Second, as the industrial uses of nickel expand to include the nanomaterials industry, the physical characteristics which often limit certain types of nickel from entering the cell may be altered during a nano-engineering process, thereby necessitating a more complete understanding of not just which forms of nickel are toxic, but why at a physical level certain forms gain entry to a cell while other forms do not. Third, the mechanisms of nickel toxicity have been difficult to pinpoint because metals target and interact with many intracellular sites, and these sites are difficult to identify once the cell is lysed. In recent years a clearer understanding has emerged which describes the mechanisms behind nickel’s cellular toxicity, but there is still much to learn with regard to pollution sources and nickel uptake.
The focus of this paper will be to review and synthesize our understanding of nickel uptake with a particular interest in the particulate forms of nickel of varying water solubility. We will review the information that is available on the physical determinants of such uptake and how it relates to particulate forms of nickel, including nanoparticles.
Epidemiologic evidence
Public concern over nickel exposure ranges from issues of allergic contact dermatitis to lung and nasal cancers (Duda-Chodak and Blaszczyk, 2008). Since the 1930’s strong evidence of nickel’s toxicity has accumulated in occupational settings as nickel mining, refining and smelting workers demonstrate significantly higher incidence of lung and nasal cancers (Doll and Morgan, 1970; Doll et al., 1977). However at times, these findings were inconsistent and inconclusive due to analytic confusion resulting from co-exposure to various forms of nickel that can occur at a single refinery, where different steps of processing involve different forms. For example, research between the 30’s and 70’s had difficulty distinguishing effects from individuals exposed to metallic nickel, nickel carbonyl, and the dust formed from calcination of impure forms of nickel, as they are all involved in a single process (Doll et al., 1977). Furthermore when ores are smelted to extract nickel, a number of forms of nickel particles and compounds having different charges can be generated-again amplifying the species involved in exposure and thwarting efforts to ascertain which species is the causative agent (Goodman et al., 2009). Recent efforts now utilize complex sampling techniques in order to generate “fingerprints” of workplaces that provide information regarding the species of Ni and their size categories (Vincent et al., 2001). Assessing workplace exposure limits still continues today and prominent now in investigations is understanding the size distribution of particles and the impact such sizing has on dose (Oller and Oberdorster, 2010). Issues of co-exposure were another confounding factor, because Ni can increase carcinogenicity of other toxins. Exposure to soluble Ni compounds, which typically do not provide evidence of carcinogenicity in epidemiologic studies, increased tumor incidence when exposure occurred simultaneously to sparingly soluble Ni and tobacco smoke (Doll et al., 1990; Andersen et al., 1996).
Forms of Ni and cellular uptake
As a transition metal Ni can exist in five oxidation states (−1, 0, +2, +3, and +4) with the +2 state being most common under normal conditions. Traditionally, Ni compounds were divided into two main categories, soluble and insoluble. More recently Ni compounds now fall into four categories: soluble nickel, sulfidic nickel, oxidic nickel and metallic nickel (Goodman et al., 2009). Soluble Ni is quickly flushed from tissue and therefore has a limited ability to enter the cell via the divalent metal transporter 1 (DMT1) and some evidence suggests that it enters through calcium channels, though this form of entry is limited (Funakoshi et al., 1997; Denkhaus and Salnikow, 2002; (Davidson et al., 2005). Ni carbonyl, a highly toxic form of Ni, is lipid soluble allowing it to pass through the cellular membrane and in turn significant absorption occurs through inhalation and skin contact (Sunderma.Fw and Selin, 1968).
This article will focus on the Ni sulfide particles which exhibit sparingly soluble properties in aqueous media, specifically the compounds Ni sulfide (NiS) and Ni subsulfide (Ni3S2). Some forms of sparingly soluble Ni compounds, such as crystalline Ni3S2, enter via endocytic routes and are among the most toxic and carcinogenic forms of all nickel compounds. Other forms of sparingly water soluble Ni compounds, such as amorphous NiS in its native form, do not penetrate the cell and as such have limited or no toxicity. The distinction between amorphous NiS and crystalline Ni3S2, and their respective abilities to penetrate the cell, will be a focal point of this article and discussed in detail. Ni3S2 is found in two forms: αNi3S2, which is the low-temperature green form; and βNi3S2, which is the high temperature bronze-yellow form. NiS is found in three forms (α, β, and amorphous) ranging in color from dark green to black and in form as crystals or powder (Goodman et al., 2009).
Different forms of nickel compounds can also be distinguished along criterion of size and structure. Particulate forms are defined by their relatively water insoluble nature, but can vary in action based on their sizes, consistency of structure (crystalline or amorphous), and chemical composition. Particulates belonging to a larger size range can be generated in industrial settings where dust is produced during processing and those on the smaller size range are now intentionally generated in the nanoparticle industry. The generation of nanoparticles comprises a unique concern in the field of toxicology and represents a burgeoning industry. Nanomaterials are now being used in many fields including biomedicine, electronics, lithographic techniques, environmental cleanup, and materials engineering; and nanomaterials can now be found in products including sporting goods, stain-resistant clothing, and sunscreens (Nel et al., 2006; Zhao et al., 2011). Thus consumer, medical, occupational and environmental exposures are all on the rise. While nickel nanoparticles have been utilized in a variety of technological areas, their usage in biomedical applications is limited, with only recent research investigating their use and toxic potential (Guo et al., 2008).
Ni exposure
Inhalation is the most significant avenue of uptake for Ni compounds, especially in occupational settings where workers are in close proximity of Ni processing. Inhalation exposure is associated with acute respiratory conditions that range from irritation and inflammation to bronchitis, pulmonary fibrosis, asthma, and pulmonary edema. Ni compound exposure is also linked to kidney and cardiovascular diseases and to allergic contact dermatitis. Ni compound carcinogenicity is the most alarming aspect of exposure and will be discussed in more detail in relation to Ni’s toxicity.
While Ni excretion does occur via feces, there is no dedicated metabolic route for processing Ni in the human body and as such differences in its action result from differences in chemical properties of the different forms of Ni. That said, there are still physiologic parameters which can be generalized regarding Ni ingestion. Isotopic studies in mice have indicated that independent of the intake location, Ni accumulates in the lung tissues (Oskarsson and Tjalve, 1979; Herlantpeers et al., 1983). Ni that is absorbed at the lungs can migrate and does so through the bloodstream, but its movement occurs through a slow release such that Ni levels measured in whole blood are not significantly elevated above control subjects (Bennett, 1984). Most control subjects in human studies have Ni present in their systems as the estimated body burden for a healthy non-exposed adult is approximately 7.3 μg Ni/kg body weight (Bennett, 1984; Denkhaus and Salnikow, 2002). Non-exposed individuals may absorb Ni through ambient air pollution or dietary sources including cocoa, spinach, and some forms of nuts (Flyvholm et al., 1984).
Endocytic Uptake of Particles
Introduction to cellular uptake
The entry of a toxin into the cell is a critical step that often determines whether or not the toxin will exert a carcinogenic effect on the cell through mutagenic or epigenetic mechanisms. Thus, to understand the varying toxicities associated with Ni we must understand how and why certain forms of Ni are endocytized by the cell while other forms are not.
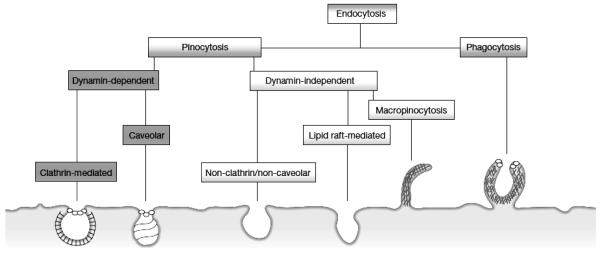
In general endocytic mechanisms (Figure 1) can be roughly divided into two categories depending on whether the uptake substrate consists mainly of fluids and solutes (pinocytosis) or whether it is composed of large particles (phagocytosis) (Mercer and Helenius, 2009). Within pinocytosis there is a further division depending on whether the mechanism is dynamin dependent (clathrin and caveolar) or dynamin independent (non clathrin/noncaveolar, lipid raft-mediated, and macropinocytosis). Much of the research that describes the molecular mechanisms involved in these processes focuses on viruses and other biologically active elements (Underhill and Ozinsky, 2002; Mailander and Landfester, 2009; Mercer and Helenius, 2009). Studies in this area highlight the complexity of understanding these processes, the specifics of which are dependent on multiple factors including virus and microbe types, receptor interactions, and signaling responses that include inflammation, activation of kinases, and cell-type specificity. When it comes to particles, studies on uptake have focused on the nano-bio interface where interactions with membranes involve a set of physically and energetically driven interactions including hydrodynamic, electrodynamic, electrostatic, solvent, steric, and polymer bridging interactions (Mailander and Landfester, 2009; Nel et al., 2009). This unique set of interactions allows for control over the entry of nanoparticles into cells, through modifications of its physical properties such as size, shape, and surface characteristics, and control over these properties has become essential in therapeutic applications (Petros and DeSimone, 2010). Attempts at classifying uptake routes for nanoparticles based on classifications of their size, shape and surface characteristics continue and require detailed investigations along multiple parameters in order to ascertain exactly which pathway is being utilized. At times review articles have classified these processes as size-dependent (Zhao et al., 2011) and while size restrictions often help to inform the route of uptake, exceptions do exist (Nel et al., 2009). Other studies have focused on aspect ratios (AR) and have determined that particles of the same chemical composition and the same shape experience differential uptake, where for example in HeLa cells, discrimination for macropinocytosis occurs due to differences in width to height ratios (AR) ranging from AR 2.1-2.5 (Meng et al., 2011).
Figure 1.
Types of Endocytosis
In general endocytic mechanisms can be roughly divided into two categories depending on whether the uptake substrate consists mainly of fluids and solutes (pinocytosis) or whether it is composed of large particles (phagocytosis). Within pinocytosis there is a further division depending on whether the mechanism is dynamin dependent (clathrin and caveolar) or dynamin independent (non clathrin/noncaveolar, lipid raft-mediated, macropinocytosis).
Figure adapted from Mercer and Helenius, 2009. Reprinted with permission of Nature Cell Biology
The lack of classification and clarity in this area of research is likely a result of the complexity of the potential forces involved in uptake and particle structure. Particles can be characterized through more technical properties than those already mentioned, including chemical composition, surface functionalization, shape, angle of curvature, porosity, surface crystallinity, heterogeneity, roughness, hydrophobicity, and hydrophilicity. Moreover, there are other properties including effective surface charge (zeta potential), particle aggregation, state of dispersion, stability/biodegradability, dissolution characteristics, hydration and valence of the surface layer that are determined by the characteristics of the suspending media (pH, temperature, and ionic strength, etc) (Nel et al., 2009). The interplay of these properties is also dynamic-for instance as the size of a particle changes, its interaction with the cellular membrane which has a surface heterogeneity on the scale of 10-50 nm can vary in charge uniformity. But if the particle is sized between 10-50 nm, then depending on its location and shape the interaction energy would vary as well; while this variation may be extreme for a 10 nm particle, a larger sized particle on the μm scale would experience an averaging of the energies (Nel et al., 2009). Meanwhile, properties such as surface area demonstrate predictive dose-response relationships for low-solubility low toxicity nanoparticles such as titanium dioxide and carbon black, while their fine particle counterparts have dose-response relationships determined on a mass basis rather than by surface area (Monteiller et al., 2007). Collectively, attempts at delineating the toxic of effects of nanoparticles are often incomplete due to the complex nature of the field and the inconsistencies between studies which asses different particles at different sizes utilizing different cell lines and evaluating different parameters (Zhao et al., 2011).
While little is known about the specific details of nano-Ni containing particle uptake, there is a significant body of information about Ni-containing particle uptake. Insoluble forms of particulate Ni compounds enter cells through endocytic processes, and due to their size are likely to utilize pathways which favor larger particles. Studies investigating the processes of particulate Ni compound uptake will be discussed in detail later in this review, and here basic principles of phagocytosis, macropinocytosis, and clathrin-mediated endocytosis will be introduced as they pertain to the later discussion.
