Abstract
Introduction and hypothesis
Vaginal tactile imaging (VTI) is based on principles similar to those of manual palpation. The objective of this study is to assess the clinical suitability of new approach for imaging and tissue elasticity quantification under normal and prolapse conditions.
Methods
The study subjects included 31 women with normal and prolapse conditions. The tissue elasticity (Young’s modulus) was calculated from spatial gradients in the resulting 3-D tactile images.
Results
Average values for tissue elasticity for the anterior and posterior compartments for normal conditions were 7.4±4.3 kPa and 6.2±3.1 kPa respectively. For Stage III prolapse the average values for tissue elasticity for anterior and posterior compartments were 1.8±0.7 kPa and 1.8±0.5 kPa respectively.
Conclusions
VTI may serve as a means for 3-D imaging of the vagina and a quantitative assessment of vaginal tissue elasticity, providing important information for furthering our understanding of pelvic organ prolapse and surgical treatment.
Keywords: Biomechanical properties, Elasticity, Prolapse, Vaginal tissue, Tactile imaging
Introduction
Pelvic organ prolapse (POP) is a highly prevalent condition affecting at least 50% of women in the USA during their lifetimes [1]. Some loss of uterine or vaginal support occurs in most adult women [2]. However, the true etiology of prolapse and differences seen among individuals are not entirely understood. Changes in the elasticity of the vaginal walls, connective support tissues, and muscles are thought to be significant factors in the development of pelvic organ prolapse [3, 4]. Characterization of the biomechanical properties of pelvic support tissues across anatomical variations in support from patient to patient will allow major progress in the field by assessing: (1) how tissue elasticity and changes in elasticity are related to the development of pelvic organ prolapse and (2) how functional elasticity can be restored and maintained through reconstructive surgery.
In the last decade, a new modality for tissue characterization termed elasticity imaging or elastography has emerged. Elasticity imaging allows the visualization and assessment of mechanical properties of soft tissue. Mechanical properties of tissues, i.e., elastic modulus and viscosity, are highly sensitive to tissue structural changes accompanying various physiological and pathological processes. Elasticity imaging is based on generating a stress in the tissue using various static or dynamic means and the measuring resulting strain by ultrasound or magnetic resonance imaging (MRI) [5–7]. The current increasing number of publications on elastography covers practically all key human organs [8–10]. Tactile imaging, a branch of elasticity imaging, yields a tissue elasticity map, similarly to other elastographic techniques [9–12]. At the same time, tactile imaging most closely mimics manual palpation because the tactile imaging probe with a pressure sensor array mounted on its face acts similarly to human fingers during a clinical examination by slightly compressing soft tissue with the probe and detecting the resulting changes in the pressure pattern on the tissue surface.
As previously described, we designed a prototype of the vaginal tactile imager (VTI) for visualization and assessment of elastic properties of pelvic floor tissues. In a pilot clinical study with 13 patients, we demonstrated that VTI has the potential for prolapse characterization and detection and allows imaging of the vaginal walls with increased rigidity due to implanted mesh grafts following reconstructive pelvic surgery [13].
The main purpose of the present study is to assess the clinical suitability of a new approach for 3-D imaging of vagina and tissue elasticity quantification under normal and prolapse conditions.
Materials and methods
Population description
In a period between March 2010 and September 2010, 31 women were enrolled in the development study (clinical trials identifier NCT01111916 at http://clinicaltrials.gov) and underwent VTI examination. The study subjects included 18 women with normal pelvic support and 13 women with pelvic organ prolapse (stage I–III). One patient in the prolapse category had previously undergone pelvic surgery for prolapse, including a vaginal hysterectomy and anterior repair. All other subjects had not undergone prior pelvic surgery for prolapse. The average age was 60±17, from 28 to 90 years old. The clinical protocol was approved by the local institutional review board, and all women gave written informed consent. The study was done in compliance with the Health Insurance Portability and Accountability Act. The VTI images were obtained and recorded at the time of scheduled routine gynecologic visits.
Total workflow comprised of the following steps:
Recruiting women who routinely undergo vaginal examination as a part of their diagnostic treatment of concerned areas;
Acquisition of clinical diagnostic information related to the studied cases by standard clinical means;
Performing a VTI examination; and
Analyzing tactile images and calculation of vaginal wall elasticity from the recorded VTI examination data.
