Abstract
The concept that developmental insults (for example, poor pre- or postnatal nutrition) can have long-term consequences on health and well-being of the offspring has been termed developmental programming. In livestock, developmental programming affects production traits, including growth, body composition, and reproduction. Although low birth weight was used as a proxy for compromised fetal development in the initial epidemiological studies, based on controlled studies using livestock and other animal models in the last two decades we now know that developmental programming can occur independently of any effects on birth weight. Studies in humans, rodents, and livestock also have confirmed the critical role of the placenta in developmental programming. In addition, the central role of epigenetic regulation in developmental programming has been confirmed. Lastly, relatively simple therapeutic/management strategies designed to ‘rescue’ placental development and function are being developed to minimize the effects of developmental programming on health and productivity of humans, livestock, and other mammals.
Keywords: Developmental programming, Health and productivity, Placenta, Epigenetics, Therapeutic/management strategies
1. Introduction and developmental programming
Infants that are growth-restricted or developmentally compromised in some other way (e.g., altered development of specific organs) have an increased risk of health complications not just as infants but throughout their lifespan, including a range of metabolic, neurological, behavioral, and reproductive disabilities. Although originally dubbed ‘the Barker hypothesis,’ or ‘fetal and infant origins of adult disease,’ more recently this concept has been re-named developmental programming, or developmental origins of health and disease (Barker, 1992, 2004; Paneth and Susser, 1995; Armitage et al., 2004; Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010b). The general idea is that something that happens while in utero or during infancy can have long-term effects on the health and well being of that individual.
Throughout the world, human epidemiological studies have provided convincing support for the concept of developmental programming by showing a strong association between low birth weight, or other developmental problems such as exposure to stress-related hormones (such as cortisol, which is important for preparing the fetus for birth and also for initiating parturition), and the subsequent risk of developing a range of pathologies as adults, including poor growth, obesity, type 2 diabetes, cardiovascular disease, immune dysfunction, and behavioral problems (Figure 1; Armitage et al., 2004; Barker, 2004; Luther et al., 2005; Wallace et al., 2006; Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010b). It is clear that these pathologies have a major impact on the quality of life and, ultimately, will reduce life expectancy. Thus, reducing the incidence of low birth weight or developmentally compromised offspring has the potential to affect both the immediate survival and lifelong health of an individual. In addition, although all of these pathologies will potentially have a negative effect on offspring productivity, perhaps the greatest impact is that the phenotype may not reflect the offspring’s genetic potential, thereby leading to poor selection decisions for breeding programs (Reynolds et al., 2010b).
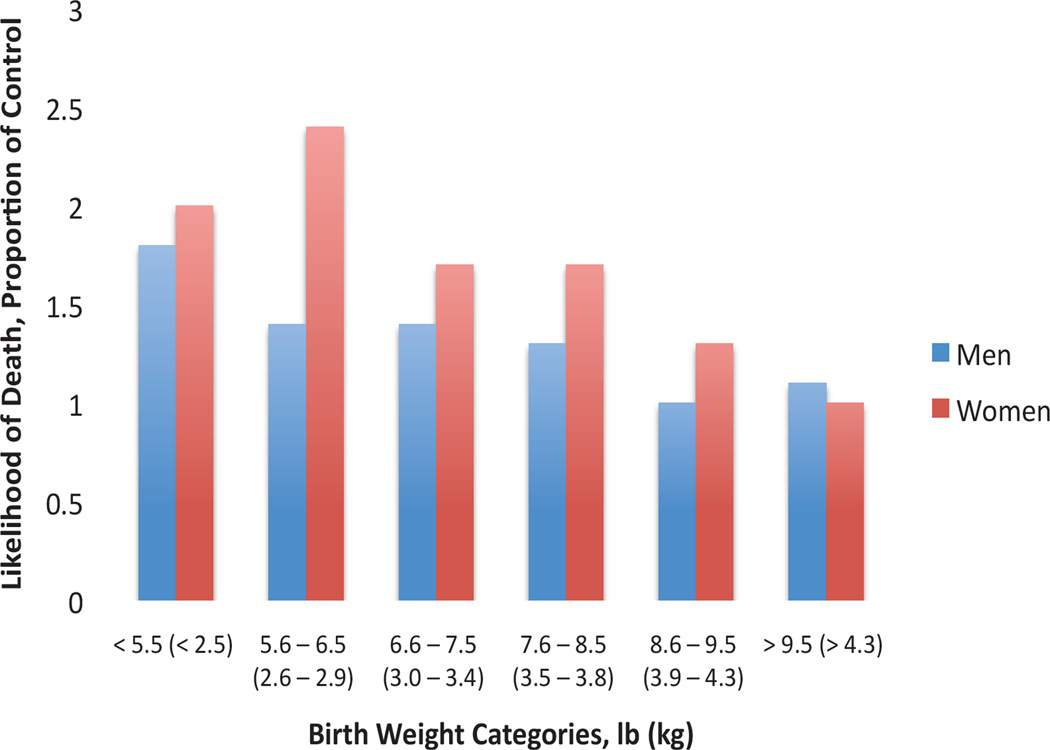
Figure 1.