Phagocytosis
Phagocytosis is the engulfment of large particles, where particles greater than 0.5 μm in diameter are considered “large” (May and Machesky, 2001; Nel et al., 2009). Phagocytosis is a process that is typically associated with an immune system response and is performed by macrophages, neutrophils and other specialized cells which utilize phagocytosis to attack microbes, viruses and other pathogens. In this regard phagocytosis has received much attention. In a generalized version of the process phagocytosis is driven by the actin cytoskeleton whose reorganization often results in the formation of membrane ruffles and a phagocytic cup. The phagocytic cup engulfs the entity and separates from the cellular membrane into its own vesicle, forming the phagosome (Ohsawa et al., 2000; Underhill and Ozinsky, 2002). Characterizing phagocytosis is difficult because machinery and signaling routes vary depending on several factors. For instance, phagocytosis can be mediated by multiple receptor types including Fc-receptors, complement receptors, non-complement receptor integrins, lectins, and lipopolysacharide receptor CD14 (May and Machesky, 2001). There are also a number of downstream signaling pathways that are involved in the phagocytic process and while much research in this area has focused on the Fc-receptors, many of these pathways implicate other receptor types as well (May and Machesky, 2001). Finally, phagocytosis can also vary based on the type of entity being phagocytized: different processes will be engaged for microbes, apoptotic cells, and other foreign particles (Gregory, 2000; Underhill and Ozinsky, 2002).
Macropinocytosis
Macropinocytosis is a process highly comparable to phagocytosis. It is defined as a transient, growth factor induced, actin-dependent endocytic process that results in the internalization of fluid and membrane into large vacuoles (Mercer and Helenius 2009). Unlike phagocytosis, which is driven by the presence of the particle, macropinocytosis can occur in the absence of a particle wherein the cell ingests large quantities of fluid (Mercer and Helenius, 2009). Additionally, the two processes are distinct in that nearly any type of cell can participate in macropinocytosis, while phagocytosis is a specialized property of immune type cells (Mercer and Helenius, 2009). Both processes involve intracellular increases in calcium (Falcone et al., 2006; Nunes and Demaurex, 2010). In macropinocytosis there is a slow intracellular increase in Ca2+ concentration that precedes the PI3K-dependent step (Falcone et al., 2006). In phagocytosis it is generally accepted that an intracellular rise in Ca2+ occurs during the phagosomal maturation phase; while reports that Ca2+ rise occur during the initial stages of phagocytic uptake are still under debate (Nunes and Demaurex, 2010). Macropinocytosis is characterized by membrane rearrangements that occur prior to uptake and that include membrane ruffling and the formation of distinct membrane structures including planar lamellipodia, circular ruffles, and blebs (Mercer and Helenius, 2009). In terms of size, because macropinosomes do not require a particle as phagosomes due to form, their conformational structure is not dependent on particle size or structure as the formation of the phagocytic cup is, and as such sizes can range from 0.5-10 μm (Mercer and Helenius, 2009) and there are macropinosomes reported endocytizing particles in the range of 0.5-5 μm (Zhao et al., 2011).
Clathrin mediated endocytosis (CME)
Clathrin mediated endocytosis is an uptake process, which occurs through the formation of clathrin coated vesicles that form from the plasma membrane. CME is involved primarily in the internalization of receptors, but can also be utilized by cells to internalize nutrients, assess the extracellular environment, regulate signaling pathways, and endocytize other cargo. Clathrin vesicle formation involves five main steps: nucleation, cargo selection, coat assembly, scission, and uncoating. A basic outline of the steps will be presented here and for a detailed review of the process see McMahon and Boucrot, 2011. In nucleation the cell membrane undergoes a morphological change via invagination through which a membrane pit is formed. The size of clathrin pits can vary widely and do so in a species dependent manner, though typically sizes do not exceed 200 nm (McMahon and Boucrot, 2011). From there the putative nucleation module forms which then initiates the process of cargo selection by recruiting AP-2 (adaptor protein-2) which then recruits cargo adaptors and receptors, forming a hub network of proteins involved in protein binding and membrane bending. AP-2 plays a critical role in the formation of vesicles and is necessary for CME to proceed-its involvement is not only necessary for cargo selection, but also in the recruitment of clathrin to the pit. Clathrin helps to organize the vesicle and undergoes polymerization which stabilizes the curvature of the vesicle. Vesicle scission occurs through a process that is not fully elucidated but involves formation of the vesicle neck, recruitment of dynamin, and a GTP hydrolysis-dependent conformational change that mediates scission. After the vesicle is separated from the membrane, the clathrin coat is disassembled in order to promote the movement and future association between the vesicle and other endosomes.
Toxicity of Particulate Ni Compounds
Nanoparticle toxicity
Nanoparticles and nanostructures are typically defined as those particles having one dimension of 100 nm or less (Nel et al., 2006). This cutoff is somewhat arbitrary, especially in the context of ecotoxicity, where aggregates can form or where particles exist across a distribution of sizes (Shaw and Handy, 2011). In vivo and in vitro studies have demonstrated that Ni containing nanoparticles are more toxic than particles greater than 3 μm (Horie et al., 2009; Zhao et al., 2009; Phillips et al., 2010). While it is sometimes the case that nanoparticles are more toxic than their larger counterparts, many studies have demonstrated that this understanding cannot be generalized (Karlsson et al., 2009) as there are noted exceptions where toxicity is not correlated with size (Warheit et al., 2006) and in some instances demonstrates correlation with surface reactivity instead (Warheit et al., 2007). The task of understanding nanotoxicity then relates to many factors as does understanding nano-uptake and these factors have been reviewed and are still in the process of being fully categorized (Nel et al., 2006; Warheit, 2010). At this time there is limited information with respect to nano-Ni containing particles both in regard to their cytotoxicity and cellular uptake. Research into the uptake of other metal nanoparticles is more abundant for metals such as titanium (Karlsson et al., 2009; Migdal et al., 2010) and gold (Chithrani and Chan, 2007; Bartneck et al., 2010). These studies more closely explore the nature of their uptake and focus on the array of properties which can affect uptake.
Nanoparticle toxicity is an area that still requires much investigation before a complete understanding is reached and likely toxicity assessments of nanoparticles will require case-by-case consideration. Even studies which assess the “core identities” of particles, such as nano-Ti02, cannot accurately classify their toxicity without considering the full range of physicochemical characteristics which may influence their behavior and which can vary with surface modifications and manufacturing choices (Warheit, 2010).
When assessments are made about nanotoxicity, oxidative stress and inflammatory markers are parameters that are often used to assess toxicity. At a preliminary level it is suspected that oxidative stress induced by nanoparticles may be a major mechanism of their toxicity and is often a focal point of studies (Nel et al., 2006). Reactive oxygen species (ROS) generation by nanoparticles can be particularly insidious as nanoparticles can be found interacting with the mitochondria, whose damage can exacerbate ROS production (Karlsson et al., 2009). A major factor in determining toxicity is the extent to which the particle enters the cell and the factors which contribute to uptake are now important considerations for drug delivery as well. In addition to uptake the level of exocytosis is also integral as it affects nanoparticle accumulation rates. Again size considerations also play a role in exocytosis and efforts are also being made to track this relationship (Chithrani and Chan, 2007).
Nano-Ni toxicity
Most studies that address the toxicity of nano-Ni focus on particles smaller than 100 nm. Recently there were several studies that assessed their effect on cell survival with respect to drug delivery and cancer treatment. When leukemia K562 cancer cells were exposed to 30 nm nano-Ni, DNA damage occurred and resulted in decreased cell viability, which was dependent on dose and exposure time (Guo et al., 2008). Treated cells were observed to undergo morphological changes which led to the formation of bumps and holes on the surface of the cell membrane and eventually these cells were observed to lyse. Additionally, dosage corresponded with multiple effects including early apoptosis, advanced apoptosis and necrosis where 12.5 μg/mL induced the highest rate of apoptosis while advanced apoptosis, and necrosis had higher rates at 25 and 50 μg/mL (Guo et al., 2008).
Fish studies found that particles sized around 6 nm at a high dose of 10 mg l−1 was not high enough to establish an LC50 in adults or larvae (Griffitt et al., 2008). While particles ranging in size from 30-100 nm with higher concentrations ranging to 1000 mg l−1 resulted in similar LD50 levels, and those forming dendritic clusters of 60 nm lead to morphologic changes (Ispas et al., 2009). In the latter study the effect of Ni nanoparticles sized at 30, 60, and 100 nm as well as aggregated particle clusters of 60 nm having dendritic structures were delivered to zebrafish embryos to assess changes in mortality and developmental defects (Ispas et al., 2009).
Treatment resulted in thinning of the intestinal epithelium and skeletal muscle fiber separation, while control treatments involving soluble Ni salts did not produce such effects. Overall the nanoparticle forms were more toxic than the soluble forms, and of the nanoparticle varieties the dendritic clusters demonstrated the highest level of toxicity indicating that shape is an important factor for toxicity of Ni nanoparticles.
Ni hydroxide nanoparticles (nano-NH), which are increasingly used in the power and energy industries, induced significant inflammatory responses in mouse lungs in a dose-dependent manner (Gillespie et al., 2010). Later research assessed the role of particle solubilization from the nanoparticles to determine if toxicity resulted from the generation of oxidative stress and inflammation. Nano-NH were compared to nickel sulfate nanoparticles (nano-NS). Results indicated that the greater toxic potential of nano-NH was not attributed to increased solubilization nor to generic properties of nanoparticles, but rather was more likely related to increased levels of deposition and a stronger inflammogenic potential than nano-NS that was independent of lung Ni burden. This indicates that the toxicity of nano-NH is chemical-specific and important factors to consider are the deposited dose and solubility.
Uptake of Particulate Ni Compounds
Particulate Ni compound uptake: phagocytosis, macropinocytosis or clathrin-mediated endocytosis?
While little is known specifically about the uptake processes involved in nano-Ni particles, a series of experiments conducted detailed research on particulate Ni compound uptake (Costa and Mollenhauer, 1980a; Costa and Mollenhauer, 1980b; Costa et al., 1980; Abbracchio et al., 1981; Costa et al., 1981a; Costa et al., 1981b; Evans et al., 1981; Abbracchio et al., 1982; Evans et al., 1982a; Evans et al., 1982b; Heck and Costa, 1982a; Heck and Costa, 1982b; Heck and Costa, 1982c). These experiments included particles sized 5 μm and smaller with the majority in the size range of 1.4 −4 μm. The uptake of these larger sized particles deserves special consideration and will be reviewed here in detail in order to more fully explore the mechanisms which were discovered and which in the context of more recent research can be more fully understood. These studies investigate the parameters of sparingly water soluble particulate uptake, providing an early model through which to assess toxin entry into the cell, and as such these experiments pertain to recent research in the fields of nanoparticle uptake and the effort to fully classify forms of endocytic uptake.
At the time, these experiments yielded the observation that the form of uptake involved was phagocytosis. However, in the context of recent research it is our opinion that the route of uptake involved was macropinocytosis and/or clathrin-mediated endocytosis (CME). It seems unlikely that the observed process was phagocytosis, as most reports indicate that this process is restricted to immune associated cells and involves a set of receptors specific to these cells. Earlier reports in this field indicated that asbestos can be phagocytized by alveolar cells (Suzuki, 1974), however this finding was likely a misnomer as detailed understanding of differences in endocytic uptake had not be established at the time of publication. Other studies in this area focused on macrophages and it is likely that these studies were describing phagocytosis (Light and Wei, 1977).
Amorphous NiS vs. Crystalline Ni3S2
Differences in carcinogenicity
Early studies attempting to understand the toxicity of Ni focused on the selective nature of its toxicity. Crystalline Ni3S2 and crystalline Ni3Se2 induced a high incidence of sarcoma development in rats while administration of amorphous NiS did not induce any cancer formation. This difference in carcinogenicity was also observed in other models including mice and Syrian hamsters receiving intrarenal and intratesticular injection (Sunderman, 1976; Sunderman and Maenza, 1976; Sunderman, 1978). Cell culture studies confirmed this difference by demonstrating that crystalline Ni3S2 induced a concentration dependent transformation of cells while amorphous NiS produced little or no effect (Costa et al., 1979; Dipaolo and Casto, 1979). Ni3S2 transformed cells grew in soft agar and clones were developed into immortal cell lines that formed three-dimensional colonies in soft agar and tumors in athymic nude (Costa et al., 1978; Costa, 1979; Costa et al., 1979; Costa, 1980). It should be noted that amorphous NiS and crystalline Ni3S2 have similar dissolution rates in serum and water. Future investigations sought to describe the mechanisms underlying these differences in toxicity and to do so assessed rates of uptake relative to transformation; properties of the particles leading to uptake including size, charge, and cell culture medium constituents; and finally the intracellular fate of particles and their role in transformation.