Prior to the VTI examination, a standard gynecologic vaginal examination was performed by a practicing urogynecologist. For prolapse description, we used POP-Q system [14]. Additionally, the patients were asked to assess comfort level of VTI examination relative to manual palpation.
Vaginal tactile imager
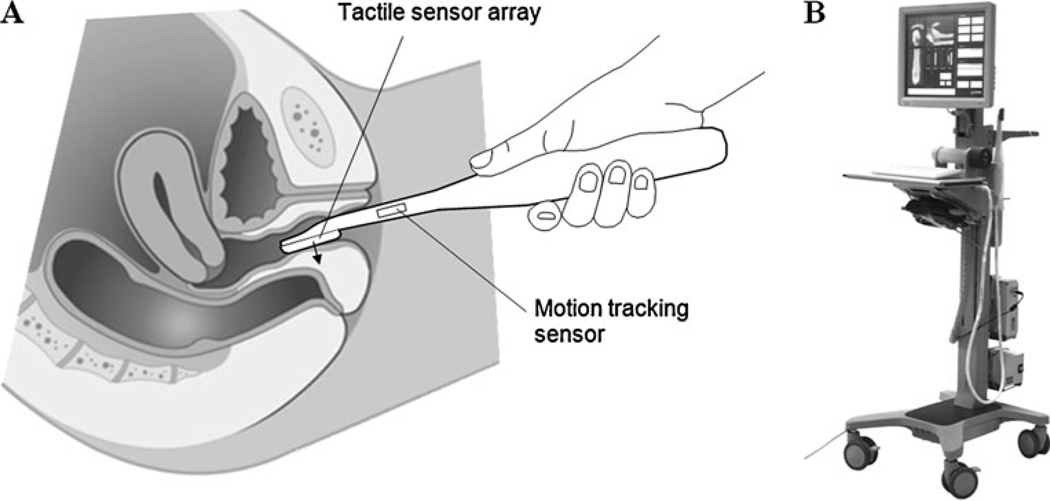
The VTI includes a transvaginal probe, a motion tracking system, data acquisition electronic unit, and a computer with touch screen monitor (see Fig. 1b). The vaginal probe is comprised of a pressure sensor array and a motion-tracking sensor. The pressure sensor array is installed on the probe head surface contacting with the vaginal wall during the examination procedure. The probe head measures 45 mm in length, 20 mm in diameter. The pressure sensor array is comprised of 128 capacitive pressure sensors which provide 2-D pressure pattern being contacted with vaginal wall. On average, each pressure sensor has a sensitivity of 20 Pa; reproducibility is about 300 Pa and operational range, 30 kPa. The six-degree-of-freedom motion-tracking system provides VTI probe positioning accuracy better that 1 mm and angular accuracy of about 0.25°. The electronic unit provides data acquisition from the pressure sensors, synchronizes data from motion tracking sensor, and communicates with the computer through a USB port. The data acquisition rate is about 25 pressure patterns per second.
Fig. 1.
The tactile sensor array mounted at the tip of the VTI probe allows recording the pressure patterns on the vaginal walls under manually applied pressure to compose 3-D tactile image of the vagina. a Vaginal examination with VTI probe; b view of the VTI
Figure 1a shows the VTI probe during imaging of the posterior wall. The VTI examination was performed on patients in a standard position for a routine gynecologic exam. Examinations were performed with an empty bladder. During examination, the VTI probe is covered by a disposable plastic sheath with a lubricant. The full VTI examination required 3–5 min for completion. Three orthogonal projections of the 3-D vaginal pressure map with VTI probe location are observed by operator in real time. The VTI clinical operators were trained on pelvic floor models prior to VTI clinical application to standardize imaging techniques. The examination procedure includes multiple compressions of the vaginal walls and allows composing a circumferential 3-D tactile image or pressure map of the vagina and storing the acquired data in digital format.
Tissue elasticity calculation
The tissue elasticity, Young’s modulus (E), was calculated from spatial gradients in the resulting 3-D tactile images. We are using a non-linear model for vaginal tissue. This approach was validated with multiple pelvic floor models built with two-component silicone (GE Silicones, Albany, NY). The VTI provides reproducible elasticity measurements with resolution better than 10% and with accuracy within 20% for the expected tissue elasticity range from 2 to 40 kPa. The capacity of a human finger to scale the magnitude of softness of objects is substantially lower [15].
