Abstract
Properdin deficiency is a rare immunological disorder inherited as an X-chromosomal recessive trait. Properdin deficiency poses a significant risk for severe meningococcal infections. About 20 mutations have been reported to underlie properdin deficiency. Here we report a large Finnish family with a novel mutation in the properdin gene (CFP). Based on the total absence of properdin activity in a 14-year-old male patient with an infection resembling meningococcal bacteraemia the coding region and splice sites of the gene were sequenced. The mutation is located in exon 9 and changes guanine to adenine at nucleotide 1164 (c.1164G>A) that causes tryptophan to change to a premature stop-codon (W388X). The mother of the patient was shown to be a carrier of the mutation. In total, the mutation was identified in six females and three young males in the family. The mutation must be inherited from the grandfather who had died of an unknown infectious disease. This is the first mutation of the properdin gene identified in Finland.
Introduction
The complement system is an important part of human innate immunity comprising altogether approximately 40 proteins in blood and on cell membranes., Complement has a significant role in protecting the body against bacterial infections, in removing debris after tissue injury and in acting as a link between innate and acquired immunity. The complement system contains three pathways (classical, alternative and lectin) that all produce the C3 convertase enzyme that participates in reactions destroying foreign cells. The alternative pathway C3 convertase enzyme is stabilised by the positive regulator, properdin, that is formed by 442 amino acids [1, 2].
Properdin deficiency was described for the first time in 1982 in three brothers in Sweden [3]. Since then three types of properdin deficiency have been found. Type I deficiency is the most common with no properdin activity, in type II properdin concentration is diminished but it is functionally active and in type III there is normal amount of properdin but it is functionally inactive. The properdin gene is located at Xp11.23-p11.3 [4] and contains 10 exons, of which 2–10 are translated. Properdin deficiency is inherited as an X-chromosomal recessive trait. If the mother is a carrier of a mutation her sons have a 50% risk to inherit the mutation and the daughter have a 50% risk to be carriers. All daughters of a male carrier are also carriers of the mutation. About twenty different mutations have been reported in CFP [5–11]. About 18% of the relatives with properdin type I deficiency suffer from meningococcal infections, usually at school age [1, 2, 8].
Case report
Index patient
Index patient (Figure 1; IV/1) was a previously healthy male, aged 14 years. He was suspected to have meningococcal infection based on fever, petecchiae and ecchymoses in the upper and lower extremities. Preceding hospital care the patient had vomiting, loose stools and worsening arthralgia on his wrists and ankles. Laboratory investigations revealed leucocytosis (20.3×109/l) and increased C- reactive protein level (348.7 mg/l). Activated partial thromboplastin time and the level of fibrinogen degradation products were slightly increased on admission (48 sec, 0.5 mg/l, respectively) but they rapidly normalized when antibiotic treatment was started. The patient’s thrombocyte count and prothrombin time were normal.
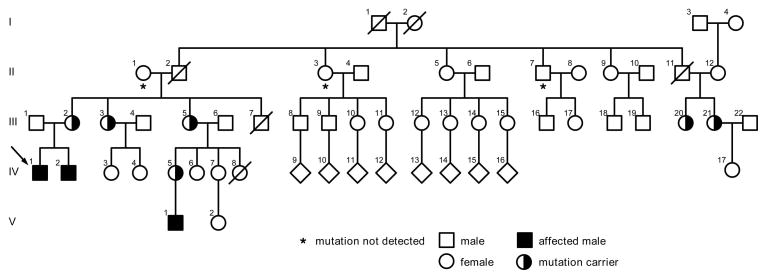
Figure 1. The family pedigree with the W388X mutation in the properdin gene.
The index-patient is marked by an arrow. Three males IV/1, IV/2 and V/1 have the mutation. A total of six females III/2, III/3, III/5, III/20, III/21 and IV/V were shown to be carriers. In subjects marked by a star the mutation was excluded. The rest of the family members did not participate in the study. The number of children and their sexes are not known in IV/9–IV/16.
The clinical picture seemed to indicate meningococcal infection and i.v. penicillin was started. The blood cultures remained negative. Because the clinical picture was compatible with meningococcal infection, the patient and his family members received prophylactic antibiotics. Immunological studies showed normal immunoglobulin and tetanus antibody levels. The level of the C3 complement protein was 1.12 g/l (reference values 0.5–1.12 g/l) and C4 0.08 g/l (reference values 0.12–0.42 g/l). The functional activity of the classical pathway was normal but the functional activities of the alternative and lectin pathway were undetectable (Wielisa®, Wieslab, Lund, Sweden).. No properdin protein could be detected by Western blot analysis. Sequencing of the coding region and splice sites of the properdin gene resulted in the identification of a mutation in exon 9 at codon 388, where tryptophan was changed to a premature stop codon (W388X). The mutation had been inherited from his mother (Figure 1; III/2). Primers are available from the authors on request.