Level of uptake corresponds to level of toxicity
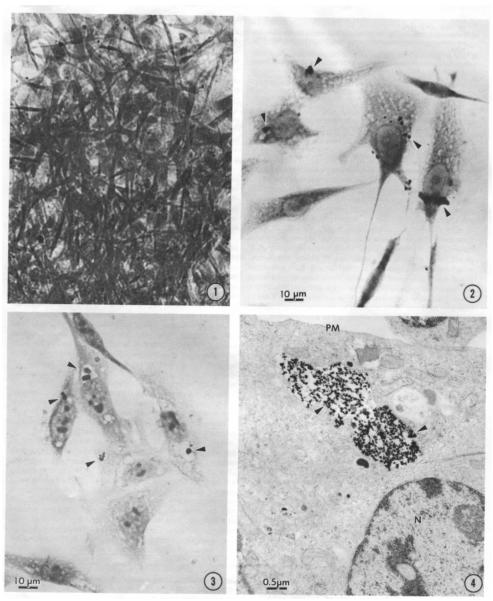
The first major finding in this endeavor was that the level of cellular uptake corresponded with the level of transformation and that both events are concentration dependent. In these studies the level of uptake was assessed through visualization experiments where uptake of the opaque particles was tracked using light microscopy and electron micrograph imaging. Images from the light microscope show Syrian Hamster Embryo (SHE) cells (Figure 2-2) and Chinese Hamster Ovary (CHO) cells (Figure 2-3) which have endocytized the Ni3S2 particles. Endocytized particles are encapsulated in a cytoplasmic vacuole, which is visible in CHO cells (Figure2-4). Transformed SHE cells demonstrated altered morphology characterized by disordered growth and criss-crossing (Figure 2-1). SHE cells treated with multiple doses of crystalline Ni3S2 and amorphous NiS were grown in a soft agar assay to assess transformation and while crystalline Ni3S2 induced formation of colonies in a dose-dependent manner, amorphous NiS did not induce any agar colony formation at any dose. When the level of uptake in these cells was assessed, 42.9% of cells had Ni3S2, while only 0.81% had NiS (Costa and Mollenhauer, 1980a). Particles for both Ni3S2 and NiS were approximately the same size (>5 μm) and demonstrated approximately the same level of insolubility in media. Ni uptake then is strongly correlated with the level of transformation and is the basis for differences observed in the toxicity of insoluble Ni compounds.
Figure 2.
Cells Endocytizing Particles
Figure 2-1 demonstrates the Ni3S2 induced morphological transformation in SHE cells. Cells exhibit disordered growth and criss-crossing. These disordered cells grow in soft agar and form tumors in athymic nude mice. Figure 2-2 is a light microscope photograph of SHE cells with arrows indicating the location of endocytized Ni3S2 particles which appear as black dots. Figure 2-3 is a light microscope photograph of CHO cells that have endocytized Ni3S2 particles with arrows indicating the location of the particles which appear as black dots are visibly encapsulated in a vesicle. Figure 2-4 is an electron micrograph of a CHO cell with endocytized Ni3S2 particles contained in a vesicle. Images from Costa and Mollenhauer 1980b. Reprinted with the permission of Cancer Research.
Soluble forms of Ni by contrast have more difficulty gaining access to the interior of the cell. More recent studies have demonstrated that longer exposure times for compounds such as NiCl2 can increase the amount that enters cells (Ke et al., 2006). Soluble forms of Ni can enter via cell membrane transporters such as the DMT1 where Ni competes with iron to enter (Chen et al., 2005; Davidson et al., 2005). Other studies have determined that soluble Ni may enter via the calcium channel, because calcium ionophore ionomicyin increases Ni uptake four to five fold and because Ni2+ is known to block the calcium channel causing an intracellular release of calcium in the cell (Refsvik and Andreassen, 1995). Uptake of soluble Ni has been observed to increase when cell cultures lack magnesium and calcium, likely due to less competition at the calcium channel (Funakoshi et al., 1997). Overall, uptake of soluble Ni is relatively low and is the reason soluble Ni compounds have little carcinogenic effect in animal and in human epidemiologic studies.
When mouse macrophages were exposed to amorphous and crystalline forms of NiS, early uptake patterns were consistent with those in CHO and SHE cells in which crystalline NiS was favored for uptake, however at later time intervals macrophages were observed to take up amorphous NiS (Heck and Costa, 1982a). This difference in cell types may suggest that phagocytosis or macropinocytosis was responsible for uptake in macrophages while either macropinocytosis or clathrin-mediated endocytosis was responsible in the other cell types-either way some factor which sensitized the non-immune cells’ uptake was no longer a mediating factor in the macrophages. Identifying this difference was a focal point of research and led to the close investigation of the route of uptake.
Characteristics of Particulate Ni Compound Endocytosis
Route of uptake
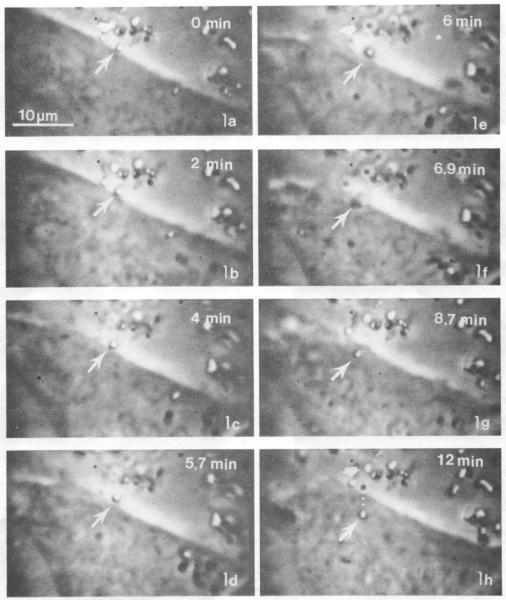
Particle uptake of crystalline NiS was visualized using video time-lapse microscopy (Figures 3A-H) and a timeline of uptake events was generated (Evans et al., 1981; Evans et al., 1982). Crystalline NiS particles entered CHO cells over the course of 7-10 minutes. First, particles bind to the membrane and within 4 minutes of contact, the membrane begins to ruffle and eventually forms a membrane envelope which internalizes the particles. Membrane ruffling was “frequently” observed at the site of particle internalization (Evans et al., 1982). Membrane ruffling can be associated with both phagocytosis and macropinocytosis and is more often associated with the latter. However, due to the types of cells in involved, CHO and SHE, it is unlikely that phagocytosis would be the mechanism since all other instances of it indicate that it is a specialized property of immune cells. From our review of research we see no associations between membrane ruffling and CME.
Figure 3.
Image of Cells Endocytizing Particles
Endocytosis of crystalline Ni3S2 particles. Images were recorded on videotape at 18/1 time lapse and photographs of the film were taken with Polaroid film. The total time lapse from images 3A-3H is 12 minutes. Ni3S2 particles appear white under phase contrast in these images. In 3a (0min) two particles are bound to the CHO cell surface. In panels A (0 min), E (6 min), and H (12 min) the particle indicated by the white long arrow is endocytized over the time course while the particle indicated by the white short arrow remains affixed to the cell surface.
Images from Evans et al. 1982. Reprinted with permission of Cancer Research.
Uptake of NiS particles was visible in CHO cells due to the formation of a distinct vacuole that formed around each particle once inside the cell. This feature provided a convenient method with which to assess the uptake of other metallic compounds in their crystalline forms relative to their amorphous forms (Table 1) (Costa et al., 1982). In all cases the crystalline forms demonstrated higher levels of uptake in CHO cells and corresponded to a higher incidence of morphological transformation in SHE cells. These results were observed across varying concentrations for cobalt, copper and cadmium (Table 1) (Costa et al., 1982).
Table 1.
Comparison of crystalline and amorphous forms of various metals and the level at which they were endocytized by cells
| Metal | Mean Particle size (μm) |
Concentration (μg/ml) |
Concentration (μg/cm2 growth area) |
Cells with endocytized particles (%) |
|---|---|---|---|---|
| Crystalline Ni3S2 |
3.75 | 5 | NA | 12.5 |
| 3.75 | 10 | NA | 23.0 | |
| Crystalline NiS | 3.20 | 5 | 2 | 12.5 |
| 3.20 | 10 | 4 | 22 | |
| Amorpous NiS | 3.35 | 5 | NA | .1 |
| 3.35 | 10 | NA | .3 | |
| Amorphous CoS |
2 | 1 | 0.13 | 0.7 |
| 2 | 5 | 0.63 | 0.9 | |
| 2 | 10 | 1.27 | 1.9 | |
| Crystalline CoS2 |
1.25 | 1 | 0.13 | 7.2 |
| 1.25 | 5 | 0.63 | 28.1 | |
| 1.25 | 10 | 1.27 | 49.3 | |
| Amorphous CuS |
0.98 | 1 | 0.13 | 1.6 |
| 0.98 | 10 | 1.27 | 6 | |
| Crystalline CuS | 0.76 | 5 | 0.63 | 21.4 |
| 0.76 | 10 | 1.27 | 43.8 | |
| Amorphoud CdS |
0.64 | 1 | 0.13 | <1 |
| 0.64 | 5 | 0.63 | <1 | |
| Crystalline CdS | 0.64 | 1 | 0.13 | 8.3 |
| 0.64 | 5 | 0.63 | 12.1 |
All cells were CHO cells treated for 24 hours.
Costa et al. 1982 (Co, Cu, and Cd); Costa et al. 1981b (Ni)
Calcium dependence
Another qualifying factor in determining the uptake route is whether the process is calcium dependent. When a calmodulin antagonist was added to the solution there was a 13% decrease in uptake for crystalline NiS, 40% decrease for crystalline Ni3S2, and 84% decrease for amorphous NiS. When calcium was removed from media, uptake of crystalline NiS declined to less than 10% of cells, and when calcium was restored uptake returned to about 55% of cells (Heck and Costa, 1982a). This association with calcium is suggestive because it indicates that the endocytic process here exhibits calcium dependence and can be enhanced through calcium supplementation. Both phagocytosis and macropinocytosis exhibit intracellular increases in calcium concentration, which can occur through the release of calcium stored in the cell or through increased influx of extracellular calcium through dedicated transporters (Falcone et al., 2006; Nunes and Demaurex, 2010). The debate on whether phagocytosis is a calcium dependent process has taken many turns and at present the consensus is that phagocytosis is likely not calcium dependent but that the later stage of phagosomal maturation is calcium dependent. During phagocytic maturation the phagosome undergoes extensive remodeling and can fuse with endosomes, lysosomes, or other secretory vesicles (May and Machesky, 2001; Nunes and Demaurex, 2010). Several forms of phagocytosis are known to be calcium dependent including Fc domain of IgG (FcγR) activation (May and Machesky, 2001). Macropinocytosis by contrast is calcium dependent at an earlier stage of the process prior to phosphotidylinositol 3-kinase (PI3K) activation and prior to particle internalization (Falcone et al., 2006). In a phagocytic process, uptake would likely still occur in the absence of calcium. Because uptake of particles was inhibited by calcium depletion, it is likely that such dependence indicates the process involved was macropinocytosis. That said, because both processes offer many possible signaling routes the determination as to whether it is macropinocytosis or phagocytosis cannot be made without further study.
To further complicate the discussion, there remains the question of whether CME is a calcium dependent or even calcium enhanced process. Reports indicate that CME also responds positively to the presence of calcium in the media, as calcium stimulates the polymerization of clathrin and the formation of clathrin baskets (Keen et al., 1979; Nandi et al., 1981). The importance of calcium in clathrin basket formation is dependent on the ionic strength of the solution. In solutions of moderate ionic strength (τ/2 = 0.11)-a strength similar to in vivo situations-CME is not dependent on calcium presence, while at low ion strength solutions (τ/2 = 0.01), it was dependent on calcium presence (Keen et al., 1979). Recent research has not utilized calcium depletion as a method of inhbition for CME (McMahon and Boucrot, 2011).