Likelihood of death due to coronary heart disease as adults for various birth weight categories as a proportion of controls (highest birth weight category). Data from Godfrey and Barker, 2000. Births from Hertfordshire, UK, from 1911–1930. N = 1033 deaths for men and 120 deaths for women.
Maternal nutritional status is implicated in programming nutrient partitioning and ultimately growth, development, and function of the major fetal organ systems (Wallace, 1948; Wallace et al., 1999a; Godfrey and Barker, 2000; Redmer et al., 2004; Luther et al., 2005; Wu et al., 2006; Caton et al., 2007; Caton and Hess 2010; Reynolds et al., 2010), and prenatal growth trajectory is sensitive to the direct and indirect effects of maternal dietary intake from the early embryonic stages (Robinson et al., 1999). These observations are highly relevant to the current discussion because both pre-term delivery and fetal growth restriction are associated with increased risk of neonatal mortality, morbidity, and in some cases growth efficiency. Additionally, neonates that were growth restricted in utero are at risk of immediate postnatal complications and may also exhibit poor growth and development, with significant consequences later in life (Barker et al., 1993; Godfrey and Barker, 2000; Barker, 2004; Wu, 2006; Caton and Hess, 2010; Reynolds et al., 2010). Moreover, across all livestock species in the U.S., neonatal mortality is approximately 10% with most occurring in the first week after delivery (Wu et al., 2006; Reynolds et al., 2010).
Although less well documented, it seems likely that developmental programming also affects livestock production, especially in extensive production systems such as those prevalent in Intermountain region of the western U.S. and similar environments (e.g., savannahs) throughout the world. In the U.S., for example, livestock are often under a poor nutritional environment during pregnancy due to: (1) breeding of young, often peripubertal, dams and the attendant competi tion for nutrients between the rapidly growing maternal and fetal systems; (2) selection for multiple fetuses (e.g., sheep) or large litter size (e.g., pigs), in which increased numbers of fetuses are strongly associated with reduced fetal size; (3) selection for increased milk production, in which the increased energy demand of lactation competes with the increased energy demand of fetal and placental growth; and (4) breeding of livestock during high environmental temperatures (e.g., summer to early fall) with the subsequent pregnancy during periods of poor pasture conditions (e.g., fall to winter; Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010b). Additionally, in livestock, just as in humans, compromised fetal or neonatal growth has been shown to lead to: (1) increased neonatal morbidity and mortality; (2) altered postnatal growth, including reduced average daily gain and weaning weight; (3) poor body composition, including increased fat, reduced muscle growth, and reduced meat quality; (4) metabolic disorders, such as poor glucose tolerance and insulin resistance; (5) cardiovascular disease; and (6) dysfunction of specific organs, including the ovaries, testes, mammary gland, liver, and small intestine (Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010b). In fact, nearly all organ systems are affected negatively in various animal models of developmental programming (Reynolds et al., 2010b; Table 1).
Table 1.
