Abstract
The red raspberry (Rubus idaeus) fruit contains bioactive polyphenols including anthocyanins and ellagitannins with reported anti-inflammatory properties. Here we sought to investigate the cartilage protecting and anti-inflammatory effects of a polyphenolic-enriched red raspberry extract (RRE; standardized to total polyphenol, anthocyanin, and ellagitannin contents) using: 1) an in vitro bovine nasal explant cell culture model and, 2) an in vivo adjuvant-induced arthritis rat model. RRE contained 20% total polyphenols (as gallic acid equivalents), 5% anthocyanins (as cyanidin-3-glucoside equivalents) and 9.25% ellagitannins (as ellagic acid equivalents). In the in vitro studies, bovine nasal explants were stimulated with 10 ng/mL IL-1β to induce the release of proteoglycan and type II collagen. On treatment with RRE (50 μg/mL), there was a decrease in the rate of degradation of both proteoglycan and type II collagen. In the in vivo antigen-induced arthritis rat model, animals were gavaged daily with RRE (at doses of 30 and 120 mg/Kg, respectively) for 30 days after adjuvant injection (750 μg of Mycobacterium tuberculosis suspension in squalene). At the higher dose, animals treated with RRE had a lower incidence and severity of arthritis compared to control animals. Also, histological analyses revealed significant inhibition of inflammation, pannus formation, cartilage damage, and bone resorption by RRE. This study suggests that red raspberry polyphenols may afford cartilage protection and/or modulate the onset and severity of arthritis.
Keywords: Rubus idaeus, red raspberry, polyphenols, rheumatoid arthritis, inflammation
INTRODUCTION
Polyphenols are abundant phytochemicals in berries, fruit and vegetables and constitute a large class of compounds which have attracted research attention due to their potential human health benefits.1 Among the various sub-classes of polyphenols, the anthocyanins (water soluble pigments) and ellagitannins (hydrolyzable tannins) have been implicated with a wide range of biological properties including anti-inflammatory effects.2, 3 Anthocyanins from berries have been shown to have anti-inflammatory effects in both in vitro and in vivo models.4, 5 Similarly, ellagitannins and their colonic derived metabolites have been shown to decrease inflammatory markers in colonic mucosa and suppress arthritis incidence and inflammatory markers in arthritic joints.6, 7
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease that affects about 1% of the US population.8 The disease is characterized by inflammation of the synovium, synovial hyperplasia with increased cell density, and infiltration of inflammatory cells leading to pannus formation and irreversible cartilage and bone destruction.9, 10 The articular cartilage is composed mainly of type II collagen (CII; 60% of dry weight) and proteoglycan (10% of dry weight). Together, they are responsible for the biomechanical properties of the cartilage and confer tensile strength and load bearing capacity respectively.11, 12 In arthritis, the destruction of the cartilage is associated to a reduced synthesis of the matrix components by articular chondrocytes and an enhanced breakdown of the matrix by proteolytic enzymes, mainly the matrix metalloproteases (MMPs).9, 10
The red raspberry (Rubus idaeus L.) fruit contains macronutrients and micronutrients including fiber and vitamin C, as well as bioactive polyphenols.13 The polyphenols in red raspberry are primarily found as ellagitannins and anthocyanins, along with ellagic acid glycosides and flavonol conjugates.14–17 While a polyphenolic-enriched red raspberry extract has been reported to show anti-inflammatory effects in vitro,4 to date, these effects have not been evaluated using in vivo models.
The purpose of the current study was to investigate the cartilage protecting and anti-inflammatory effects of a polyphenolic-enriched red raspberry extract (RRE) using: 1) an in vitro bovine nasal explant cell culture model and, 2) an in vivo adjuvant-induced arthritis rat model. The RRE was evaluated for vitamin C as well as total polyphenol, anthocyanin, and ellagitannin contents using a combination of spectrophotometric, HPLC-UV, and HPLC-MS/MS methods.
MATERIALS AND METHODS
General Experimental Procedures
Analytical HPLC was performed on a Hitachi Elite LaChrom system consisting of a L2130 pump, L-2200 autosampler, and a L-2455 Diode Array Detector (DAD), all operated by EZChrom Elite software. LC-MS/MS analyses were carried out on a Q-Star Elite (Applied Biosystems MDS) time-of-flight mass spectrometer equipped with a Turbo Ionspray source and coupled to an HP1100 series HPLC system consisting of an autosampler/injector, quaternary pump, and DAD. Data handling for the LC-MS was carried out using Analyst QS version 2.0 software (Applied Biosystems). Methanol (HPLC grade) was obtained from Wilkem Scientific (Pawtucket, RI, USA). Recombinant IL-1β was purchased from Peprotech (Rocky Hill, NJ, USA). All cell culture reagents and 1,9-dimethyl-methylene blue (DMMB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Horseradish peroxidase-conjugated anti-mouse IgG, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate and electrophoresis supplies were purchased from Biorad (Hercules, CA, USA). All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Red Raspberry Fruit
The red raspberry fruit was provided to our laboratory by the Washington Red Raspberry Commission and was obtained from HoneyVille farms (Brigham City, UT, USA) as freeze-dried berries (226.8 g per container; Lot # 09236-07; Sherco Products). The berries were packed, stored, and shipped in sealed cans. Based on the labeling information, 100 g of freeze dried red raspberries contain 21.8 g of fiber and 128.2 mg vitamin C.