The lack of membrane ruffling and possibly calcium dependence associated with CME, provides evidence that CME is not the dominant process occurring here. However, studies in the following section do provide evidence of CME. Important to note is that the process described here may involve multiple mechanisms of uptake, which are simultaneously working and which may be acting to discriminate particles based on size or other physical characteristics.
Factors Affecting Uptake of Particulate Ni Compounds
Size
Size is one factor that can influence the route of uptake, but because other factors such as charge can play a significant role as well, there are no clear rules that predict route of uptake based on size. While some guidelines have been suggested (Zhao et al., 2011), there are also numerous exceptions and the general consensus is that such determinations cannot yet be made (Nel et al., 2009).
In the context of crystalline Ni3S2, particle size plays a clear role in mediating uptake. According to recent research, particles greater than 0.5 μm can be expected to enter via phagocytosis (Nel et al., 2009) and similarly for macropinocytosis, particles are expected to be in the range of 0.5-5 μm (Zhao et al., 2011). When CME occurs, particles are expected to be no greater than 200 μm (McMahon and Boucrot, 2011). In the studies conducted with crystalline Ni3S2, particles of different sizes were generated by grinding crystalline Ni3S2 with a Spex 5100 mixing mill after which particles were filtered through nucleopore filters that had defined pore sizes (1 μm, 2 μm, and 3 μm, etc)(Costa et al., 1981a). Particle sizes were confirmed using an electron microscope and a light microscope and ranged between 1.8 μm and 5.8 μm. The effect of size on endocytosis was evidenced in CHO cells where most particles between 1.8 μm and 4 μm were actively endocytized with more frequency than those between 4 μm and 5 μm, while particles exceeding 5 μm were rarely endocytized. 70% of cells had particles at 1.8 μm and 60% had particles at 4 μm, while 10% had particles that were 5 μm and only 5% had particles that were 5.8 μm. The steep drop in uptake at 5 μm indicates that there is some threshold after which cells experience difficulty endocytizing these particles. The size-dependent change in uptake also correlated with increased colony formation, which was also dependent on the concentration of particles in solution where higher concentrations demonstrated increased toxicity. The effect of concentration was likely the result of increased contact between particulates and cell walls and was not a result of increased particle number as particles were scaled back in relation to their mean diameter. Amorphous NiS uptake did not change in relation to size in any experiments.
Charge/structure
The role of surface charge in uptake was first addressed in studies which assessed the toxicity of asbestos particles where it was determined that these particles were most toxic with negative surface charges (Light and Wei, 1977). Various forms of crystalline Ni were compared to noncrystalline forms of Ni and in all cases the crystalline forms which included α Ni3S2, α NiS, and Ni3Se2 were more actively endocytized and induced higher levels of transformation than the noncrystalline forms which included metallic Ni, Ni oxide, and amorphous NiS. The crystalline forms of Ni had a negative surface charge while amorphous forms of Ni retained a positive surface charge (Abbracchio et al., 1981). The importance of a crystalline structure was also demonstrated in relation to other metals and their amorphous counterparts (Table 1).
To assess the effect of this charge difference, forms of Ni compounds with a positive surface charge, crystalline NiS and amorphous NiS, were reduced to forms with a greater negative surface charge. In these studies particles were either washed with a solvent pyridine and/or were reduced with LiAlH4 reduction. Those reduced with LiAlH4 were also washed with the solvent pyridine prior to reduction. The solvent washing and reduction with LiAlH4, independently and in conjunction, increased uptake of amorphous NiS and subsequently resulted in increased transformation of cells that was proportional to their uptake, indicating that uptake is a critical step in determining carcinogenicity (Table 2). Once the amorphous NiS was taken in, its eventual dissolution to ionic forms resulted in increased Ni2+ concentrations within the cell which lead to transformation (Heck and Costa, 1982c). These studies demonstrated that surface charge of the particles is a critical factor in determining their level of uptake.
Table 2.
Summary of the effect of surface treatments on Ni particulate uptake
| Metal | Charge (mV) |
Particle size (μm) |
Concentrati on(μg/ml) |
Concentration (μg/cm2 growth area) |
Treatme nt time (hours) |
Cells with endocytized particles (%) |
|---|---|---|---|---|---|---|
| Untreated crystalline NiS | −27 | 2.37 | 10 | 1.78 | 24 | 29.2 |
| Untreated amorphous NiS | +9 | 1 | 10 | 1.78 | 24 | 4.8 |
| Solvent-washed crystalline NiS |
NA | 2.07 | 10 | 1.78 | 24 | 33.3 |
| LiAlH4 reduced crystalline NiS |
NA | 3.76 | 10 | 1.78 | 24 | 51 |
| Solvent-washed amorpohous NiS |
NA | 2.66 | 10 | 1.78 | 24 | 9.9 |
| LiAH4-reduced amorpohus NiS |
NA | 2.26 | 10 | 1.78 | 24 | 27.7 |
CHO cells were treated with particles for 24 hours.
Another factor that may play a role in selective endocytosis is the structure of the atoms on the surface of these compounds. X-ray photoelectron spectroscopy revealed differences in Ni/S ratios where crystalline NiS had more sulfur in the negative two oxidation state compared with amorphous NiS, which had more sulfur in the positive six oxidation state on the surface as well as less Ni content. Reduction of these compounds did not significantly alter the Ni-S stoichiometrics or sulfur oxidation states, and the role of these characteristics in uptake was not determined (Abbracchio et al., 1981).
While it is clear that negative surface charge plays a significant role in uptake selectivity, questions remain as to whether the magnitude of negative charge influences that uptake level. In studies which addressed uptake where reduction was promoted with washing samples in a solvent, the magnitude of increased uptake was considerably less than in the experiments where solvent washing occurred indicating that other factors (magnitude of charge, surface chemistry) require further consideration in order to understand the influence of charge, structure, and stoichiometries in Ni particulate uptake.
Extracellular requirements
The extracellular environment plays an important role in determining other characteristics of the particle and thereby influencing its interaction with the cellular membrane. Properties such as surface charge, particle aggregation, state of dispersion, stability/biodegradability, dissolution characteristics, hydration, and valence of the surface layer can be influenced through the extracellular environment. When considering cell culture media, characteristics such as temperature, pH, ionic strength, and the presence of large organic molecules or detergents can alter the character of the particle. For a detailed review of these interactions and the importance of such characteristics see Nel 2009.
In the context of NiS and Ni3S2, several components of the extracellular environment were assessed and evaluated in order to understand the role of the suspending media and other organic and inorganic components on uptake of Ni3S2.
Media consisted of a simple salts/glucose maintenance medium or a complex culture medium fortified with 10% fetal bovine serum (FBS), which contains serum proteins. Changes in media had little effect on the uptake of particles including crystalline NiS and Ni3S2 as well as amorphous NiS (Heck and Costa, 1982a). Temperature reduction to 4°C significantly decreased uptake by CHO cells (Heck and Costa, 1982a) and other studies have observed that macropinocytosis and CME can be inhibited at 4°C (Meng et al., 2011; Thurn et al., 2011).
In the presence of manganese dust with a mean particle size of 4.1 μm, there was inhibition of transformation by crystalline Ni3S2 in SHE. Both manganese (Mn) dust and Mn2+ ions inhibited the endocytosis of crystalline NiS. Mn compounds were not actively endocytized and inhibition was likely the result of a change produced by these compounds in the extracellular environment. Similarly, amorphous NiS which was not endocytized also inhibited phagocytosis of crystalline NiS (Costa et al., 1981b).
Influence of inhibitors
Dansylcadaverine, known as monodansylcadaverine (MDC), inhibited the endocytosis of crystalline αNi3S2 by 66% at 100 μm and did so in a dose-dependent manner (Costa et al., 1981a). The meaning of this inhibition is unclear and points to CME, but to some to extent to macropinocytosis and phagocytosis as well. The impact of MDC is contested with some studies indicating that it can inhibit macropinocytosis and phagocytosis while other studies report that it does not (Ivanov, 2008). It is clear however that pre-treatment with 200 μM MDC inhibits CME (Panicker et al., 2006), and further research in this area confirmed that treatment with MDC and knockdown of clathrin results in similar reductions of uptake in both THP-1 cells and macrophages (Lunov et al., 2011). It is unclear however whether MDC at elevated doses could completely inhibit clathrin-mediated endocytosis as later studies have used only one dose (200 μM) and its presence does not completely eliminate uptake (Panicker et al., 2006). Despite the evidence supporting CME here, MDC is considered to be among several inhibitors of CME (potassium depletion, phenylarsie oxide, cytosolic acidification, hypertonic shock, and chlorpromazine) which lack specificity; current recommendations for assessing the role of CME include utilizing an RNAi strategy to target clathrin heavy chain and the α-subunit of AP-2 or an overexpression strategy to target domains that are specific for nucleation and clathrin-coated pit formation (McMahon and Boucrot, 2011).
Inhibition via MDC does not provide definitive evidence of a particular pathway and this lack of specificity is a common problem with uptake inhibitors used to determine uptake route. One class of inhibitors that is commonly used to determine route of uptake are those which target actin. Cytochalasin D, for example, targets and disrupts serine/threonine protein kinase PAK1 (PAK1), a protein which plays a critical role in macropinocytosis by promoting the dorsal ruffling that precedes macropinocytosis (Dharmawardhane et al., 2000). While cytochalasin D inhibits actin polymerization, it lacks selectivity because actin polymerization plays a role not only in macropinocytosis but also in CME and phagocytosis in which it is involved in reshaping the membrane and promoting the formation of lamellipodia and fillipodia (Sarkar et al., 2005; (Kaksonen et al., 2006). In CME, actin does not necessarily play a critical role, but it can be involvled in the late-stage process of membrane scission (McMahon and Boucrot, 2011). Future studies may further evaluate this question by investigating whether PAK1 is in involved in uptake as it is a specific regulator of macropinocytosis and can be utilized to distinguish macropinocytosis from clathrin or receptor mediated endocytosis (Dharmawardhane et al., 2000). PAK1 plays a role in inducing cytoskeletal changes and is particularly associated with changes that lead to the formation of circular ruffles. Rotterlin, which is a more selective inhibitor of macropinocytosis than cytochalasin D and amiloride, can also be used to assess the role of macropinocytosis in uptake (Sarkar et al., 2005).
Particulate nickel compound uptake: clathrin-mediated endocytosis and/or macropinocytosis
We return to the original question: what type of endocytosis occurs for insoluble Ni particles? Many experiments were conducted which provided multiple criteria through which to assess the parameters that influence uptake (Table 3). The evidence presented thus far has provided support for three types of uptake, of which two are more likely. Phagocytosis is highly unlikely due to the selective nature of cells which utilize it and we hypothesize that further study will confirm that CME and/or macropinocytosis are involved instead. The calcium dependence, the membrane ruffling, the predominantly larger particles sizes used in these experiments 1.8-5 μm, the inhibition at 4°C and possibly the inhibition via MDC support macropinocytosis as the dominant process. The inhibition by MDC however strongly implicates CME, as does the inhibition at 4°C, but the calcium sensitivity and the predominance of larger sized particles provide some evidence against CME as the form of uptake. That said, the overlap and the conflict in the results may be the result of both processes occurring simultaneously. The combination of macropinocytosis and CME has been observed in PC-3M cells with nanoparticle, nanoconjugate, and nano-titanium dioxide (Thurn et al., 2011). Final determinations however, cannot be made at this time and require further studies utilizing a carefully chosen selection of uptake inhibitors and/or other strategies which target the unique processes involved in different uptake routes. Last, all the studies mentioned in this section discuss particle uptake in vitro and this may not mimic the in vivo situation. Thus there is a need to conduct uptake studies in the lung epithelial of animal models or perhaps also in organ culture models using human lung epithelial cells to assess how what form of uptake is involved in vivo.
Table 3.