Statistical analysis
For visual evaluation of the analyzed clinical data distributions within the normal and prolapse patient samples, we used boxplots. In descriptive statistics, the boxplot is a convenient and widely accepted way of graphically depicting groups of numerical data or data samples. Boxplots are able to visually show distinctions of data samples without making any assumptions about the underlying statistical distribution. We have used a notched boxplot [16] showing a confidence interval for the median value (central horizontal line), and 25% and 75% quartiles. The spacings between the different parts of the box help to compare variance. The boxplot also identifies skewness (asymmetry) and outliers. The intersection or divergence of confidence intervals for two patient samples is a visual analog of the paired t test. To determine whether there were differences among tissue elasticity subsamples, one-way analysis of variance (ANOVA) was performed by using MATLAB 6.1 (MathWorks, Natick, MA). A significance level of 0.05 (P≤0.05) was chosen as an indication that parameter distributions were different among subgroups. A significant result indicated that at least two of the subgroups (normal, prolapse stage I, prolapse stage II, prolapse stage III) differed significantly.
Results
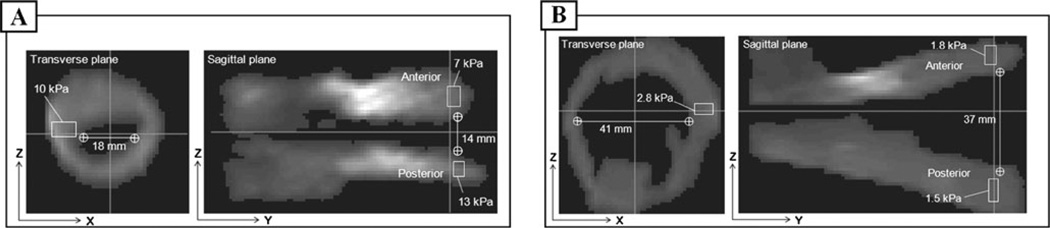
All 31 enrolled women were successfully examined with the VTI, and 3-D images of vagina were recorded and stored. We found substantial differences in the anatomy and tissue elasticity between normal and prolapse conditions. Figure 2 presents common examples of examination results for normal and prolapse conditions. Specifically, Fig. 2a shows transverse and sagittal cross-sections (planes) of 3-D vaginal tactile image received with VTI for a patient (63 years) with normal pelvic floor conditions as was detected by manual palpation during physical examination. Young’s modulus (E) was calculated for areas specified by the rectangles. We found that E=7 kPa at apical anterior and E=13 kPa at apical posterior respectively. The right side of the apical vagina demonstrated E=10 kPa (transverse plane in Fig. 2a). The anterior/posterior spacing at the apical vagina was measured as 14 mm (sagittal plane in Fig. 2a). Figure 2b shows transverse and sagittal planes of 3-D vaginal tactile image received with VTI for a patient (77 years) with stage III prolapse in the anterior and upper half of the posterior compartment that recurred less than a year from a vaginal hysterectomy and anterior repair. We found, for this case, that E=1.8 kPa at the apical anterior wall and E=1.5 kPa at the apical posterior wall. The left side of the apical vaginal wall demonstrated E=2.8 kPa (transverse plane in Fig. 2b). The anterior/posterior spacing at the apical vagina was measured as 37 mm (sagittal plane in Fig. 2b).
Fig. 2.