Phytochemical Evaluation and Standardization of RRE
Preparation of Red Raspberry Extract (RRE)
The freeze dried berries were enriched in polyphenol content as previously described.5 Briefly, a portion of the ground berry powder (1 Kg) was exhaustively extracted with 0.1% HCL in methanol (1 L × 3; by stirring). The combined methanol filtrate was concentrated in vacuo and enriched in polyphenolic content by using Amberlite XAD-16 resin adsorption chromatography.5 A portion (500 g) of the methanol extract was reconstituted in water, adsorbed onto the XAD-16 resin column, and then eluted with copious amounts of water to remove the natural fruit sugars and acids. The column was then eluted with acidic methanol (0.1% HCl) to yield the polyphenolic-enriched red raspberry extract (RRE) after drying in vacuo.
Standardization of RRE to Total Phenolic Content
The total phenolic contents of the RRE was determined according to the Folin-Ciocalteau method18 and was measured as gallic acid equivalents (GAEs). Briefly, the extract was diluted 1:100 with methanol/H2O (1:1, v/v), and 200 μL of sample was incubated with 3 mL of methanol/H2O (1:1, v/v) and 200 μL of Folin-Ciocalteau reagent for 10 min at 25 °C. After this, 600 μL of a 20% Na2CO3 aqueous solution was added to each tube and vortexed. Tubes were further incubated for 20 min at 40 °C. After incubation, samples were immediately cooled in an ice bath to room temperature. Samples and standard (gallic acid) were processed identically. The absorbance was determined at 755 nm, and final results were calculated from the standard curve obtained from a Spectramax M2 plate reader operated by SoftmaxPro v.4.6 software (Molecular Devices, Sunnyvale, CA, USA).
Standardization of RRE to Total Anthocyanin Content
The anthocyanin content was determined by using the pH differential method and was calculated as equivalents of cyanidin-3-glucoside, using the extinction coefficient of 26900 L mol−1 cm−1 and a molecular mass of 449.2 g/mol as previously reported.19 Briefly, an accurately weighed sample of RRE was used to make a stock solution in a 100 mL volumetric flask. Two aliquots of this stock solution (1.0 mL each) were removed and placed into separate 25 mL volumetric flasks which were made up to mark with pre-prepared buffers of pH 1.0 or pH 4.5, respectively. Absorbance of these two buffer solutions were recorded at 510 nm using a Spectramax M2 plate reader operated by SoftmaxPro v.4.6 software (Molecular Devices, Sunnyvale, CA, USA). According to this assay protocol, absorbance can also be recorded at 700 nm if there is sample turbidity. The difference in absorbance between the two samples was then calculated using the formula: Absorbance = (A510nm pH 1.0 - A700nm pH 1.0) - (A510nm pH 4.5 - A700nm pH 4.5). Finally the % w/w of total anthocyanins in the sample was calculated by using the formula: % w/w anthocyanins = A/εL × MW × DF × V/Wt × 100% where A = Absorbance; ε = cyanidin-3-glucoside molar absorptivity (26,900 L mol−1 cm−1); MW = anthocyanin molecular weight (449.2 g/mol); DF = dilution factor; V = final volume (mL); Wt = sample weight (mg); L = cell path length (1 cm).
Standardization of RRE to Total Ellagitannin Content
The total ellagitannin concentration was determined by the quantification of ellagic acid released during acidic hydrolysis as previously described with minor modifications.20 Briefly, 5 mg RRE was dissolved in 10 mL acidic methanol (methanol: 6N HCl, 9:1, v/v) and hydrolyzed at 110 °C for 3 h. A standard curve was constructed for ellagic acid (concentrations of 1, 0.5, 0.25, 0.125, 0.0625, 0.03125 mg/mL in DMSO) using HPLC-UV methods (described below). The original RRE (50.8 mg/mL in methanol) and hydrolyzed RRE mixture (0.5 mg/mL in methanol) were individually subjected to HPLC-UV analyses to evaluate for free ellagic acid contents. The HPLC analyses were carried out at 25 °C using a Phenomenex Luna C18 column (250 × 4.6 mm i.d., 5 μm) at a flow rate of 0.75 mL/min, eluted with a water (A)/methanol (B) linear gradient system as follows: 0–30 min, 10% to 60% B; 30–35 min, 60% to 100% B; 35–40 min, 100% B; 40–41 min, 100% to 10% B; 41–51 min, 10% B. The injection volume was 20 μL and the detection wavelength was monitored at 360 nm.