Summary of experiments conducted that assess influence of extracellular and particulate factors on Ni particulate uptake
| Extracellular Factors |
Conditions | Relationship to uptake | Study |
|---|---|---|---|
| Media |
|
No effect |
Heck and Costa, 1982a |
| Temperature |
|
Lower temperatures decrease uptake for NiS, may be related to decrease in cell metabolism |
Heck and Costa, 1982a |
| Calcium |
|
Significant reduction in uptake for amorphous NiS and crystalline Ni3S2 |
Heck and Costa, 1982a |
|
Presence of Other
Metals |
|
Significant reduction in uptake for crystalline Ni3S2 in all cases |
Costa et. al., 1981b |
| Dansylcadaverine |
0, 25, 50, 60, 75, 100 μm |
Significant reduction in uptake for crystalline Ni3S2 at all concentrations |
Costa et. al., 1981a |
| Actinomycin D |
0.1, 1.0, 4.0 μg/mL |
Significant reduction in uptake for crystalline Ni3S2 at all concentrations |
Costa et. al., 1981a |
| Cycloheximide |
1.0, 5.0, 20.0 μg/mL |
Significant reduction in uptake for crystalline Ni3S2 at all concentrations |
Costa et. al., 1981a |
|
Particulate
Factors |
|||
|
Mean Particle
Diameter |
|
<4 μm: actively endocytized 4-5 μm: reduced endocytosis relative to <4 μm >5 μm: not actively endocytized |
Costa et. al., 1981a |
| Structure |
|
Crystalline forms are actively endocytized and amorphous forms are not |
Costa et al, 1982a |
| Charge |
Chemical reduction via LiAlH4 for crystalline and amorphous forms |
Increased uptake |
Heck and Costa, 1982c |
|
Surface
properties |
Solvent cleaning and storage in inert atmosphere |
Increased uptake |
Heck and Costa, 1982c |
|
Ni-S ratio on
surface |
Crystalline NiS had more nickel atoms on surface relative to amorphous NiS |
Unknown |
Abbracchio et al, 1981 |
|
Primary sulfur
oxidation state |
|
Unknown |
Abbracchio et al, 1981 |
| Primary oxidation state not affected by charge reduction with LiAlH4 |
Intracellular Fate of Endocytized Ni Particles
Intracellular movement
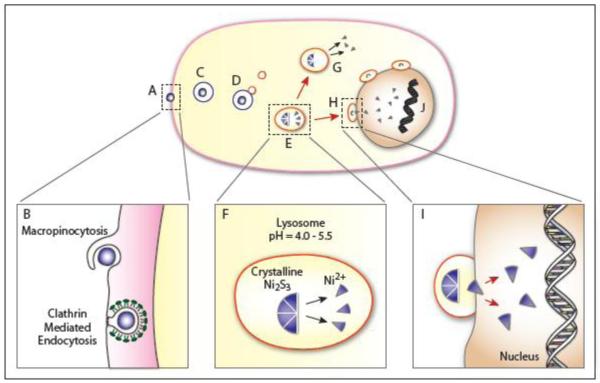
Figure 4 provides a summary of particle endocytosis and intracellular movement based on experimental results and hypotheses. Video time-lapse footage revealed that once inside the cell, the particle begins saltatory movement through the perinuclear area (Figure 4-C) and during which time lysosomes are observed to bind, detach, and then reattach to these particles (Figure 4-D). The particle is soon observed inside of a large vacuole that may be the result of lysosomal fusion (Figure 4-E). Multiple particles accumulate in the cytosol time, and particles aggregate around the nucleus (Figure 4-H). At 24 hours, particles remained aggregated but had ceased most saltatory motion. At 48 hours, particles remained aggregated around the nucleus and demonstrated an aggregation pattern that was commonly observed (Evans et al., 1982). Phagocytized Ni particles were observed in the cytoplasm of cells via light and electron microscopy, but were not visible in the nucleus. Ni presence in the nucleus however was confirmed via chemical analysis, wherein a substantial portion of Ni was coprecipitated with trichloroacetic acid-insoluble material, indicating that Ni was bound to macromolecules (Costa et al., 1981b). The visible presence of Ni in the cytoplasm and lack of such visual confirmation for the nucleus suggest that mechanistically Ni, after it is endocytized, is broken down into smaller particles or more likely water soluble Ni compounds which can enter into the nucleus (Figure 4-I).
Figure 4.
Proposed Model of Particulate Nickel Uptake and Intracellular Distribution
The nickel particle, crystalline Ni3S2 affixes at the cell surface (A). We hypothesize that the particle enters via macropinocytosis and/or clathrin mediated endocytosis - where the different forms of uptake may be related to the size of the particle (B). In macropinocytosis the membrane exhibits ruffling prior to uptake, a feature that was frequently observed during Ni3S2 endocytosis. In clathrin-mediated endocytosis the membrane undergoes a morphological change via invagination and forms a membrane pit. A number of proteins are involved in CME, pictured here are the clathrin proteins that stabilize the pit curvature and the dynamin that aggregates at the neck prior to scission. The endocytized particle moves via saltatory motion towards the nucleus inside some form of vesicle (the specific form will vary in accordance with the form of endocytosis - macropinosome or clathrin coated vesicle) (C). Lysosomes then interact with the vesicle in a process of lysosomal attack (D). These interactions often lead to lysosomal fusion (E). Once fused, the pH of the vesicle may be altered through proton pumps leading to acidification of the vesicle and the dissolution of the crystalline Ni3S2 particle. This process produces high concentrations of Ni2+ (F). In some cases the Ni2+ may exit the vesicle into the cytoplasm where they can interact with biomolecules (G); while in other cases the vesicles will continue to travel towards the nucleus where they will aggregate at the nuclear membrane (H). Those aggregated at the membrane will promote the transfer of Ni2+ ions into the nucleus (I). The presence of Ni2+ can lead to multiple effects including DNA condensation and the modification of epigenetic marks including increased DNA methylation and loss of histone acetylation in H2A, H2B, H3, and H4 as well as an increase in H3K9 dimethylation, H3K4 trimethylation and ubiquitylation of H2A and H2B.
Lysosomal dissolution
The eventual movement of Ni containing particles into lysosomes that appears to occur through fusion events does not provide clear evidence of any particular path as this event can occur during many types of endocytosis. What is important about this event is the eventual dissolution of particulate Ni compounds that likely occurs through lysosomal acidification-a process which has been observed in relation to zinc oxide nanoparticles (Nel et al., 2009). With zinc oxide and other particles, the lysosome proton pump promotes acidification inside the lysosome and the change in pH leads to the dissolution of the particle. The lysosome swells and eventually ruptures, leading to the release of the now ionic form of the metal into the cytoplasm. These ions can now penetrate the nucleus and other organelles. This mechanism would explain how insoluble Ni particles can be responsible for high levels of intracellular Ni2+. Estimates indicate that endocytosis of a 1.5 or 4.0 μm crystalline NiS particle can result in the eventual dissolution and distribution of Ni such that the intracellular concentration of Ni2+ is 0.25 and 4.75 M, respectively (Costa et al., 1981a; Costa and Mollenhauer, 1981). For more soluble forms of Ni where Ni ions enter the cell via transport systems, the concentrations reached within the cell are significantly lower. Cells that were exposed to the soluble form 63NiCl2 were found to concentrate Ni 100 times less than cells exposed to the insoluble 63NiS form (Costa et al., 1981b). The intracellular accumulation of insoluble forms of Ni and the eventual dissolution and dissemination of Ni ions is integral in understanding Ni’s toxic effect. These ions then go on to participate in a number of events in the cell, including oxidative chemistry and binding to biomolecules in the nucleus and other organelles-collectively these events have the capacity to transform cells into carcinogenic forms.
Cell transformation by endocytized Ni particles
Ni’s ability to transform cells has been demonstrated in numerous in vitro studies. Crystalline Ni3S2 transforms Syrian hamster embryo cells resulting in morphological changes which promote colonial growth in soft agar and the formation of tumors in athymic nude mice with exposure times between 24-48 hours (Costa et al., 1979). Another crystalline form, crystalline Ni3Se2, was also effectively endocytized and consequently transformative (Costa and Mollenhauer, 1980a; Costa and Mollenhauer, 1980b). By contrast amorphous NiS, NiCl2, and metallic Ni have significantly lower levels of uptake and very little ability to transform cells when cells are exposed for short periods of time. Thus, at short exposure times the level of uptake is directly related to the level of transformation in SHE where crystalline Ni compounds all demonstrate the highest level of uptake and induce the highest level of transformation. These findings pertain to studies involving short exposure times in which rapid uptake is critical to induce transformation, however in chronic exposures scenarios where soluble forms, such as NiCl2, are maintained in the culture for two months, cellular transformation can and does occur (Pan et al., 2011). While sparingly soluble nickel compounds demonstrate a unique ability to transform cells rapidly, soluble and sparingly soluble forms are both capable of transformation and this commonality points to the shared mechanisms of toxicity for nickel compounds.
Mechanisms of Particulate Ni Toxicity
In vitro cell transformation assays demonstrate that both soluble and sparingly water soluble particulate Ni compounds are toxic to cells and interfere with long-term viability (Dipaolo and Casto, 1979; Costa et al., 1981b; Miura et al., 1989; Patierno et al., 1993; Pan et al,, 2011). However, the persistence of soluble Ni in a cell culture is an artifical situation, which is not natively replicated in vivo because soluble forms are rapidly cleared from tissues. That said, the capacity of soluble forms to induce cell transformation is important because it points to the underlying agent of action that is involved in the toxicity of low and high water soluble nickel compounds, the Ni(II) ions which are the ultimate cellular carcinogen. Because particulate Ni compounds are digested in the lysosome, Ni ions are delivered into the cell at high concentrations, a situation that is similar to prolonged and forced exposure of cells to soluble Ni compounds. In both cases the active agent is Ni(II).
In the context of nano-Ni, Ni(II) are likely to be the active agent as well, as uptake often leads to the eventual dissolution of nanoparticles to smaller particles (Nel et al., 2009). Ni(II) toxicity then concerns all forms of Ni uptake with the possible exception of nickel carbonyl.
Ni(II) behavior however is distinct from that expected of a carcinogen, as it does not demonstrate a high affinity for DNA nor does it act as a mutagen in in vitro assays. It has a low mutagenic activity in a variety of systems (Biggart and Costa, 1986; Arrouijal et al., 1990; Kargacin et al., 1993). The aberrant behavior of this carcinogen prompted investigations into the molecular nature of its toxicity and the primary areas of focus were oxidative damage, epigenetic changes, and its role as a comutagen.
Ni (II) oxidative damage
Ni(II) is an oxidant that promotes oxidative chemistry in the cytosol and nucleus through participation in Fenton chemistry, a process which generates the production of hydroxyl radicals and hydrogen peroxide. These compounds are highly reactive and can go on to participate in reactions with biomolecules leading to lipid peroxidation and membrane damage. Additionally, the redox chemistry of Ni can change when it is complexed into amino acids sequences (Bal et al., 2000). While Ni does participate in Fenton chemistry, its participation is somewhat restricted and requires the presence of a strong oxidizer such as hydrogen peroxide or ascorbate as well as ligand binding to reduce its oxidative potential.
Initially Ni’s involvement in oxidative chemistry was observed by tracking the formation of oxidative base damage (8-hydroxydeoxyguanosine) and depurination of the DNA which was achieved with both Ni3S2 and NiCl2, however only in the presence of H2O2 (Kasprzak and Hernandez, 1989). The first observations of Ni oxidative damage in whole cells were observed in CHO cells exposed to NiCl2 and crystalline Ni3S2 that were probed with 2′, 7′-dichlorofluorescein (DCHF), which fluoresces when oxidized (DCF) and is responsive to the presence of intracellular oxidants particularly H2O2 (Huang et al., 1994a). After only 6 hours both NiCl2 and Ni3S2 induced formation of oxidants in the cytosol. After 18 hours Ni3S2 demonstrated a decline in activity in the cytosol, but increased activity in oxidant production in the nucleus. Additionally oxidant production generated by crystalline Ni3S2 was reduced through treatment with catalase indicating that H2O2 is one of the primary oxidants generated by Ni(II) (Huang et al., 1994b). Later studies demonstrate that in addition to catalase, treatment with superoxide dismutase, n-acetylcysteine, and glutathione all offer protection from oxidative damage induced lymphocyte death in response to Ni3S2 (M’Bemba-Meka et al., 2006). Despite the evidence that oxidative damage is occurring as a result of Ni compound treatment, studies suggest that the magnitude and effects of such damage are not sufficient to implicate oxidative damage as the primary mechanism. The damage does not induce point mutations and therefore cannot explain the magnitude of chromosomal anomalies that are associated with Ni treated cells (Mayer et al., 1998).