The patient was immunised with a quadrivalent polysaccharide meningococcal vaccine (Mencevax ACWY Novuma) and his meningococcal antibody levels were measured before and after vaccination (MenY 14.2–18.2 ug/ml, MenW135 0.4–6.9 ug/ml, MenC 0.6–111.5 ug/ml, MenA 1.3–9.7 ug/ml). The patient had good antibody levels against all serotypes after vaccination and against serotype Y already before vaccination. The patient was given amoxicillin for home as pre-emptive therapy for fever.
The healthy brother of the index patient (IV/2 in Figure 1), aged 12 years, lacked functional activity of the alternative pathway. However, the activity of his lectin pathway was normal. He turned out to have inherited the same mutation from his mother. The mutation was excluded from the DNA of the grandmother. Thus, the mutation was inherited from the grandfather who had died of an unknown infectious disease. Further analyses of the family confirmed that it is not a de novo mutation.
The brother was also immunised with quadrivalent polysaccharide meningococcal vaccine and showed good antibody response after immunisation (MenY 0.3–46.9 ug/ml, MenW135 0.2–4.2 ug/ml, MenC 0.3–96.4 ug/ml, MenA 2.5–37.2 ug/ml). He has amoxicillin at home to be used in case of fever.
In further analyses of the family both sisters of the carrier mother (III/2) and two female cousins (III/20 and III/21) were found to be mutation carriers. The carriership of the IV/17 female has not been studied due to her young age. One female cousin (IV/5) and her newborn son (V/1) were also found to carry the mutation. One paternal uncle (II/7) and aunt (II/3) did not have the mutation. To our knowledge, this family is the first family where CFP mutation has been characterised in Finland. All the family members that could be contacted were analysed.
Summary
Neisseria meningitidis is a rare but severe cause of fulminant sepsis and meningitis. Properdin deficiency and low levels or dysfunction of the terminal components of complement have been associated with increased risk of meningococcal infection.[12] However, most patients with meningococcal infection have a normal immune system and complement deficiency rarely predisposes to the infection. An immune deficiency is more likely if the family has a history of meningococcal infections or the causative meningococcal serotype is W-135, X, Y or Z, which rarely cause infections in healthy individuals [8, 13]. The causative pathogen of the index patient of the study could not be cultured. However, since antibodies against serotype Y were elevated already before vaccination, this serotype, which only occasionally causes infections in healthy individuals, was the most likely cause. Since properdin deficiency is extremely rare and many of the deficiencies probably are undiagnosed, it is very difficult to describe typical features of meningococcal infection in these patients. Another important feature, which should raise suspicion of properdin deficiency, is the age of the patient. The risk of meningococcal infection in healthy individuals is usually highest in children less than two years of age when protective antibodies against meningococcal serotypes have not yet developed. In patients with properdin deficiency the median age at the time of meningococcal infection is much higher [14].
Recurrent meningococcal infections even in patients with properdin deficiency are extremely rare. This may reflect the fact that protective antibodies are produced following infection. Therefore, patients with properdin deficiency should be immunised with tetravalent meningococcal vaccine, which gives protection against infections caused by serotype A, C, W135 and Y. Since it is a polysaccharide vaccine, children younger than two years of age only develop antibodies against serotype A. Therefore vaccinations should be considered only in children older than two. A quadrivalent conjugate meningococcal vaccine has been licenced in the United States and is recommended for children over two years of age and with increased risk of meningococcal infection [15]. Because meningococcal infection could most likely be prevented by vaccinations; male members of the family should be identified and vaccinated. In children less than two years of age antibiotic prophylaxis with penicillin or amoxicillin should be used before protective antibodies have accumulated after vaccination.
Although the risk of contracting meningococcal infection is significantly higher in individuals with properdin deficiency not all have meningococcal infection during their lifetimes. Additional risk factors may be needed or may increase the risk. Both a low activity of the lectin pathway and a lack of the immunoglobulin G2 m(n) which weakens antibody response against polysaccharide antigens, have been connected to increased risk of meningococcal infection in patients with properdin deficiency [8, 16]. The index patient with meningitis in this study had absent lectin pathway activity. Although no further analysis was done, the most likely explanation was mannan-binding lectin (MBL) deficiency. The presence of variant MBL alleles with absent or low protein activity has been associated with an increased risk of infection especially with concomitant immunodeficiency [17]. In early childhood MBL deficiency has been associated with meningococcal infection [18]. In an analysis of a Danish family with recurrent meningococcal disease, Bathum et al. showed that combined deficiency of both MBL and properdin increased the risk of infection [16]. However, properdin deficiency is such a rare event and variant MBL alleles so common in the population that it is difficult to show that these immunological associations do not occur only by chance [19].