Analyses of RRE by Liquid Chromatography Mass Spectrometry (LC-MS)
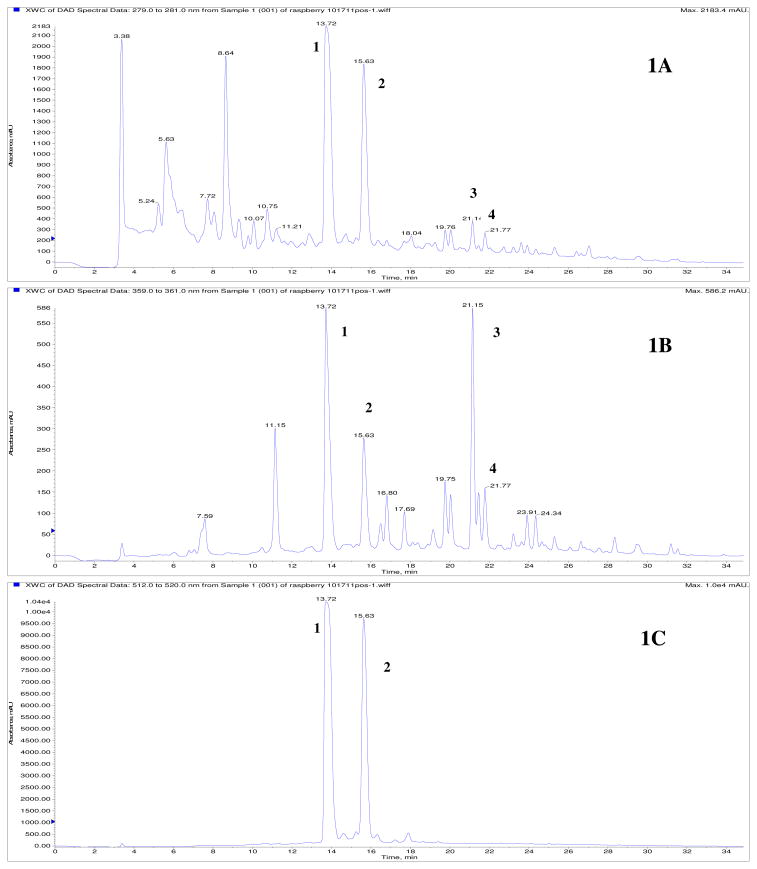
RRE was subjected to LC-MS/MS analyses as follows. Column: XBridge C18, 150 × 4.6 mm i.d., 5 μm (Waters Corp., Milford, MA). The solvent system consisted of 1% aqueous formic acid (A)/1% formic acid in methanol (B) linear gradient as follows: 0–30 min, 80% to 35% A; 31–33 min, 100% B with a total run time of 33 min, a flow rate of 0.5 mL/min and injection volume of 5 μL. The column temperature was 25 °C; DAD range was 210-600 nm; MS parameters were positive ionization mode (for anthocyanins) and negative ionization mode (for ellagitannins); scan range, 400–2000 amu; scan rate, 1 scan/s. Peak identities were obtained by matching their molecular M+ and MS/MS ions to literature data.21 HPLC chromatograms were extracted at 280 (characteristic wavelength of phenolics including ellagitannins), 360 (characteristic wavelength of phenolic compounds including ellagic acid glycosides and flavonols) and 520 nm (characteristic wavelength of anthocyanins) as shown in Figs. 1A, 1B, and 1C, respectively.
Figure 1.
HPLC-UV chromatogram of the polyphenolic-enriched red raspberry extract (RRE). Fig 1A: 280 nm (characteristic of phenolics including ellagitannins); Fig. 1B: 360 nm (characteristic of phenolics including flavonols and ellagic acid glycosides); Fig. 1C: 520 nm (characteristic of anthocyanins). Compounds were identified based on comparison to published reports and LC-MS/MS data as follows: Peak 1 = cyanidin-3-O-sophoroside, Peak 2 = cyanidin-3-O-glucoside, Peak 3 = quercetin glycoside, Peak 4 = ellagic acid. Based on literature, unlabelled/unknown peaks correspond to flavonols, ellagic acid glycosides and ellagitannins which are known to be present in the red raspberry fruit.
Analysis of RRE for Vitamin C
RRE was evaluated for vitamin C using HPLC methods as previously reported with minor modifications.21 The column consisted of an Allure® (Restek, Bellefonte, PA, USA) organic acid column (150 × 4.6 mm), eluted with an isocratic solvent system of 100 mM KH2PO4 at a flow rate of 0.5 mL/min. HPLC chromatograms were extracted at 226 nm and a standard of vitamin C was injected for comparison of retention times.
In Vitro Anti-inflammatory Effects of RRE
RRE Effects on Nasal Bovine Cartilage Degradation
Bovine nasal septa were obtained from a local slaughterhouse (Canterbury, CT, USA) immediately after sacrifice. Upon weighing, full thickness cartilage discs (3 mm) were placed (3 discs/well) in each well of a 48-well flat-bottomed plate. The cartilage explants were cultured for 24 h in 600 μL of DMEM-F12 (Dulbecco's Modified Eagle Medium Nutrient Mixture F-12) media supplemented with antibiotics (wash out period). On day 0, the media was harvested and quadruplicate wells were cultured with media alone or media supplemented with 10 ng/mL IL-1β alone or in the presence of 50 μg/mL of RRE. The media was replaced at different time points (every four days up to 28 days; see Fig. 2A) and the wells replenished with identical treatments as day 0. Harvested media was kept at -80 °C until analyzed. Total proteoglycan and CII present in the culture supernatant were quantified as described below.