Transverse and sagittal cross-sections of 3-D vaginal tactile image received with VTI for a patient with normal pelvic floor conditions (a) and stage III prolapse (b). Young’s modulus was calculated for areas specified by a rectangle
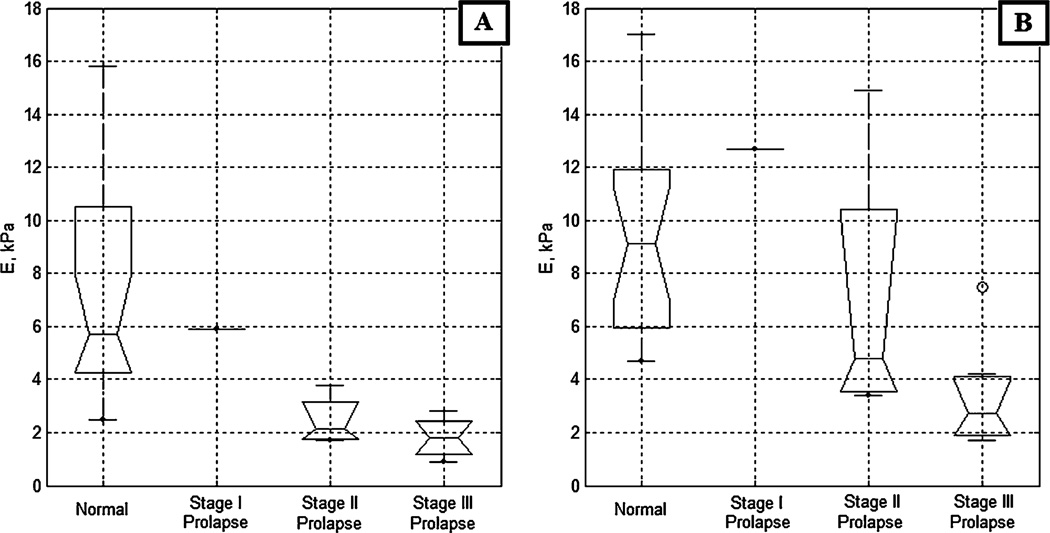
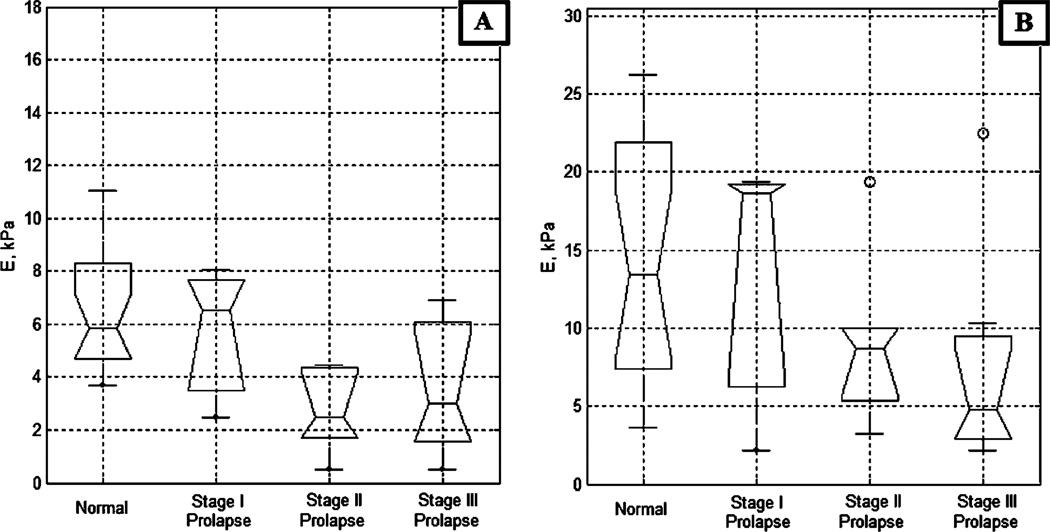
Figure 3 presents the vaginal tissue elasticity distribution for the apical and mid-anterior aspects of the vagina across varying POP conditions; among them, 19 normal (average age, 55 years), one stage I, four stage II, and seven stage III (average age, 70 years) cases. The data demonstrate the respective elasticity modulus decrease up to 320% for the apical anterior walls and up to 340% for the mid-anterior walls with stage III prolapse relative to the normal conditions. From the apical to the mid-anterior vaginal walls, the elasticity modulus is increased in average by 80%. Figure 4 presents the vaginal tissue elasticity distribution for the apical and mid-posterior vaginal walls across varying POP conditions; among them, 23 normal (average age, 58 years), two stage I, two stage II, and four stage III (average age, 70 years) cases. The data demonstrate the respective elasticity modulus decrease up to 310% for the apical posterior and up to 220% for the mid-posterior with stage III prolapse relative to the normal conditions. From the apical to the mid-posterior vaginal walls, the elasticity modulus is increased in average by 95%. The average values for tissue elasticity for the anterior and posterior compartments for normal conditions were 7.4±4.3 and 6.2±3.1 kPa, respectively. For stage III prolapse, the average values for tissue elasticity for the anterior and posterior compartments were 1.8±0.7 and 1.8±0.5 kPa, respectively.
Fig. 3.
Anterior tissue elasticity, Young’s modulus (E), vs anterior conditions defined by physical examination. a Apical anterior; b Mid-anterior
Fig. 4.