Systems and bodily processes affected in the fetus and(or) offspring during late pregnancy or postnatally in a variety of animal models of compromised pregnancy.1
| Maternal stressor | System(s) and processes affected |
Authors2 |
|---|---|---|
| Overfeeding of adolescent or adult dams | Body composition, growth, energy balance, adipose, endocrine, gastro-intestinal tract, muscle, placenta, gonads | Abbott DH et al.; |
| Bartol FF et al.; | ||
| Alexander BT et al.; | ||
| Anthony RV et al.; | ||
| Bloomfield FH et al.; | ||
| Caton JS et al.; | ||
| Underfeeding of adolescent or adult dams | Adipose, brain, cardiovascular, endocrine, gastrointestinal tract, kidney, muscle, placenta | Cunningham CP et al.; |
| Du M et al.; | ||
| Ford SP et al.; | ||
| Foxcroft G et al.; | ||
| Funston RN et al.; | ||
| Multiple pregnancy | Behavior, endocrine, placenta | Glover V et al.; |
| Greenwood P et al.; | ||
| McMillen IC et al.; | ||
| Heat-stressed or behaviorally-stressed adult (including prenatal steroid exposure) | Behavior, growth, cardiovascular, endocrine, immune, placenta, gonads | Nathanielsz PW et al.; |
| Padmanabhan V et al.; | ||
| Poston L et al.; | ||
| Reynolds LP et al.; | ||
| Adolescent vs. Adult dams | Endocrine, placenta, gonads | Robinson J et al.; |
| Symonds ME et al.; | ||
| Maternal genotype (adult only) | Growth, gastro-intestinal tract, placenta | Vonnahme KA et al.; |
| Wallace JM et al.; and | ||
| Wintour EM et al. | ||
| Specific nutrients (e.g., protein, se, etc.) | Body composition, growth, endocrine, gastro-intestinal tract, placenta, gonads |
This table is not comprehensive but rather describes some of the major and well-documented animal models of developmental programming. Adapted from Reynolds et al., 2010b.
These author’s names are in a format that can be searched in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/).
Whereas the initial epidemiological studies that led to the concept of developmental programming focused on individuals with low birth weight, we now know that birth weight per se is only a reflection of an insult or multiple insults to the fetus during development; that is, developmental programing can occur independently of birth weight (Barker, 2004; Reynolds et al., 2010b). For example, in humans and animal models, offspring of mothers who experience nutrient restriction early in pregnancy but receive adequate nutrition later in pregnancy, resulting in normal birth weights, still exhibit many of the same phenotypes as offspring from mothers that are undernourished for the whole of pregnancy. These phenotypes include poor growth, increased adiposity, poor glucose tolerance, and dislipidemia (Barker, 2004; Ford et al., 2007; Vonnahme et al., 2007; Dong et al., 2008).
These and similar observations emphasize the importance of interventions designed to correct the developmental defects during fetal or early postnatal life, because if the organ systems are indeed programmed, then later interventions may be much less effective (Greenwood et al., 2000, 2004; Barker, 2004, 2007; Reynolds et al., 2010b). These observations also emphasize the importance of understanding how to manage offspring from compromised pregnancies. For example, in humans and animal models, rapid body weight gain during infancy further impairs body composition, leading to obesity in the offspring (Barker, 2004, 2007; Reynolds et al., 2010b). Additionally, it has been shown in humans and in animal models that developmental insults in one generation can have consequences for later generations even in the absence of further insults, and it has been suggested that these transgenerational effects may depend on epigenetic alterations (see next section, Mechanisms of Developmental Programming; Anderson et al., 2006; Ismail-Beigi et al., 2006; Wu et al., 2006; Reynolds et al., 2010b). Lastly, these observations emphasize the critical need for increased research efforts to understand the basis (i.e., the mechanisms) of developmental programming in terms of a variety of maternal stressors (e.g., nutritional, hormonal, or both), effects at various developmental stages (i.e., pre- as well as postnatally), intergenerational consequences, and potential therapeutic interventions (Barker, 2007; Caton and Hess, 2010; Reynolds et al., 2010b).
2. Mechanisms of developmental programming
Based on epidemiological studies in humans and corroborated by numerous controlled studies in animal models, such as those described above, the most likely explanation for the long-term effects of various insults during fetal or postnatal life is two-fold: (1) irreversible alterations in tissue and organ structure (i.e., a structural defect), and (2) permanent changes in tissue function (i.e., a permanent change in gene expression leading to a functional defect).
The first mechanism, a structural defect, is perhaps best exemplified by muscle. This is because the number of individual muscle cells (myocytes), or muscle fibers, is established before birth; after that, muscle fiber size can increase by the addition of nuclei [from muscle satellite cells] and subsequent hypertrophy, but no new muscle fibers can be added and muscle growth is therefore limited (Du et al., 2010a,b). Thus, factors affecting fetal muscle development can lead to permanent, irreversible changes in muscle structure and its growth potential (Du et al., 2010,b).