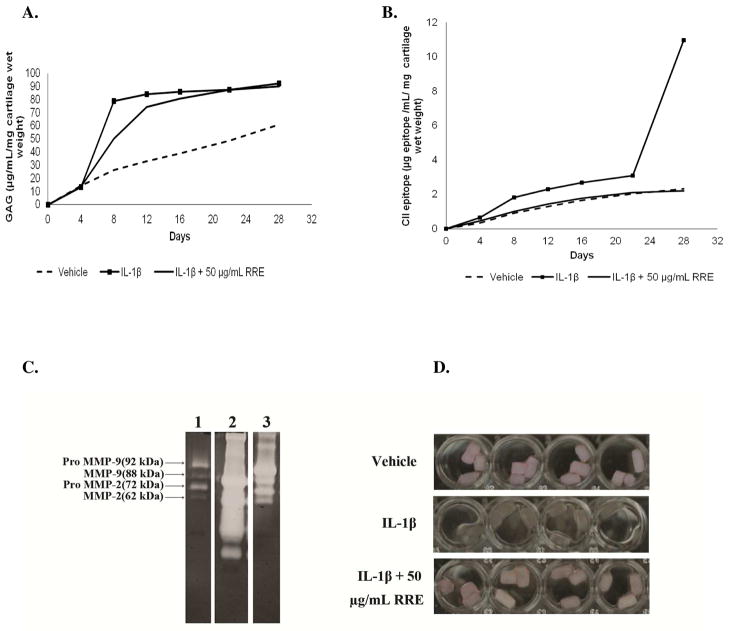
Figure 2.
In vitro effects of the polyphenol-enriched red raspberry extract (RRE) on nasal bovine cartilage degradation. Fig. 2A: Cumulative release of the GAG; Fig. 2B: Cumulative release of CII epitopes; Fig. 2C: Gelatynolitic activity of culture media collected at day 28. Lane 1, un-stimulated; lane 2 IL-1β alone, lane 3, IL-1β + RRE. *represent un-identified MMP bands; Fig. 2D: Picture showing the appearance of the cartilage plugs at the end of the culture period with and without treatment with RRE. Values as shown in Figs. 2A and 2B are reported as the mean ± SD (n = 4).
Quantification of Proteoglycan Degradation
The major cartilage proteoglycan is aggrecan, which consists of a core protein covalently attached to one or more negatively charged polysaccharides chains called glycosaminoglycan (GAG).22 The amount of proteoglycan released into the culture supernatant was estimated by measuring the levels of GAG using the colorimetric DMMB assay according to the published procedure.23 This assay is based on the metachromatic shift of the cationic DMMB dye upon binding to the negatively charged GAG molecules. 200 μL of DMMB solution (40 mM glycine, 40 mM NaCl, 16 mg/mL DMMB, pH 3.0) was mixed with 50 μL of diluted culture media in a 96-well plate. The absorbance was immediately measured at 525 nm using a Spectramax M2 plate reader operated by SoftmaxPro v.4.6 software (Molecular Devices, Sunnyvale, CA, USA). Chondroitin sulfate from shark cartilage was used as a standard. Values are expressed as the concentration of GAG (mg/mL) per mg of wet cartilage tissue.
Quantification of Type II Collagen (CII) Degradation
The degradation of type II collagen (CII) was measured by a well-established inhibition ELISA method using a mouse monoclonal antibody raised against CII epitopes generated by cyanogen bromide (CNBr) cleavage as previously described.24, 25 The CNBr-derived CII peptides were also used as standard. Equal volumes of CNBr peptides and primary antibody diluted in PBS-T (0.05% Tween-20 in phosphate buffered saline) were incubated at room temperature overnight. 50 μL of the mixture was added in triplicate to a high binding microtiter plate (Corning Inc., NY, USA) pre-coated with 50 μL of 0.5 μg/mL CII CNBr-peptides in coating buffer (50 mM sodium carbonate buffer, pH 9.5) and blocked with blocking buffer (1% bovine serum albumin in PBS). The plate was incubated for 20 min at room temperature and washed with PBS-T. Secondary antibody, horseradish peroxidase-labelled goat anti-mouse IgG, in blocking buffer was added (50 μL) and incubated for 1 h at room temperature. The plate was washed with PBS-T before the addition of 50 μL of TMB substrate. After 15 min, the reaction was stopped with 50 μL of 0.2 M sulfuric acid and absorbance was measured at 450 nm using a Spectramax M2 plate reader operated by SoftmaxPro v.4.6 software (Molecular Devices, Sunnyvale, CA, USA). Values are expressed as the concentration of CII epitope (μg/mL) per mg of wet cartilage tissue.
Gelatinase Activity
Explant media (5 μL) was separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE; 10% gels) containing 1 mg/mL gelatin as substrate. The gels were washed twice (15 min each) with 100 mL of 2.5% Triton X-100 (v/v) and incubated overnight at 37 °C in MMP buffer (50 mM Tris-HCl buffer, pH 7.6, 5 mM CaCl2, 150 mM NaCl, and 0.05% Brij). The gels were stained with Coomassie R250 (10% acetic acid) and destained in water. The gels were scanned using an Odysee Li-cor scanner.