In a recent study, BEAS-2B cells were transformed using low doses of NiCl2 and investigators found evidence suggesting that mechanisms other than oxidative damage are of primary importance in cellular transformation via nickel. Here transformation was associated with alterations in signaling that support apoptosis resistance, including the activation of serine-threonine kinase Akt (Akt), the subsequent inhibition of glycogen synthase kinase 3β (GSK3β), the upregulation of the anti-apoptotic proteins, Bcl-2 and Bcl-xL, and the decreased production of ROS (Pan et al., 2011). Further research in this area is required to fully understand the mechanisms of long-term transformation by nickel, but the induction of apoptosis resistance via activation of Akt is likely an important component as Akt activation is found in many cancers and apoptosis resistance would promote the proliferation of cells with aberrant epigenetic profiles, a common feature of nickel treatment.
Ni(II) Epigenetic effects
Ni(II) transformed cells exhibit tumorigenic behavior with morphological transformation, anchorage independent growth, and immortalization due to the inactivation of a senescence gene on the short arm of the X chromosome (Klein et al., 1991). Ni transformation is distinct from that evidenced with other mutagens, as Ni demonstrates low affinity for DNA and does not act as a mutagen in vitro, suggesting another mechanism by which a tumorigenic phenotype is obtained. Beginning with the discovery that Ni-induced methylation silences a senescence gene on the X-chromosome, thereby inhibiting senescence and promoting aberrant growth, the importance of Ni-induced epigenetic effects have been investigated. Epigenetic mechanisms are now considered to be responsible for the onset of carcinogenicity from Ni exposure and at present multiple mechanisms appear to be involved including alterations in DNA methylation, histone modifications (acetylation, methylation or ubiquitylation), structural changes in chromatin, and alteration in the activity of transcription factors (Broday et al., 2000; Karaczyn et al., 2005; Karaczyn et al., 2006; Salnikow and Zhitkovich, 2008; Fragou et al., 2011).
DNA methylation alters gene expression and leads to gene silencing by interfering with DNA binding at promoter sites and promoting the condensation of chromatin (Baylin and Herman, 2000). These epigenetic effects can result in or influence the development of numerous disease outcomes, including cancer (Feinberg et al., 2002). Ni’s interaction with DNA at a structural level is most evident through the G12 gpt system in which Ni was observed to increase DNA methylation, condense chromatin, and lead to heterochromatization of the gpt integration site which occurred if the gene was near heterochromatin but not when it was placed distant from a heterochromatic region (Lee et al., 1995). Methylation changes were also observed in vivo at the p16 promoter which was hypermethylated in Ni-induced tumors in mice (Govindarajan et al., 2002). Mechanistically the process by which Ni induces DNA methylation is unknown. However a proposed model suggests that Ni substitutes for magnesium in the phosphate backbone of DNA, and because Ni is known to have a superior ability to condense DNA such substitution leads to chromatin condensation which triggers de novo DNA methylation in regions adjacent to heterochromatin. Formation of heterochromatin can then lead to the silencing of genes, which are in the newly condensed region, such as the gpt and p16. Silenced genes will be passed along to daughter cells and retain their methylation patterns (Lee et al., 1995).
Ni-induced changes in histone modifications are another mechanism by which Ni alters epigenetic profiles. Changes include loss of histone acetylation in H2A, H2B, H3, and H4 as well as an increase in H3K9 dimethylation, H3K4 trimethylation and ubiquitylation of H2A and H2B at a global level (Arita and Costa, 2009). At a mechanistic level, changes in acetylation are reportedly due to Ni induced inhibition of histone acetyltransferase (Kang et al., 2003), while changes at H2A and H3 may be related to Ni induced oxidative damage caused by Ni binding H3 and H2A, disrupting the structural integrity of the nucleosome (Bal et al., 1999; Bal et al., 2000; Arita and Costa, 2009). Ni can also directly bind to histone H4 and this results in a conformational change that decreases enzyme recognition, and is likely responsible for Ni’s ability to decrease lysine acetylation (Broday et al., 2000; Zoroddu et al., 2010).
Ni has been shown to possess the ability to replace the ferrous iron in the catalytic centers of the iron and 2-oxoglutarate-dependent dioxygenase enzymes, an important group of enzymes which includes the HIF-prolylhydroxylase PHD2, histone demethylase JHD2A/JMD1A, and the DNA repair enzyme ABH3 (Chen and Costa, 2009). Ni’s affinity for these enzymes can lead to a host of interactions between Ni and cellular physiology which includes changes in epigenetic programs and DNA repair. Inhibition of HIF-prolyl-hydroxylases by Ni occurs with a high level of sensitivity and leads to the induction of persistent hypoxic signaling via the stabilization of HIF1α (Davidson et al., 2005; Davidson et al., 2006). For the H3K9 demethylase enzymes, inhibition promotes an increase in global H3K9 dimethylation, which is an important mark for DNA methylation and gene silencing, and Ni-induced increases may explain why Ni promotes global DNA methylation (Chen et al., 2006; Chen and Costa, 2009).
Particulate nickel compounds in vivo
Ni compounds exhibit toxicity in experimental animals and injection of these compounds results in localized formation of tumors at any number of injection sites. As with cellular toxicity, the distinction between soluble and particulate compounds occurs here as well with soluble compounds exhibiting far lower levels of toxicity relative to the particulate compounds. Mechanistically, this difference is likely related to cellular uptake and also to rapid clearance that occurs with soluble compounds relative to particulates (Onkelinx et al., 1973). Persistence of Ni at the site of injection is a critical factor that determines whether tumor formation will occur (Kasprzak et al., 1983) and tumor formation from soluble compounds has been experimentally observed with repeated intraperitoneal injections that increase the dose beyond the amount necessary to induce tumor formation via insoluble compounds (Pott et al., 1992). Intraperitoneal injections of NiCl2 occurring at a dose of fifty 1 mg injections twice weekly induced tumor formation in 12.5% of female Wistar rats. This dose occurs in contrast with the more potent carcinogen Ni3S2, which induced tumor formation with one 6 mg injection in 55.6% of female Wistar rats (Pott et al., 1992). Other studies have confirmed the ability of Ni3S2 to induce tumors via intramuscular injection in Fischer F34 rats, and demonstrated that this process can be inhibited through supplementation with magnesium (Kasprzak et al., 1985). Mouse strains demonstrate differences in susceptibility to the formation of tumors from intramuscular injection with Ni3S2, where variation occurs in conjunction with low basal levels of GSH and high levels of LPO in muscle tissue, indicating that differences in response may be linked to oxidative damage (Rodriguez et al., 1996) and are not likely associated with endogenous levels of metallothionein (Waalkes et al., 2005). Inhalation studies in which exposure to Ni3S2 occurred at multiple doses for two years in rats supported epidemiologic findings that Ni3S2 induced chronic inflammatory conditions, fibrosis of the lung and the development of tumors (Dunnick et al., 1995; Oller et al., 1997). Tumorigenic responses were not observed in mice but there were noncarcinogenic lung effects. The soluble form nickel sulfate (NiSO4) did induce noncarcinogenic lung effects, but to a much lesser extent than Ni3S2. In a recent review article Goodman et. al. suggest that the lack of carcinogenicity found for soluble nickel compounds in inhalations studies results from the fact that soluble nickel compounds are not likely to deliver a large enough dose of nickel ions to respiratory epithelial cells to promote tumorigenesis due to the rapid lung clearance and the fact that endocytic processes favor uptake of sparingly soluble forms (2011).
Most research supports the notion that sparingly soluble forms of nickel present the greatest threat, but there is some experimental evidence indicating that soluble forms of nickel also demonstrate transformative and tumorigenic capacities. The biological relevance of these findings is unclear and debate remains about how to evaluate the biological effects of soluble nickel compounds, especially in the context of inhalation studies and workplace exposure assessments. In an attempt to clarify understanding in this area, the nickel ion bioavailability model has been proposed which suggests that the dose of nickel ions available does not directly correspond with solubility of nickel compounds but instead depends on the release of nickel ions from the compound and their subsequent delivery to the nucleus of epithelial respiratory cells (Goodman et al., 2011). In turn, if the concentration of ions reaching the nucleus is not sufficient then tumor formation is unlikely. Again this returns us to the question of uptake and whether the nickel compounds in question persist long enough at the site of injection or inhalation to gain entry into the cell. Clearly further study is still required in order to fully understand the impact of these processes in humans.
Conclusion
Through a detailed review of particulate nickel uptake we have demonstrated that parameters which determine uptake are variable - and result from the confluence of many factors. Surface charge, physical structure, size thresholds, calcium levels, and metabolic activity are all factors which influence the level of particulate uptake. The adjustment of any one parameter can alter uptake and thereby alter in vitro toxicity of a Ni compound. This sensitivity in the parameters governing uptake mandate that a clear understanding of uptake is integral in toxicity assessments, and as research in the area of nano-Ni proceeds foreword it is imperative that such considerations be kept in the forefront of particle engineering.
Acknowledgments
This work was supported by grant number 5T32 ES007324-12, ES000260, ES010344, ES014454 and ES005512 from NIH/NIEHS.