Muscle fiber number in offspring is affected by maternal nutrient restriction during pregnancy (Du et al., 2010), and it has been argued that developing muscle is especially vulnerable to nutrient availability because of its low priority in terms of nutrient partitioning during fetal development, due primarily to its lower metabolic demands compared with tissues such as brain, gut, and placenta, a concept first articulated by Sir Joseph Barcroft; Barcroft (1946). Maternal nutrient restriction, or other factors that limit nutrient availability such as multiple fetuses, also affect development of other muscle cells in addition to myocytes, including adipocytes (which regulate intramuscular fat, or marbling) and fibroblasts (connective tissue-producing cells), leading to alterations in not only muscle size but also muscle marbling and connective tissue content in the offspring (Du et al., 2010,b). As with most other organ systems, the period of pregnancy during which nutrient restriction is experienced determines which of the cell types (i.e., myocytes, adipocytes, or fibroblasts) is most affected (Du et al., 2010,b).
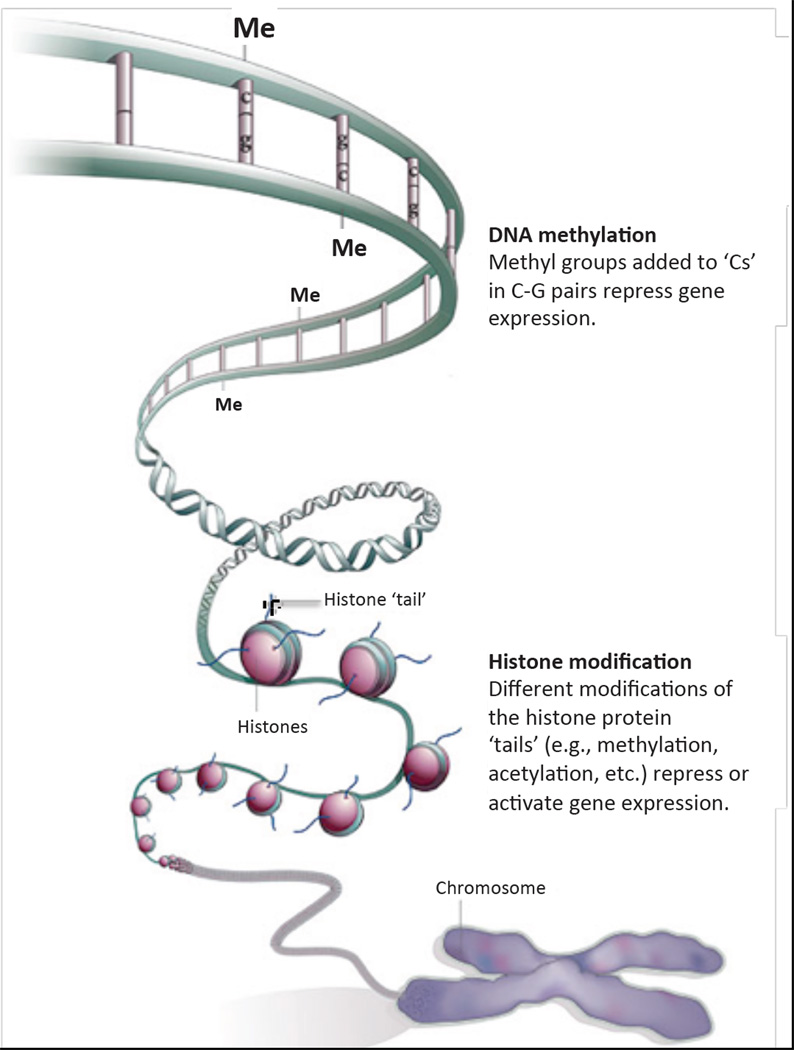
The second mechanism, a functional defect due to altered gene expression, is best explained by a relatively novel concept termed ‘epigenetics.’ The term epigenetics comes from the Greek epi, meaning besides, and thus literally means in addition to genetics. In modern parlance, epigenetics refers specifically to the mechanisms for silencing or activating gene expression independent of the gene’s DNA sequence. These mechanisms involve primarily two processes, methylation of DNA on Cs (cytosines) in CG pairs, and various modifications (methylation, acetylation, etc.) of the proteins around which DNA is wrapped, which are called histones; all of these processes determine whether a particular gene is ‘condensed’ and unavailable for transcription or, conversely, ‘open’ and ready to be transcribed (Fig. 2; Jaenisch and Bird, 2003; Qiu, 2006). These epigenetic processes are not only altered by environmental factors, but also are heritable (Jaenisch and Bird, 2003; Skinner et al., 2010). Environmental factors that affect the ‘epigenome,’ and thereby potentially have long term and even transgenerational effects on gene expression, include nutrition, various toxicants (e.g., cigarette smoke, plasticizers, pesticides, herbicides), social interactions (e.g., spousal abuse, maternal care), etc. (Jaenisch and Bird, 2003; Skinner et al., 2010). Most interestingly in the context of this discussion, when a pregnant woman or animal is subjected to these various environmental factors during pregnancy, the epigenome of her offspring is affected (Skinner et al., 2010).