In Vivo Anti-Inflammatory Effects of RRE
Animal Protocol
The animal study was conducted at Washington Biotechnology Inc. (WBI; Baltimore, MD, USA) according to approved guidelines for the care and use of laboratory animals. The protocol (1070.4 Adjuvant-Induced Arthritis in Rats; IACUC Approval No: 10-124; Expiration Date: 08/02/13) was approved by WBI's Office for Laboratory Animal Welfare (OLAW) Assurance Statement Number A4192-01. The study was conducted according to a well established antigen-induced arthritis (AIA) rat model.26 Briefly, male Lewis rats (130-150 g) were obtained from Harlan Sprague-Dawley Inc. (Indianapolis, IN, USA) and were allowed to acclimatize for 1 week. The rats were divided based on average weight into 4 groups (n = 6): vehicle control (non-diseased), positive control (diseased), RRE at 30 mg/Kg (low dose), and RRE at 120 mg/Kg (high dose). On day 0, rats in all groups except the non-diseased group were anesthetized and injected (100 μL) intradermally at the base of their tail with 750 μg of Mycobacterium tuberculosis emulsified in squalene. From day 0, RRE was administered daily by oral gavage until the end of the study. Control groups received daily doses of water (5 mL/Kg). Food intake measurement was not performed but all rats had access to food in the bottom of the cage since as the disease progressed they were not able to reach the cage-top feeders. Development of arthritis was clinically assessed by monitoring the average weight per group and paw volumes (by the water displacement method) daily. At the end of the study, the animals were sacrificed, both knee joints excised and stored in formalin for histopathology evaluation.
Histopathology Evaluation
The histological joint scoring was performed by Dr. Bendele (Washington Biotechnology Inc., Baltimore, MD, USA). The limbs were labeled with 'group' and 'animal number', only. Limbs were embedded in paraffin, sectioned, and stained with toluidine blue. The joints were scored for inflammation, pannus formation, cartilage destruction and bone resorption according to the following visual semiquantitative scoring system: 0-normal; 1-very minimal; 2-minimal; 3-mild; 4-moderate; 5-severe; 6-very severe; 7-severe infiltration (for inflammation and pannus formation) or near total destruction (for cartilage damage and bone resorption). Average scores ± SEM are reported.
Statistical Analyses
Two-tailed unpaired student’s t-test using Graph Pad Prism 5.0 (Graphpad Software Inc, San Diego, CA, USA) was used for statistical analysis of the data. Values of P ≤ 0.05 was considered significant.
RESULTS
Phytochemical Evaluation and Standardization of RRE
The red raspberry fruit contains phenolic compounds primarily found as anthocyanins and ellagitannins as well as vitamin C.14–17 Thus, we evaluated RRE for total polyphenol, anthocyanin, ellagitannin and vitamin C contents using a combination of spectrophotometric and chromatographic methods. Quantitative analyses of RRE revealed that the total polyphenol content was 20% (as gallic acid equivalents) and total anthocyanin content was 5% cyanidin-3-glucoside equivalents. Free ellagic acid in RRE before and after hydrolysis was 0.06% and 9.31%, respectively, suggesting that total ellagitannin content in RRE was 9.25%. Thus, anthocyanins and ellagitannins were indeed the major phenolic constituents in red raspberry which is in agreement with literature.14–17 Vitamin C was not found in RRE despite its reported presence in the red raspberry fruit.14 The loss in vitamin C may be due to the extraction procedure used to prepare RRE in the current study.
According to published studies, the phenolics present in red raspberry are found as ellagitannins, anthocyanins, ellagic acid glycosides, and flavonol derivatives.14–17 Indeed, the HPLC-UV chromatograms of RRE at 280 (characteristic of phenolics including ellagitannins), 360 (characteristic of phenolics including flavonols and ellagic acid glycosides), and 520 nm (characteristic of anthocyanins) showed multiple peaks indicating these compounds. 14–17 These chromatograms are shown in Figs. 1A, B and C, respectively. In the absence of authentic commercial standards of red raspberry ellagitannins (sanguiin H-6 and lambertianin C) and anthocyanins [cyanidin-3-O-sophoroside, cyanidin-3-O-(2"-O-glucosyl)rutinoside, cyanidin-3-O-glucoside, and other minor cyanidin and pelargonidin glycosides]14 we conducted further LC-MS/MS analyses of RRE. These were in agreement with literature for the anthocyanins21 and revealed cyanidin-3-O-sophoroside (tR 13.72 min, m/z M+ 611.1908, 287.0315) and cyanidin-3-O-glucoside (tR 15.63 min, m/z M+ 449.1279, 287.0307). Multiple peaks in the HPLC chromatography at 520 nm (characteristic of anthocyanins, Fig. 1C), suggested that there were minor anthocyanins present in RRE which is in agreement with literature.21 Despite our inability to confirm the presence of the parent ellagitannins by LC-MS/MS methods, it was apparent that these compounds were present in RRE based on the HPLC chromatogram at 280 nm (characteristic of ellagitannins; Fig. 1A), as well as our quantification of free ellagic acid based on acid hydrolysis. 20
In Vitro Effects of RRE on Proteoglycan and Type II Collagen (CII) Degradation
To evaluate the effects of RRE on cartilage degradation, bovine nasal septa were stimulated with 10 ng/mL IL-1β in the absence or presence of 50 μg/mL RRE. This dose of RRE (i.e. 50 μg/mL) is equivalent to 58.8 μM of polyphenols (based on gallic acid), 5.6 μM anthocyanins (based on cyanidin-3-glucoside) and 15.3 μM ellagitannins (based on ellagic acid). The cumulative release of proteoglycan (measured as GAG) and CII epitope are presented in Figs. 2A and 2B, respectively. Fig 2C shows the gelatynolitic activity of culture media collected at day 28 and Fig. 2D shows a picture of the appearance of the cartilage explants at the end of the culture period after treatment with vehicle, IL-1β alone, and IL-1β + 50 μg/mL RRE.