Abbreviations
- CME
clathrin mediated endocytosis
- Ni3S2
nickel subsulfide
- NiS
nickel sulfide
- NiSO4
nickel sulfate
- MDC
monodansylcadaverine
- DMT1
divalent metal transporter 1
- CHO
Chinese hamster ovary
- SHE
Syrian hamster embryo
- ROS
reactive oxygen species
- nano-NH
nano-nickel hydroxide
- nano-NS
nano-nickel sulfate
- nano-ni
nano-nickel
- AP-2
adaptor protein
- FcγR
Fc domain of IgG
- PI3K
phosphotidylinositol 3-kinase
- PAK1
serine/threonine protein kinase PAK1
- Akt
serine-threonine kinase Akt
- GSK3β
glycogen synthase kinase 3β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Abbracchio MP, Evans RM, Heck JD, Cantoni O, Costa M. The regulation of ionic nickel uptake and cyto-toxicity by specific amino-acids and serum components. Biological Trace Element Research. 1982;4:289–301. doi: 10.1007/BF02786543. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Heck JD, Caprioli RM, Costa M. Differences in surface-properties of amorphous and crystalline metal sulfides may explain their toxicological potency. Chemosphere. 1981;10:897–908. [Google Scholar]
- Andersen A, Berge SR, Engeland A, Norseth T. Exposure to nickel compounds and smoking in relation to incidence of lung and nasal cancer among nickel refinery workers. Occupational and Environmental Medicine. 1996;53:708–713. doi: 10.1136/oem.53.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anke M, Angelow L, Glei M, Muller M, Illing H. The biological importance of nickel in the food-chain. Fresenius J. Anal. Chem. 1995;352:92–96. [Google Scholar]
- Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1:222–228. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrouijal FZ, Hildebrand HF, Vophi H, Marzin D. Genotoxic activity of nickel subsulphide alpha-ni3s2. Mutagenesis. 1990;5:583–589. doi: 10.1093/mutage/5.6.583. [DOI] [PubMed] [Google Scholar]
- Bal W, Karantza V, Moudrianakis EN, Kasprzak KS. Interaction of nickel(II) with histones: In vitro binding of nickel(II) to the core histone tetramer. Arch. Biochem. Biophys. 1999;364:161–166. doi: 10.1006/abbi.1999.1137. [DOI] [PubMed] [Google Scholar]
- Bal W, Liang RT, Lukszo J, Lee SH, Dizdaroglu M, Kasprzak KS. Ni(II) specifically cleaves the C-terminal tail of the major variant of histone H2A and forms an oxidative damage mediating complex with the cleaved-off octapeptide. Chemical Research in Toxicology. 2000;13:616–624. doi: 10.1021/tx000044l. [DOI] [PubMed] [Google Scholar]
- Bartneck M, Keul HA, Singh S, Czaja K, Bornemann J, Bockstaller M, Moeller M, Zwadlo-Klarwasser G, Groll J. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry. ACS Nano. 2010;4:3073–3086. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis - epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- Bennett BG. Environmental nickel pathways to man. Iarc Scientific Publications. 1984:487–495. [PubMed] [Google Scholar]
- Biggart NW, Costa M. Assessment of the uptake and mutagenicity of nickel chloride in salmonella tester strains. Mutation Research. 1986;175:209–215. doi: 10.1016/0165-7992(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Broday L, Peng W, Kuo MH, Salnikov K, Zoroddu M, Costa M. Nickel compounds are novel inhibitors of histone H4 acetylation. Cancer Research. 2000;60:238–241. [PubMed] [Google Scholar]
- Chen H, Costa M. Iron- and 2-oxoglutarate-dependent Dioxygenases: an emerging group of molecular targets for nickel toxicity and carcinogenicity. Biometals. 2009;22:191–196. doi: 10.1007/s10534-008-9190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HB, Davidson T, Singleton S, Garrick MD, Costa M. Nickel decreases cellular iron level and converts cytosolic aconitase to iron-regulatory protein 1 in A549 cells. Toxicology and Applied Pharmacology. 2005;206:275–287. doi: 10.1016/j.taap.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Chen HB, Giri NC, Zhang RH, Yamane K, Zhang Y, Maroney M, Costa M. Nickel Ions Inhibit Histone Demethylase JMJD1A and DNA Repair Enzyme ABH2 by Replacing the Ferrous Iron in the Catalytic Centers. Journal of Biological Chemistry. 2010;285:7374–7383. doi: 10.1074/jbc.M109.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HB, Ke QD, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Molecular and Cellular Biology. 2006;26:3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- Costa M, Abbracchio MP, Simmonshansen J. Factors influencing the phagocytosis, neoplastic transformation, and cyto-toxicity of particulate nickel compounds in tissue-culture systems. Toxicology and Applied Pharmacology. 1981a;60:313–323. doi: 10.1016/0041-008x(91)90234-6. [DOI] [PubMed] [Google Scholar]
- Costa M, Heck JD, Robison SH. Selective phagocytosis of crystalline metal sulfide particles and dna strand breaks as a mechanism for the induction of cellular-transformation. Cancer Research. 1982;42:2757–2763. [PubMed] [Google Scholar]
- Costa M, Mollenhauer HH. Carcinogenic activity of particulate nickel compounds is proportional to their cellular uptake. Science. 1980a;209:515–517. doi: 10.1126/science.7394519. [DOI] [PubMed] [Google Scholar]
- Costa M, Mollenhauer HH. Phagocytosis of nickel subsulfide particles during the early stages of neoplastic transformation in tissue-culture. Cancer Research. 1980b;40:2688–2694. [PubMed] [Google Scholar]
- Costa M, Mollenhauer HH. Phagocytosis of particulate nickel compounds is related to their carcinogenic activity. Annals of Clinical and Laboratory Science. 1981;11:85–85. [Google Scholar]
- Costa M, Mollenhauer HH, Jones MK. Carcinogenic activity of nickel compounds may be related to their cellular uptake. Federation Proceedings. 1980;39:395–395. [Google Scholar]
- Costa M, Nye J, Sunderman FWJ. Morphological transformation of syrian hamster fetal cells induced by nickel compounds. Annals of Clinical and Laboratory Science. 1978;8:502. [Google Scholar]
- Costa M, Nye JS, Sunderman FW, Allpass PR, Gondos B. Induction of sarcomas in nude-mice by implantation of syrian-hamster fetal cells exposed invitro to nickel subsulfide. Cancer Research. 1979;39:3591–3597. [PubMed] [Google Scholar]
- Costa M, Simmonshansen J, Bedrossian CWM, Bonura J, Caprioli RM. Phagocytosis, cellular-distribution, and carcinogenic activity of particulate nickel compounds in tissue-culture. Cancer Research. 1981b;41:2868–2876. [PubMed] [Google Scholar]
- Davidson T, Chen HB, Garrick MD, D’Angelo G, Costa M. Soluble nickel interferes with cellular iron homeostasis. Molecular and Cellular Biochemistry. 2005;279:157–162. doi: 10.1007/s11010-005-8288-y. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chen H, Di Toro DM, D’Angelo G, Costa M. Soluble nickel inhibits HIF-prolyl-hydroxylases creating persistent hypoxic signaling in A549 cells. Molecular Carcinogenesis. 2006;45:479–489. doi: 10.1002/mc.20176. [DOI] [PubMed] [Google Scholar]
- Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Critical Reviews in Oncology Hematology. 2002;42:35–56. doi: 10.1016/s1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Molecular Biology of the Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipaolo JA, Casto BC. Quantitative studies of invitro morphological transformation of syrian-hamster cells by inorganic metal-salts. Cancer Research. 1979;39:1008–1013. [PubMed] [Google Scholar]
- Doll R, Andersen A, Cooper WC, Cosmatos I, Cragle DL, Easton D, Enterline P, Goldberg M, Metcalfe L, Norseth T, Peto J, Rigaut JP, Roberts R, Seilkop SK, Shannon H, Speizer F, Sunderman FW, Thornhill P, Warner JS, Weglo J, Wright M. Report of the international-committee-on-nickel-carcinogenesis-in-man. Scandinavian Journal of Work Environment & Health. 1990;16:1–82. doi: 10.5271/sjweh.1813. [DOI] [PubMed] [Google Scholar]
- Doll R, Mathews JD, Morgan LG. Cancers of lung and nasal sinuses in nickel workers - reassessment of period of risk. British Journal of Industrial Medicine. 1977;34:102–105. doi: 10.1136/oem.34.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Morgan LG. Cancers of lung and nasal sinuses in nickel workers. British Journal of Cancer. 1970;24:623. doi: 10.1038/bjc.1970.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda-Chodak A, Blaszczyk U. The impact of nickel on human health. J. Elem. 2008;13:685–696. [Google Scholar]
- Dunnick JK, Elwell MR, Radovsky AE, Benson JM, Hahn FF, Nikula KJ, Barr EB, Hobbs CH. Comparative carcinogenic effects of nickel subsulfide, nickel-oxide, or nickel sulfate hexahydrate chronic exposures in the lung. Cancer Research. 1995;55:5251–5256. [PubMed] [Google Scholar]
- Evans RM, Davies PJA, Costa M. Visualization of cellular uptake and dissolution of carcinogenic alpha-nis particles by video intensification microscopy. Journal of Cell Biology. 1981;91:A419–A419. [Google Scholar]
- Evans RM, Davies PJA, Costa M. Uptake and intracellular fate of nickel compounds in cultured-cells. Federation Proceedings. 1982a;41:1225–1225. [Google Scholar]
- Evans RM, Davies PJA, Costa M. Video time-lapse microscopy of phagocytosis and intracellular fate of crystalline nickel sulfide particles in cultured mammalian-cells. Cancer Research. 1982;42:2729–2735. [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. Journal of Cell Science. 2006;119:4758–4769. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Oshimura M, Barrett JC. Epigenetic mechanisms in human disease. Cancer Research. 2002;62:6784–6787. [PubMed] [Google Scholar]
- Flyvholm MA, Nielsen GD, Andersen A. Nickel content of food and estimation of dietary-intake. Z. Lebensm.-Unters.-Forsch. 1984;179:427–437. doi: 10.1007/BF01043419. [DOI] [PubMed] [Google Scholar]
- Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicology Mechanisms and Methods. 2011;21:343–352. doi: 10.3109/15376516.2011.557878. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Inoue T, Shimada H, Kojima S. The mechanisms of nickel uptake by rat primary hepatocyte cultures: role of calcium channels. Toxicology. 1997;124:21–26. doi: 10.1016/s0300-483x(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Gillespie PA, Kang GS, Elder A, Gelein R, Chen L, Moreira AL, Koberstein J, Tchou-Wong KM, Gordon T, Chen LC. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology. 2010;4:106–119. doi: 10.3109/17435390903470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JE, Prueitt RL, Dodge DG, Thakali S. Carcinogenicity assessment of water-soluble nickel compounds. Critical Reviews in Toxicology. 2009;39:365–417. doi: 10.1080/10408440902762777. [DOI] [PubMed] [Google Scholar]
- Goodman JE, Prueitt RL, Thakali S, Oller AR. The nickel ion bioavailability model of the carcinogenic potential of nickel-containing substances in the lung. Critical Reviews in Toxicology. 2011;41:142–174. doi: 10.3109/10408444.2010.531460. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, Bai XH, LaMontagne K, Arbiser JL. Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Molecular Medicine. 2002;8:1–8. [PMC free article] [PubMed] [Google Scholar]
- Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Current Opinion in Immunology. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environmental Toxicology and Chemistry. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- Guo DD, Wu CH, Li XM, Jiang H, Wang XM, Chen BA. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. Journal of Nanoscience and Nanotechnology. 2008;8:2301–2307. doi: 10.1166/jnn.2008.311. [DOI] [PubMed] [Google Scholar]
- Heck JD, Costa M. Extracellular requirements for the endocytosis of carcinogenic crystalline nickel sulfide particles by facultative phagocytes. Toxicology Letters. 1982a;12:243–250. doi: 10.1016/0378-4274(82)90247-8. [DOI] [PubMed] [Google Scholar]
- Heck JD, Costa M. Factors which modulate phagocytosis of potentially carcinogenic nickel sulfide particles. Federation Proceedings. 1982b;41:1225–1225. [Google Scholar]
- Heck JD, Costa M. Surface reduction of amorphous nis particles potentiates their phagocytosis and subsequent induction of morphological transformation in syrian-hamster embryo cells. Cancer Letters. 1982c;15:19–26. doi: 10.1016/0304-3835(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Herlantpeers MC, Hildebrand HF, Kerckaert JP. Invitro and invivo incorporation of Ni-63 ii into lung and liver subcellular-fractions of balb/c mice. Carcinogenesis. 1983;4:387–392. doi: 10.1093/carcin/4.4.387. [DOI] [PubMed] [Google Scholar]
- Horak E, Sunderma Fw. Fecal nickel excretion by healthy adults. Clinical Chemistry. 1973;19:429–430. [PubMed] [Google Scholar]
- Horie M, Nishio K, Fujita K, Kato H, Nakamura A, Kinugasa S, Endoh S, Miyauchi A, Yamamoto K, Murayama H, Niki E, Iwahashi H, Yoshida Y, Nakanishi J. Ultrafine NiO Particles Induce Cytotoxicity in Vitro by Cellular Uptake and Subsequent Ni(II) Release. Chemical Research in Toxicology. 2009;22:1415–1426. doi: 10.1021/tx900171n. [DOI] [PubMed] [Google Scholar]