Figure 2.
The 2 main components of the ‘epigenetic code.’ Modified from Nature 441, 143–145, 2006.
3. The placenta in developmental programming
The placenta is the organ of exchange between the fetal and maternal systems. That is, the fetus receives all of the nutrients and oxygen needed to support its development and also gives up its metabolic wastes to the mother via the placenta (Reynolds and Redmer, 1995; Reynolds et al., 2010a). Because it is primarily an organ of transport, the placenta also develops an extensive blood supply (i.e., it is highly ‘vascular’), which causes gravid uterine and umbilical blood flows to increase exponentially throughout pregnancy. Gravid uterine and umbilical blood flows represent the blood supplies to the maternal and fetal sides of the placenta, respectively.
Because we had earlier shown that the absolute rates of maternal and fetal placental blood flows are key determinants of the rate at which nutrients, oxygen, and metabolic wastes are transferred between the fetal and maternal systems, our research program has focused on placental vascular development and blood flow for many years (Reynolds and Redmer, 1995; Reynolds et al., 2010a). As part of this focus, in 2001 we received a grant from the US National Institutes of Health to develop sheep models of compromised pregnancy (that is, pregnancies in which fetal or placental growth, or both, is reduced). In the intervening decade we have shown that in every model of compromised pregnancy placental vascularity and blood flows also are affected (Table 2), leading us to conclude that altered placental vascular development and function is a critical factor leading to reduced fetal growth (Reynolds et al., 2006). These observations agree with those in women, in which placental perfusion is reduced in pregnancies with growth restricted fetuses (Poston, 1997; Moore et al., 2004; Redmer et al., 2004; Huppertz and Peeters, 2005). Similar observations by various authors in several mammalian species, including humans, have lead to the concept of ‘placental programming’ by maternal stressors (Mayhew et al., 2004; Redmer et al., 2005, 2009; Jansson and Powell, 2007; Borowicz and Reynolds, 2010; Coan et al., 2010; Burton et al., 2011).
Table 2.
Changes (compared with controls) in fetal and placental weights, placental (uterine and umbilical) blood flows, and placental vascular development in a variety of models of compromised pregnancy in sheep.1
| Model | Fetal Weight |
Placental Weight |
Gravid Uterine Blood Flow |
Umbilical Blood Flow |
Placental Vascularity2 |
|---|---|---|---|---|---|
| Overfed Adolescent | − 20–40% | − 20–45% | − 36% | − 37% | − 31% |
| Underfeeding of adolescent or adult dams | − 14% | NSE3 (adolescent) or ND3 (adult) | − 25% | ND (adolescent) or NSE (adult) | − 17% |
| Multiple pregnancy | − 30% | − 37% | − 23% | --- | − 30% |
| Heat-stressed adult | − 42% | − 51% | − 26% | − 60% | --- |
| Adolescent vs. adult | − 16% | − 26% | --- | --- | − 24% |
| Maternal genotype (adult only) | − 44% | − 28% | --- | --- | − 33% |
| High dietary Se | NSE | − 24% | --- | --- | + 20% |
A minus sign indicates a reduction and a plus sign indicates an increase compared with controls. All data were obtained between days 130 and 140 (i.e., between approximately 90 to 95%) of gestation. Adapted from Reynolds et al., 2010b.
Vascularity is a general term for altered placental vascular development, including capillary number density, capillary area density, and (or) capillary surface density (see Borowicz et al., 2007, 2008).
NSE = no significant effect observed; and ND = not determined.