In the vehicle control, basal release of GAG was observed. However, treatment with IL-1β caused a rapid and significant release of GAG from the explants within 8 days of culture compared to the vehicle control (Fig. 2A). On day 8, RRE inhibited release of GAG by 54% but beyond that, no significant difference was seen between the RRE-treated and IL-1β-treated explants. This data suggests that RRE reduced the rate of proteoglycan degradation. Levels of the CII epitope were not measurable until day 28 at which point, more than a 10 fold epitope release was observed in IL-1β-treated explants (Fig. 2B). RRE completely inhibited the release of CII epitope into the media slightly below basal levels. Significant dissolution was evident in the IL-1β-treated explants whereas the appearance of RRE-treated explants was comparable to the vehicle control explants (Fig. 2D). Thus, this data suggest that RRE may afford cartilage protection by slowing down the rate of proteoglycan and CII degradation.
In Vitro Effects of RRE on Gelatinase Activity in Explant Culture Media
Considerable evidence has associated the degradation of cartilage to high levels of MMP, mainly the collagenases, which degrade fibrillar collagen into respective ¾ and ¼ fragments.27 Another class of MMPs, the gelatinases (MMP-2 and MMP-9), further degrade the collagen fragments into small peptides. We assessed gelatinase activities in the explant culture media collected periodically by gelatin zymography (Fig. 2C). Purified recombinant pro- and activated MMP-2 and MMP-9 were used as standards. In the first two weeks (days 4, 8 and 12) Pro-MMP-2 and Pro-MMP-9 were detected in both un-stimulated and IL-1β stimulated explants at almost comparable levels. At day 16, activated MMP-2 and MMP-9 were also detected in IL-1β treated explants and further increased until the end of the culture period. By day 28, IL-1β-stimulated explants exhibited significantly higher MMP-2 and MMP-9 activities (both pro and active forms) compared to un-stimulated explants (Fig. 2C). RRE caused a downregulation in MMP-2 and MMP-9 activation and also completely down regulated the levels of an unidentified MMP which we suspect to be MMP-3, based on apparent molecular weight and literature.27
In Vivo Effects of RRE in Adjuvant-Induced Arthritis (AIA) Rat Model
We evaluated the anti-inflammatory effects of prophylactic administration of RRE at doses of 30 and 120 mg/Kg, respectively. Unfortunately, we could not correlate the level of RRE used in the in vitro experiments (i.e. 50 μg/mL which equals 58.8, 5.6, and 15.3 μM of total polyphenols, anthocyanins and ellagitannins, respectively) to a physiological relevant dose which we could utilize for the animal studies. This criticism is common for many in vitro studies which are not usually translatable to the in vivo situation and commonly use non-physiologically relevant doses. Thus, for the animal study, we sought to test RRE at similar doses previously reported for polyphenol-enriched fruit extracts in the AIA rat model. We based our dosage regimen on a previous AIA rat study where a cherry anthocyanin extract was delivered at 75, 150, and 300 mg/Kg.28 It should be noted that high doses of up to 400 mg/Kg of tart cherry anthocyanins have been previously used for anti-inflammatory animal studies.5
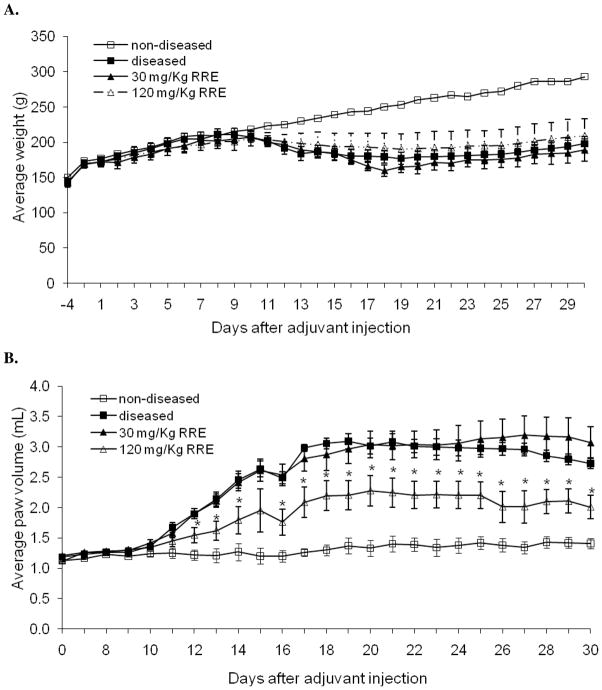
Following a single injection of Mycobacterium tuberculosis, the rats were gavaged daily with the two different doses of RRE or water. Average weights and paw volumes were monitored daily and are presented in Figs. 3A and 3B respectively. Induction of arthritis had no effect on the average weight of the diseased rats in the first 9 days but on day 20, a 15% weight loss relative to day 9 was observed. The RRE had no effect on the associated weight loss at 30 mg/Kg or 120 mg/Kg. Paw volume was significantly higher in diseased rats versus non-diseased rats, which showed no significant change in paw volume throughout the experiment. In diseased rats, a continuous increase in paw volume was observed from day 10, which reached a 2.35 fold plateau on day 18. This plateau in edema was maintained until day 27 at which point paw volumes started to gradually decline. At 30 mg/Kg, RRE had no effect on paw edema. At 120 mg/Kg, RRE caused an overall 23% reduction in paw edema compared to the diseased rats. The data for left and right limbs were comparable for each rat.