In this study, we describe the first family in Finland with a mutation in the properdin gene. Because of the homogeneous genetic background of the Finnish population, it is probable that only a few mutations will be found in Finland. It is important to look for properdin deficiency after meningococcal infection, especially if the causative serotype is a rare one or the family has a history of meningococcal infections. Vaccination with tetravalent vaccine may prevent additional life-threatening infections in family members with properdin deficiency.
Acknowledgments
We thank the family members for their participation. This study was funded by grants from the Mary and Georg Ehrnrooth Foundation, Helsinki, Finland; The Eye Foundation, Helsinki, Finland; The Academy of Finland (Comevac), Helsinki, Finland; Helsinki University Central Hospital Research Funds (TLE82G004, TYH5117, TYH2008235) Helsinki, Finland; the National Institutes of Health (EY11515) and the National Institutes of Health (R24EY017404) of the National Eye Institute.
References
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Sjöholm AG, Braconier JH, Söderström C. Properdin deficiency in a family with fulminant meningococcal infections. Clin Exp Immunol. 1982;50:291–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Goonewardena P, Sjöholm AG, Nilsson LA, Pettersson U. Linkage analysis of the properdin deficiency gene: suggestion of a locus in the proximal part of the short arm of the X chromosome. Genomics. 1988;2:115–8. doi: 10.1016/0888-7543(88)90092-4. [DOI] [PubMed] [Google Scholar]
- 5.Fredrikson GN, Westberg J, Kuijper EJ, et al. Molecular characterization of properdin deficiency type III: dysfunction produced by a single point mutation in exon 9 of the structural gene causing a tyrosine to aspartic acid interchange. J Immunol. 1996;157:3666–71. [PubMed] [Google Scholar]
- 6.Truedsson L, Westberg J, Fredrikson GN, et al. Human properdin deficiency has a heterogeneous genetic background. Immunopharmacol. 1997;38:203–6. doi: 10.1016/s0162-3109(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 7.Fijen CA, van den Bogaard R, Schipper M, et al. Properdin deficiency: molecular basis and disease association. Mol Immunol. 1999;36:863–7. doi: 10.1016/s0161-5890(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 8.Späth PJ, Sjöholm AG, Fredrikson GN, et al. Properdin deficiency in a large Swiss family: identification of a stop codon in the properdin gene, and association of meningococcal disease with lack of the IgG2 allotype marker G2m(n) Clin Exp Immunol. 1999;118:278–84. doi: 10.1046/j.1365-2249.1999.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bogaard R, Fijen CA, Schipper MG, de Galan L, Kuijper EJ, Mannens MM. Molecular characterisation of 10 Dutch properdin type I deficient families: mutation analysis and X-inactivation studies. Eur J Hum Genet. 2000;8:513–8. doi: 10.1038/sj.ejhg.5200496. [DOI] [PubMed] [Google Scholar]
- 10.Mathew S, Overturf GD. Complement and properidin deficiencies in meningococcal disease. Pediatr Infect Dis J. 2006;25:255–6. doi: 10.1097/01.inf.0000209215.65445.04. [DOI] [PubMed] [Google Scholar]
- 11.Schejbel L, Rosenfeldt V, Marquart H, Valerius NH, Garred P. Properdin deficiency associated with recurrent otitis media and pneumonia, and identification of male carrier with Klinefelter syndrome. Clin Immunol. 2009;131:456–62. doi: 10.1016/j.clim.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Sjöholm AH, Jönsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Linton SM, Morgan BP. Properdin deficiency and meningococcal disease – identifying those most at risk. Clin Exp Immunol. 1999;118:189–91. doi: 10.1046/j.1365-2249.1999.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overturf GD. Indication for the immunological evaluation of patients with meningitis. Clin Infect Dis. 2003;36:189–94. doi: 10.1086/345527. [DOI] [PubMed] [Google Scholar]
- 15.Updated Recommendations for Use of Meningococcal Conjugate Vaccines --- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Weekly. 2011;60:72–6. [PubMed] [Google Scholar]
- 16.Bathum L, Hansen H, Teisner B, et al. Association between combined properdin and mannose-binding lectin deficiency and infection with Neisseria meningitidis. Mol Immunol. 2006;43:473–9. doi: 10.1016/j.molimm.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 18.Faber J, Schuessler T, Finn A, et al. Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr Infect Dis J. 2007;26:243–6. doi: 10.1097/01.inf.0000256751.76218.7c. [DOI] [PubMed] [Google Scholar]
- 19.Seppänen M, Lokki M-L, Lappalainen M, et al. Mannose-binding lectin 2 gene polymorphism in recurrent herpes simplex virus 2 infection. Hum Immunol. 2009;70:218–21. doi: 10.1016/j.humimm.2009.01.022. [DOI] [PubMed] [Google Scholar]