4. Therapeutic/management approaches to developmental programming
The most obvious way to minimize the negative consequences of developmental programming is to simply ensure that maternal nutrition is optimal, and this approach has a great deal of merit. For example, we have shown that the level of metabolizable protein in the maternal diet affects birth weights of the offspring in both cattle and sheep (Sletmoen-Olson et al., 2000; O’Rourke et al., 2010). Recent studies have also shown that beef cattle grazing dormant range that receive protein supplementation during the last one-third of pregnancy may produce heifer calves with reduced age at puberty and increased first pregnancy rate compared with heifer calves from non-supplemented dams (Funston et al., 2010). We also have found that maternal dietary selenium supplementation at ‘supernutritional,’ but subtoxic, levels may protect, in some cases, fetal or neonatal organ systems from the negative effects of dietary restriction during pregnancy (Reed et al., 2007; Meyer et al., 2010).
Another approach is to use dietary supplementation that targets specific processes. For example, several amino acids, including many of the ‘essential’ amino acids, have been termed ‘functional amino acids’ because they not only serve as building blocks of proteins but also regulate, directly or indirectly, key metabolic pathways necessary for growth, reproduction, immunity, etc. (Wu, 2009). The amino acid Arginine (Arg) is the immediate precursor for at least two critical processes: (1) the production of nitric oxide (NO), which is an important vasodilator (i.e., it causes blood vessels to dilate and thus increases blood flow to many organs), and (2) the production of polyamines, which are critical for normal cell and tissue growth (Wu, 2009). It has been shown that dietary supplementation of gilts with Arg during pregnancy increases the number of piglets born alive and live litter birth weights (Mateo et al., 2007). More recently, Lassala et al. (2010) showed that intravenous infusion of Arg three times daily from day 60 until parturition was able to rescue birth weights of lambs in ewes receiving only 50% of NRC nutrient requirements from day 28 of pregnancy onwards. Similarly, we have shown that intravenous administration of Arg once daily from the day of mating through day 15 increases embryo survival and decreases embryo loss in ewes of low prolificacy (Luther et al., 2008).
Other essential amino acids and vitamins, including methionine, serine, glycine, and folate (also known as vitamin B9) are involved in DNA methylation, or 1-carbon metabolism. It has been shown that dietary levels of these amino acids and vitamins can dramatically affect epigenetic regulation of gene expression and can reduce the incidence of diseases or developmental defects (Jaenisch and Bird, 2003; Skinner et al., 2010). Whether these dietary factors can minimize the epigenetic-dependent negative consequences of developmental programming remains to be determined.
Lastly, in terms of therapeutic/management approaches, are those designed to directly impact placental blood flow using pharmacological agents. For example, in a recent study, Satterfield et al. (2010) administered sildenafil, which is marketed under the trade name Viagra, at 150 mg/day from day 28 until day 115 in pregnant, nutrient-restricted ewes and showed that fetal weights were similar to those of control-fed ewes but greater than those of nutrient-restricted ewes not receiving sildenafil. In fact, fetal weight of control-fed ewes receiving sildenafil was greater than in those not receiving sildenafil.
5. Future directions
Our immediate goals are to begin to characterize the epigenetic changes in key fetal organs (intestine, adrenal, brain, pituitary, bone, muscle, pancreas, etc.) that we previously have shown to be ‘programmed’ by maternal nutritional or other insults. As part of this effort, we also will evaluate epigenetic responses to various therapies (sildenafil or dietary Arg, amino acids, protein, etc.) designed to overcome the deleterious effects of developmental programming. We also are pursuing studies of ‘rumen-protected’ Arg and other amino acids, which will allow us to deliver these in the diet in ruminants.
A more long-term goal includes establishing, using large data sets, whether birth weights are associated with long-term negative effects on animal productivity in livestock in real-world, production settings.
Of course, all of these efforts will require the continued commitment of funding agencies at all levels. They also will require talented and committed individuals from undergraduate research interns through senior scientists to livestock producers. Together, we may be able to understand and manage the consequences of developmental programming, which is emerging as a major health issue for livestock and humans alike.
Highlights.
This review highlights the importance of and recent research on Developmental Programming, especially in large animals including livestock, as well as the now well-established role of the placenta. We also discuss some recent work on relatively simply and easily applied therapeutic/management strategies designed to minimize the negative consequences of Developmental Programming.