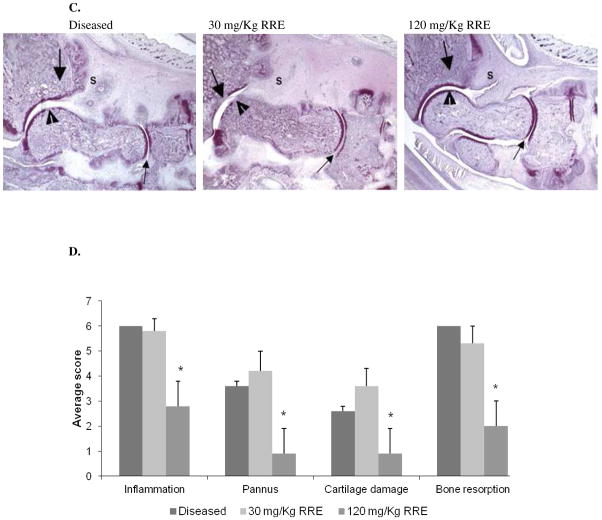
Figure 3.
In vivo effects of the polyphenol-enriched red raspberry extract (RRE) in the antigen-induced arthritis (AIA) rat model. Fig. 3A: Effects of RRE on body weight; Fig. 3B: Effects of RRE on left paw volume; Fig. 3C: Representative toluidine blue-stained histology sections (16 X magnification) showing the ability of RRE to decrease inflammation (S), pannus formation (large arrow), cartilage damage (arrow head), and bone resorption (large arrow); Fig. 3D: Mean histology scores. Data is expressed as the mean ± SEM (n = 6).
Histological sections of representative joints collected from each group are presented in Fig. 3C. Ankles of animals from the non-treated diseased control showed severe inflammation and bone resorption with marked pannus and moderate cartilage damage. Ankles of animals treated with 30 mg/Kg RRE showed severe inflammation and bone resorption with severe pannus and marked cartilage damage. However, ankles of animals from the 120 mg/Kg RRE treatment showed mild inflammation and marked bone resorption with minimal pannus and cartilage damage. Clinical scoring revealed that at the higher dose, RRE significantly inhibited inflammation (54%), pannus formation (74%), cartilage damage (67%), and bone resorption (67 %), whereas rats treated with the lower dose of RRE showed no improvement (Fig. 3D).
DISCUSSION
In the present study, a polyphenolic-enriched red raspberry extract showed cartilage protecting and anti-inflammatory properties using a combination of in vitro and in vivo models. Analyses revealed that anthocyanins and ellagitannins are among the predominant polyphenols in the RRE studied here which is in agreement with literature.14–16
Rheumatoid arthritis (RA) is characterized by joint inflammation with concomitant cartilage destruction,9 which results from the loss of its two major protein components, proteoglycans and type-II collagen (CII). While loss of proteoglycan is an early and reversible process, CII degradation is irreversible and leads to loss of joint function.27 We evaluated the effects of RRE on nasal bovine cartilage degradation using an explant model in which degradation was stimulated IL-1β. As expected, stimulation of bovine nasal septa with IL-1β caused a rapid degradation of proteoglycan followed by a late phase degradation of CII. The RRE was able to delay the progression of both proteoglycan and CII degradation as evidenced by a decrease in GAG and CII epitope in the culture media, respectively. Moreover, the anti-inflammatory effects of RRE were evaluated in an AIA rat model at two doses of 30 mg/Kg and 120 mg/Kg. Daily prophylactic oral administration of the higher dose of RRE resulted in a decreased rate of disease development (as measured by paw volume) and resulted in a significant 23% reduction in hind limb edema with no effect on the weight loss associated with this model. Histological analysis also showed that RRE significantly (P ≤ 0.05) reduced inflammation, pannus formation, cartilage damage and bone resorption. However, at the lower dose, RRE had no effect on disease progression nor on paw edema.
It should be noted that the AIA animal model shares many features with human RA and has been widely used for preclinical evaluation of novel anti-inflammatory agents. This animal model is characterized by rapid and reliable onset (within 9–11 days) and progression of polyarticular inflammation marked by bone resorption, periosteal bone proliferation, and mild cartilage degradation.26 The AIA animal model is well known to be accompanied by weight loss as the disease develops and furthermore, RA symptoms are often accompanied by a loss in body mass (rheumatoid cachexia) that precedes the onset of joint inflammation.29 While we did not record body composition nor feed intake in the current study, the RRE treatment was not expected to alleviate the cachexia associated with this animal model (Fig. 3A).