- Huang X, Klein CB, Costa M. Crystalline ni3s2 specifically enhances the formation of oxidants in the nuclei of cho cells as detected by dichlorofluorescein. Carcinogenesis. 1994a;15:545–548. doi: 10.1093/carcin/15.3.545. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhuang ZX, Frenkel K, Klein CB, Costa M. The role of nickel and nickel-mediated reactive oxygen species in the mechanism of nickel carcinogenesis. Environmental Health Perspectives. 1994b;102:281–284. doi: 10.1289/ehp.94102s3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN. Toxicity and Developmental Defects of Different Sizes and Shape Nickel Nanoparticles in Zebrafish. Environmental Science & Technology. 2009;43:6349–6356. doi: 10.1021/es9010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI. Pharmacological Inhibition of Endocytic Pathways: Is it specific enough to be useful? Methods in Molecular Biology. 2008;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nature Reviews Molecular Cell Biology. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kang J, Zhang YT, Chen J, Chen HF, Lin CJ, Wang Q, Ou YX. Nickel-induced histone hypoacetylation: The role of reactive oxygen species. Toxicological Sciences. 2003;74:279–286. doi: 10.1093/toxsci/kfg137. [DOI] [PubMed] [Google Scholar]
- Karaczyn AA, Golebiowski F, Kasprzak KS. Truncation, deamidation, and oxidation of histone H2B in cells cultured with nickel(II) Chemical Research in Toxicology. 2005;18:1934–1942. doi: 10.1021/tx050122a. [DOI] [PubMed] [Google Scholar]
- Karaczyn AA, Golebiowski F, Kasprzak KS. Ni(II) affects ubiquitination of core histones H2B and H2A. Exp. Cell Res. 2006;312:3252–3259. doi: 10.1016/j.yexcr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Kargacin B, Klein CB, Costa M. Mutagenic responses of nickel oxides and nickel sulfides in chinese-hamster v79 cell-lines at the xanthine guanine phosphoribosyl transferase locus. Mutation Research. 1993;300:63–72. doi: 10.1016/0165-1218(93)90141-y. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Gustafsson J, Cronholm P, Moller L. Size-dependent toxicity of metal oxide particles-A comparison between nano- and micrometer size. Toxicology Letters. 2009;188:112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Kasprzak KS, Gabryel P, Jarczewska K. Carcinogenicity of nickel(ii)hydroxides and nickel(ii)sulfate in Wistar rats and its relation to the invitro dissolution rates. Carcinogenesis. 1983;4:275–279. doi: 10.1093/carcin/4.3.275. [DOI] [PubMed] [Google Scholar]
- Kasprzak KS, Quander RV, Poirier LA. Effects of calcium and magnesium salts on nickel subsulfide carcinogenicity in fischer rats. Carcinogenesis. 1985;6:1161–1166. doi: 10.1093/carcin/6.8.1161. [DOI] [PubMed] [Google Scholar]
- Kasprzak KS, Hernandez L. Enhancement of hydroxylation and deglycosylation of 2′-deoxyguanosine by carcinogenic nickel compounds. Cancer Research. 1989;49:5964–5968. [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, Inoue S, Nishino K. Distinct mechanisms of oxidative DNA damage induced by carcinogenic nickel subsulfide and nickel oxides. Environmental Health Perspectives. 2002;110:789–791. doi: 10.1289/ehp.02110s5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q, Davidson T, Chen H, Kluz T, Costa M. Alterations of histone modifications and transgene silencing by nickel chloride. Carcinogenesis. 2006;27:1481–1488. doi: 10.1093/carcin/bgl004. [DOI] [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles - isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Klein CB, Conway K, Wang XW, Bhamra RK, Lin XH, Cohen MD, Annab L, Barrett JC, Costa M. Senescence of nickel-transformed cells by an X-chromosome - possible epigenetic control. Science. 1991;251:796–799. doi: 10.1126/science.1990442. [DOI] [PubMed] [Google Scholar]
- Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, Zhitkovich A, Christie NT, Costa M. Carcinogenic nickel silences gene-expression by chromatin condensation and dna methylation - a new model for epigenetic carcinogens. Molecular and Cellular Biology. 1995;15:2547–2557. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light WG, Wei ET. Surface-charge and hemolytic activity of asbestos. Environmental Research. 1977;13:135–145. doi: 10.1016/0013-9351(77)90012-3. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang J-S, Maciejczyk P, Chen L-C. Cardiovascular effects of nickel in ambient air. Environmental Health Perspectives. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunov O, Syrovets T, Loos C, Beil J, Delecher M, Tron K, Nienhaus GU, Musyanovych A, Mailander V, Landfester K, Simmet T. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano. 2011;5:1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- M’Bemba-Meka P, Lemieux N, Chakrabarti SK. Role of oxidative stress, mitochondrial membrane potential, and calcium homeostasis in nickel subsulfide-induced human lymphocyte death in vitro. Science of the Total Environment. 2006;369:21–34. doi: 10.1016/j.scitotenv.2006.04.007. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Reviews Molecular Cell Biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Mailander V, Landfester K. Interaction of Nanoparticles with Cells. Biomacromolecules. 2009;10:2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. Journal of Cell Science. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- Mayer C, Klein RG, Wesch H, Schmezer P. Nickel subsulfide is genotoxic in vitro but shows no mutagenic potential in respiratory tract tissues of Big Blue (TM) rats and Muta (TM) Mouse mice in vivo after inhalation. Mutation Research-Genetic Toxicology and Environmental Mutagenesis. 1998;420:85–98. doi: 10.1016/s1383-5718(98)00140-5. [DOI] [PubMed] [Google Scholar]
- Meng H, Yang S, Li ZX, Xia T, Chen J, Ji ZX, Zhang HY, Wang X, Lin SJ, Huang C, Zhou ZH, Zink JI, Nel AE. Aspect Ratio Determines the Quantity of Mesoporous Silica Nanoparticle Uptake by a Small GTPase-Dependent Macropinocytosis Mechanism. ACS Nano. 2011;5:4434–4447. doi: 10.1021/nn103344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nature Cell Biology. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Migdal C, Rahal R, Rubod A, Callejon S, Colomb E, Atrux-Tallau N, Haftek M, Vincent C, Serres M, Daniele S. Internalisation of hybrid titanium dioxide/para-amino benzoic acid nanoparticles in human dendritic cells did not induce toxicity and changes in their functions. Toxicology Letters. 2010;199:34–42. doi: 10.1016/j.toxlet.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Miura T, Patierno SR, Sakuramoto T, Landolph JR. Morphological and neoplastic transformation of C3H-10T1/2 CL-8 mouse embryo cells by insoluble carcinogenic nickel compounds. Environ. Mol. Mutagen. 1989;14:65–78. doi: 10.1002/em.2850140202. [DOI] [PubMed] [Google Scholar]
- Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occupational and Environmental Medicine. 2007;64:609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi PK, Vanjaarsveld PP, Lippoldt RE, Edelhoch H. Effect of basic compounds on the polymerization of clathrin. Biochemistry. 1981;20:6706–6710. doi: 10.1021/bi00526a028. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Nunes P, Demaurex N. The role of calcium signaling in phagocytosis. Journal of Leukocyte Biology. 2010;88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. Journal of Cell Science. 2000;113:3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- Oller AR, Costa M, Oberdorster G. Carcinogenicity assessment of selected nickel compounds. Toxicology and Applied Pharmacology. 1997;143:152–166. doi: 10.1006/taap.1996.8075. [DOI] [PubMed] [Google Scholar]
- Oller AR, Oberdorster G. Incorporation of particle size differences between animal studies and human workplace aerosols for deriving exposure limit values. Regul. Toxicol. Pharmacol. 2010;57:181–194. doi: 10.1016/j.yrtph.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Onkelinx C, Becker J, Sunderma Fw. Compartmental analysis of metabolism of Ni-63(ii) in rats and rabbits. Research Communications in Chemical Pathology and Pharmacology. 1973;6:663–676. [PubMed] [Google Scholar]
- Oskarsson A, Tjalve H. Autoradiographic study on the distribution of (Ni-63)-CL - 2 in mice. Annals of Clinical and Laboratory Science. 1979;9:47–59. [PubMed] [Google Scholar]
- Pan J, Chang Q, Wang X, Son Y, Liu J, Zhang Z, Bi Y, Shi X. Activation of Akt/GSK3β and Akt/Bcl-2 signaling pathways in nickel-transformed BEAS-2B cells. International Journal of Oncology. 2011;39:1285–1294. doi: 10.3892/ijo.2011.1157. [DOI] [PubMed] [Google Scholar]
- Panicker AK, Buhusi M, Erickson A, Maness PF. Endocytosis of beta 1 integrins is an early event in migration promoted by the cell adhesion molecule L1. Exp. Cell Res. 2006;312:299–307. doi: 10.1016/j.yexcr.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Patierno SR, Dirscherl LA, Xu J. Transformation of rat tracheal epithelial-cells to immortal growth variants by particulate and soluble nickel compounds. Mutation Research. 1993;300:179–193. doi: 10.1016/0165-1218(93)90049-j. [DOI] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Phillips JI, Green FY, Davies JCA, Murray J. Pulmonary and Systemic Toxicity Following Exposure to Nickel Nanoparticles. American Journal of Industrial Medicine. 2010;53:763–767. doi: 10.1002/ajim.20855. [DOI] [PubMed] [Google Scholar]
- Pott F, Rippe RM, Roller M, Csicsaky M, Rosenbruch M, Huth F. Carcinogenicity of nickel compounds and nickel alloys in rats by I.P. injection. In: Nieboer E, Nriagu JO, editors. Nickel and Human Health: Current Perspectives. John Wiley and Sons; New York: 1992. pp. 491–502. [Google Scholar]
- Rauch JN, Pacyna JM. Earth’s global Ag, Al, Cr, Cu, Fe, Ni, Pb, and Zn cycles. Glob. Biogeochem. Cycle. 2009;23:16. [Google Scholar]
- Refsvik T, Andreassen T. Surface binding and uptake of nickel(ii) in human epithelial kidney-cells - modulation by ionomycin, nicardipine and metals. Carcinogenesis. 1995;16:1107–1112. doi: 10.1093/carcin/16.5.1107. [DOI] [PubMed] [Google Scholar]
- Rodriguez RE, Misra M, Diwan BA, Riggs CW, Kasprzak KS. Relative susceptibilities of C57BL/6, (C57BL/6xC3H/He)F-1, and C3H/He mice to acute toxicity and carcinogenicity of nickel subsulfide. Toxicology. 1996;107:131–140. doi: 10.1016/0300-483x(95)03251-a. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: Nickel, arsenic, and chromium. Chemical Research in Toxicology. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K, Kruhlak MJ, Erlandsen SL, Shaw S. Selective inhibition by rottlerin of macropinocytosis in monocyte-derived dendritic cells. Immunology. 2005;116:513–524. doi: 10.1111/j.1365-2567.2005.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BJ, Handy RD. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environment International. 2011;37:1083–1097. doi: 10.1016/j.envint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Sunderma Fw, Selin CE. Metabolism of nickel-63 carbonyl. Toxicology and Applied Pharmacology. 1968;12:207. doi: 10.1016/0041-008x(68)90033-1. [DOI] [PubMed] [Google Scholar]
- Sunderman FW. Review of carcinogenicities of nickel, chromium and arsenic compounds in man and animals. Prev. Med. 1976;5:279–294. doi: 10.1016/0091-7435(76)90045-1. [DOI] [PubMed] [Google Scholar]
- Sunderman FW. Carcinogenic effects of metals. Federation Proceedings. 1978;37:40–46. [PubMed] [Google Scholar]
- Sunderman FW, Maenza RM. Comparisons of carcinogenicities of nickel compounds in rats. Research Communications in Chemical Pathology and Pharmacology. 1976;14:319–330. [PubMed] [Google Scholar]
- Sunderman FW, Hopfer SM, Sweeney KR, Marcus AH, Most BM, Creason J. Nickel absorption and kinetics in human volunteers. Proceedings of the Society for Experimental Biology and Medicine. 1989;191:5–11. doi: 10.3181/00379727-191-42881. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Interaction of asbestos with alveolar cells. Environmental Health Perspectives. 1974;9:241–252. doi: 10.1289/ehp.749241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurn KT, Arora H, Paunesku T, Wu AG, Brown EMB, Doty C, Kremer J, Woloschak G. Endocytosis of titanium dioxide nanoparticles in prostate cancer PC-3M cells. Nanomedicine-Nanotechnology Biology and Medicine. 2011;7:123–130. doi: 10.1016/j.nano.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: Complexity in action. Annual Review of Immunology. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Vincent JH, Ramachandran G, Kerr SM. Particle size and chemical species ‘fingerprinting’ of aerosols in primary nickel production industry workplaces. J. Environ. Monit. 2001;3:565–574. doi: 10.1039/b105848g. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Kasprzak KS, Diwan BA. Metallothionein-I/II double knockout mice are no more sensitive to the carcinogenic effects of nickel subsulfide than wild-type mice. International Journal of Toxicology. 2005;24:215–220. doi: 10.1080/10915810591000668. [DOI] [PubMed] [Google Scholar]
- Warheit DB. Debunking Some Misconceptions about Nanotoxicology. Nano Lett. 2010;10:4777–4782. doi: 10.1021/nl103432w. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Colvin VL, Reed KL, Sayes CR. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicological Sciences. 2007;95:270–280. doi: 10.1093/toxsci/kfl128. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicological Sciences. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao Y, Liu Y, Chang XL, Chen CY, Zhao YL. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- Zhao JS, Shi XL, Castranova V, Ding M. Occupational Toxicology of Nickel and Nickel Compounds. Journal of Environmental Pathology Toxicology and Oncology. 2009;28:177–208. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i3.10. [DOI] [PubMed] [Google Scholar]
- Zoroddu MA, Peana M, Medici S, Casella L, Monzani E, Costa M. Nickel binding to histone H4. Dalton Transactions. 2010;39:787–793. doi: 10.1039/b916019c. [DOI] [PubMed] [Google Scholar]