Acknowledgements
Our sincere thanks to the many colleagues, postdoctoral fellows, and graduate students who have made important contributions to this work, especially the following: Drs. Daniel Arnold, Ewa Borowczyk, Pawel Borowicz, David Buchanan, David Carlson, Stephen Ford, Anna Grazul-Bilska, Shireen Hafez, Carrie Hammer, Bret Hess, Mary Lynn Johnson, Leslie Lekatz, Justin Luther, Kasey Maddock Carlin, Allison Meyer, Tammi Neville, Dale Redmer, Jake Reed, Chris Schauer, Abraham Scheaffer, Sergio Soto-Navarro, Brett Taylor, Kimberly Vonnahme, Jacqueline Wallace, Marcy Ward, and Guoyao Wu. We also acknowledge the support of the following funding agencies: Agriculture and Food Research Initiative of the U.S. National Institute of Food and Agriculture; National Heart, Lung and Blood Institute, and National Institute of Child Health and Human Development of the U.S. National Institutes of Health; the North Dakota Agricultural Experiment Station; and the U.S. National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Lopez F, Zimmer A, Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol. Reprod. 2006;74:538–544. doi: 10.1095/biolreprod.105.045807. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J. Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir Joseph Barcroft. Researches on Pre-Natal Life. Blackwell, Oxford: 1946. [Google Scholar]
- Barker DJP, editor. Fetal and Infant Origins of Adult Disease. London: BMJ Publishing Group; 1992. [Google Scholar]
- Barker DJP. Philos. Trans. Royal Soc. London. Vol. 359. 2004. Developmental origins of well being; pp. 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Introduction: The window of opportunity. Symposium: Novel concepts in the developmental origins of adult health and disease. J. Nutr. 2007;137:1058–1059. doi: 10.1093/jn/137.4.1073. [DOI] [PubMed] [Google Scholar]
- Borowicz P, Reynolds LP. ‘Placental programming’: more may still be less. J Physiol. 2010;588:393. doi: 10.1113/jphysiol.2009.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two-thirds of pregnancy in sheep: Vascular development and angiogenic factor expression. Biol Reprod. 2007;76:259–267. doi: 10.1095/biolreprod.106.054684. [DOI] [PubMed] [Google Scholar]
- Borowicz PP, Hafez S, Redmer DA, Reynolds LP. Chapter 10. Methods for evaluating uteroplacental angiogenesis and their application using animal models. In: Cheresh D, editor. Methods in Enzymology, Vol. 445, Angiogenesis, In Vivo, Part B. NY: Elsevier; 2008. pp. 229–253. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Braker DJP, Moffett A, Thornburg K, editors. The Placenta and Human Developmental Programming. Cambridge University Press; 2011. p. 258. [Google Scholar]
- Caton JS, Hess BW. Maternal plane of nutrition: Impacts on fetal outcomes and postnatal offspring responses. Page 104–122. In: Hess BW, DelCurto T, Bowman JGP, Waterman RC, editors. Proc. 4th Grazing Livestock Nutrition Conference; West. Sect. Am. Soc. Anim. Sci.; Champaign, IL. 2010. http://coronasc.nmsu.edu/documents/proceedings_of_the_fourth_glnc_2010.pdf#page=112. [Google Scholar]
- Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588:527–538. doi: 10.1113/jphysiol.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J. Nutritional Biochem. 2008;19:409–414. doi: 10.1016/j.jnutbio.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Tong J, Zhao J, Underwood KR, Zhu M, Ford SP, Nathanielsz PW. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010a;88:E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- Du M, Zhao JX, Yan X, Huang Y, Nicodemus LV, Yue W, McCormick RJ, Zhu MJ. Fetal muscle development, mesenchymal multipotent cell differentiation and associated signaling pathways. J Anim Sci. 2011;89:583–590. doi: 10.2527/jas.2010-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 2007;85:1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- Funston RN, Larson DM, Vonnahme KA. Effects of maternal nutrition on conceptus growth and offspring performance: Implications for beef cattle production. J Anim Sci. 2010;88:E205-215E. doi: 10.2527/jas.2009-2351. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J. Anim. Sci. 2000;78:50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: IV. Organ growth. J. Anim. Sci. 2004;82:422–428. doi: 10.2527/2004.822422x. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Peeters LL. Vascular biology in implantation and placentation. Angiogenesis. 2005;8:157–167. doi: 10.1007/s10456-005-9007-8. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F, Catalano PM, Hanson RW. Metabolic programming: fetal origins of obesity and metabolic syndrome in the adult. Am. J. Physiol. 2006;291:E439–E440. doi: 10.1152/ajpendo.00105.2006. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 Suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical Science. 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr. 2010;140(7):1242–1248. doi: 10.3945/jn.110.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JS, Redmer DA, Reynolds LP, Wallace JM. Nutritional paradigms of ovine fetal growth restriction: implications for human pregnancy. Human Fertil. 2005;8:179–187. doi: 10.1080/14647270500320121. [DOI] [PubMed] [Google Scholar]