Unfortunately, we did not collect tissues or blood in the current study and thus were not able to evaluate for measures of inflammation such as inflammatory cytokines or cyclooxygenase expression etc. to correlate with the histological data. Thus, the mechanism underlying these observed effects needs further investigation. Also, it is noteworthy that after ingestion, polyphenols are known to be poorly bioavailable and extensively metabolized.21, 30, 31 Thus, the colonic catabolism of red raspberry polyphenols, and the formation of further bioactive metabolites, could contribute towards the health benefits observed for this fruit.21, 31 Therefore while it is likely that the effects seen here are resulting from RRE polyphenol derived metabolites, further studies would be required to investigate this.
In summary, this study provides insights into the anti-inflammatory effects of the red raspberry fruit and adds to the growing body of biological data on polyphenolic-enriched berry extracts. However, whether regular consumption of red raspberry fruit may have beneficial effects on joint health will require future human clinical studies.
Acknowledgments
This project was supported by the Washington Red Raspberry Commission. Spectrometric data were acquired from an instrument located in the RI-INBRE core facility located at the University of Rhode Island (Kingston, RI, USA) obtained from Grant # P20RR016457 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Part of the Berry Health Symposium 2011
References
- 1.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 2.De Pascual-Teresa S, Sanchez-Ballesta MS. Anthocyanins: from plant to health. Phytochemistry Rev. 2008;7:281–299. [Google Scholar]
- 3.Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspect Med. 2010;31:513–539. doi: 10.1016/j.mam.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–369. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 5.Tall JM, Seeram NP, Zhao C, Nair MG, Meyer RA, Raja SN. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav Brain Res. 2004;153:181–188. doi: 10.1016/j.bbr.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán FA, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolysable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–743. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 9.Ibold Y, Frauenschuh S, Kaps C, Sittinger M, Ringe J, Goetz PM. Development of a high-throughput screening assay based on the 3-dimensional pannus model for rheumatoid arthritis. J Biomol Screen. 2007;12:956–965. doi: 10.1177/1087057107307147. [DOI] [PubMed] [Google Scholar]
- 10.Strietholt S, Maurer B, Peters MA, Pap T, Gay S. Epigenetic modifications in rheumatoid arthritis. Arthritis Res Ther. 2008;10:219–227. doi: 10.1186/ar2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 12.Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421–430. doi: 10.5435/00124635-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rao AV, Snyder DM. Raspberries and human health: A review. J Agric Food Chem. 2010;58:3871–3883. doi: 10.1021/jf903484g. [DOI] [PubMed] [Google Scholar]
- 14.Mullen W, Stewart A, Lean M, Gardener P, Duthie G, Crozier A. Effect of freezing storage on the phenolics ellagitannins flavonoids antioxidant capacity of red raspberries. J Agric Food Chem. 2002;50:5197–5201. doi: 10.1021/jf020141f. [DOI] [PubMed] [Google Scholar]
- 15.Mullen W, McGinn J, Lean ME, MacLean MR, Gardner P, Duthie GG, Yokota T, Crozier A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J Agric Food Chem. 2002;50:5191–5196. doi: 10.1021/jf020140n. [DOI] [PubMed] [Google Scholar]
- 16.Mullen W, Yokota T, Lean ME, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 17.Zafrilla P, Ferreres F, Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem. 2001;49:3651–3655. doi: 10.1021/jf010192x. [DOI] [PubMed] [Google Scholar]
- 18.Singleton VL, Esau P. Phenolic substances in grapes and wine, and their significance. Adv Food Res Suppl. 1969;1:61–111. [PubMed] [Google Scholar]
- 19.Li L, Adams LS, Chen S, Killian C, Ahmed A, Seeram NP. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem. 2009;57:826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S, Scalbert A, Monties B. Insoluble ellagitannins in Castanea sativa and Quercus petraea woods. Phytochemistry. 1991;30:775–778. [Google Scholar]
- 21.Gonzalez-Barrio R, Borges G, Mullen W, Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem. 2010;58:3933–3939. doi: 10.1021/jf100315d. [DOI] [PubMed] [Google Scholar]
- 22.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 23.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 24.Felice BR, Chichester CO, Barrach HJ. Type II collagen peptide release from rabbit articular cartilage. Ann NY Acad Sci. 1999;878:590–593. doi: 10.1111/j.1749-6632.1999.tb07736.x. [DOI] [PubMed] [Google Scholar]
- 25.Barrach HJ, Chichester CO, Sargent DA. Quantification of collagen type II peptides in synovial fluid by inhibition ELISA. Trans Orthop Res Soc. 1996;21:218–237. [Google Scholar]
- 26.Bendele AM. Animal models of rheumatoid arthritis. J Musculoskel Neuron Interact. 2001;1:377–385. [PubMed] [Google Scholar]
- 27.Murphy G, Knäuper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4:S39–S49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y, Zhou J, Wang Y, Xiao C, Tong Y, Tang JC, Chan AS, Lu A. Immunomodulation and antioxidant effects of anthocyanins from cherries on adjuvant-induced arthritis in rats. Evidence Based Integr Med. 2005;2:95–99. [Google Scholar]
- 29.Walsmith JM, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 30.Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 31.González-Barrio R, Edwards CA, Crozier A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: in vivo and in vitro studies. Drug Metab Dispos. 2011;39:1680–1688. doi: 10.1124/dmd.111.039651. [DOI] [PubMed] [Google Scholar]