- Luther JS, Windorski EJ, Schauer CS, Kirsch JD, Vonnahme KA, Reynolds LP, Caton JS, Wu G. Impacts of L-arginine on ovarian function and reproductive performance in ewes. J Anim Sci. 2008;86 E-Suppl. 2:ii. (Abstr.) [Google Scholar]
- Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007 Mar;137(3):652–656. doi: 10.1093/jn/137.3.652. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Reed JJ, Neville TL, Taylor JB, Hammer CJ, Reynolds LP, Redmer DA, Vonnahme KA, Caton JS. Effects of plane of nutrition and selenium supply during gestation on ewe and neonatal offspring performance, body composition, and serum selenium. J Anim Sci. 2010;88(5):1786–1800. doi: 10.2527/jas.2009-2435. [DOI] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: An experiment of nature – A review. Placenta. 2004;25 Suppl. A:S60–S71. doi: 10.1016/j.placenta.2004.01.008. [Trophoblast Research 18] [DOI] [PubMed] [Google Scholar]
- O'Rourke ST, Modgil A, Sun C, Vonnahme KA, Caton JS, Reynolds LP. Maternal dietary protein level alters function of large-conductance, calcium-activated k (bkca) channels in fetal coronary arterial smooth muscle cells. Pediatric Research. 2010;68:177. [Google Scholar]
- Paneth N, Susser M. Early origin of coronary heart disease (the “Barker hypothesis”) Brit Med J. 1995;310:411–412. doi: 10.1136/bmj.310.6977.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L. The control of blood flow to the placenta. Exp Physiol. 1997;82:377–387. doi: 10.1113/expphysiol.1997.sp004033. [DOI] [PubMed] [Google Scholar]
- Qui J. Unfinished symphony. Nature. 2006;441:143–145. doi: 10.1038/441143a. [DOI] [PubMed] [Google Scholar]
- Reed JJ, Ward MA, Vonnahme KA, Neville TL, Julius SL, Borowicz PP, Taylor JB, Redmer DA, Grazul-Bilska AT, Reynolds LP, Caton JS. Effects of selenium supply and dietary restriction on maternal and fetal body weight, visceral organ mass and cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J Anim Sci. 2007;85(10):2721–2733. doi: 10.2527/jas.2006-785. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP. Effects of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domestic Anim Endocrinol. 2004;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Aitken RP, Milne JS, Reynolds LP, Wallace JM. Influence of maternal nutrition on messenger rna expression of placental angiogenic factors and their receptors at mid-gestation in adolescent sheep. Biol Reprod. 2005;72:1004–1009. doi: 10.1095/biolreprod.104.037234. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Luther J, Milne J, Aitken R, Johnson M, Borowicz P, Borowicz M, Reynolds LP, Wallace J. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction. 2009;137:749–757. doi: 10.1530/REP-08-0516. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J. Anim. Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. Invited (Topical) review. J. Physiol. (1) 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Uteroplacental vascular development and placental function: an update. Int J Dev Biol. 2010a;54(2–3):355–366. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Hammer CJ, Maddock Carlin KR, Grazul-Bilska AT, Redmer DA. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J Anim Sci. 2010b;88(13) Suppl:E61–E72. doi: 10.2527/jas.2009-2359. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr. 2010 Feb;140(2):251–258. doi: 10.3945/jn.109.114678. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010 Apr;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletmoen-Olson KE, Caton JS, Olson KC, Reynolds LP. Undegraded intake protein supplementation: I. Effects on forage utilization and performance of periparturient beef cows fed low-quality hay. J Anim Sci. 2000;78:449–455. doi: 10.2527/2000.782449x. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Zhu MJ, Borowicz PP, Geary TW, Hess BW, Reynolds LP, Caton JS, Means WJ, Ford SP. Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J. Anim. Sci. 2007;85:2464–2472. doi: 10.2527/jas.2006-805. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW., Jr Nutritional modulation of adolescent pregnancy outcome -- a review. Placenta. 2006;27 Suppl. A:S61–S68. doi: 10.1016/j.placenta.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Wallace JM, Spencer TE. BOARD-INVITED REVIEW: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]