Posterior tissue elasticity, Young’s modulus (E), vs posterior conditions defined by physical examination. a Apical posterior, b Mid-posterior
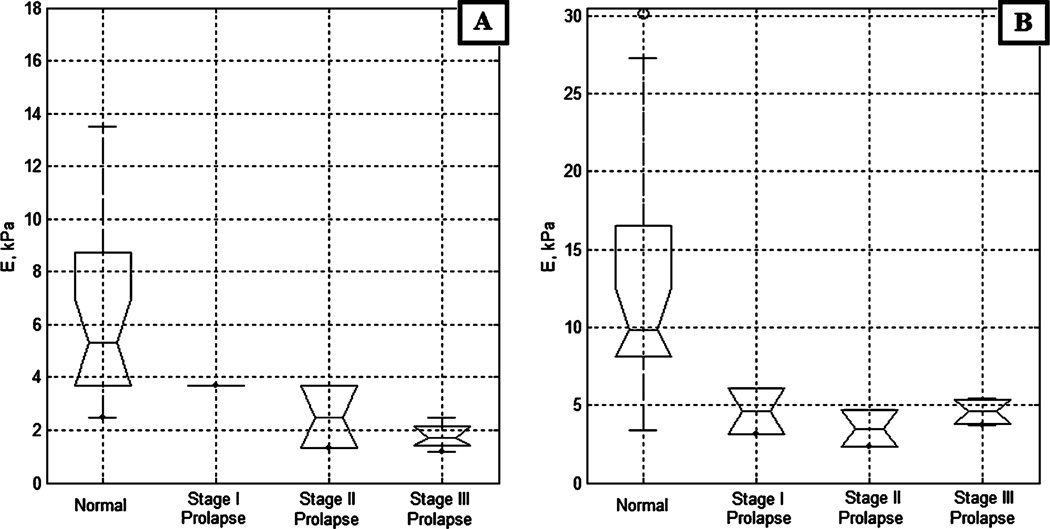
Figure 5 presents the vaginal tissue elasticity distribution for the apical and mid-vagina side walls across varying POP conditions; among them, 18 normal (average age, 54 years), three stage I, six stage II, and seven stage III (average age, 69 years) cases. The anterior and posterior POP conditions were combined together. We observe on Fig. 5a a decrease in elasticity modulus for apical side walls from normal conditions (E=6 kPa) to POP conditions (E=3 kPa). The results of ANOVA support this observation (p<0.0044). For the mid-vagina (Fig. 5b), despite of the visible difference in median values, ANOVA cannot guarantee that we have statistically significant result in elasticity change among four groups (p<0.21).
Fig. 5.
Side wall tissue elasticity, Young’s modulus (E), vs conditions defined by physical examination. a Apical sides, b Mid sides
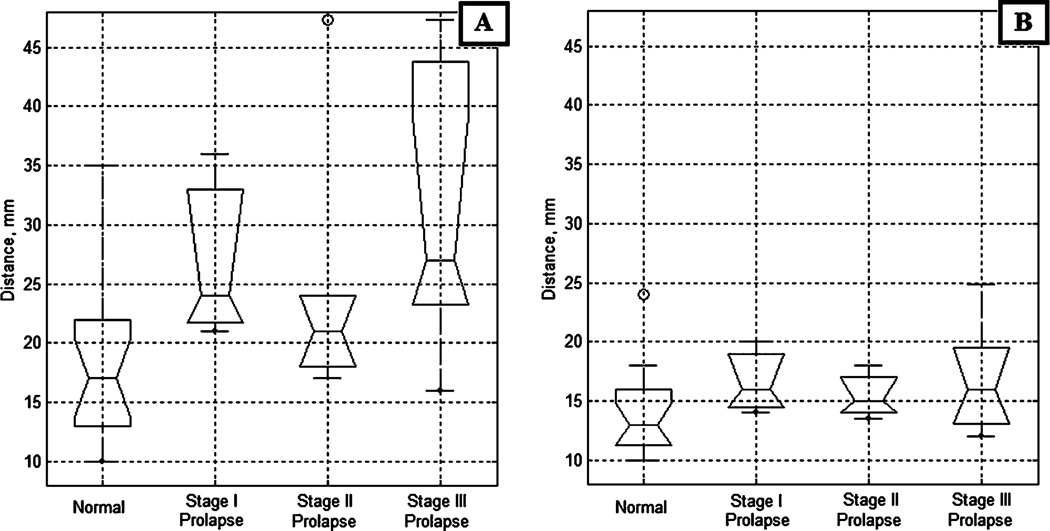
Figure 6 presents the anterior/posterior spacing at the apical and mid-vagina across varying POP conditions (the same group distribution as in Fig. 5). The anterior/posterior spacing was measured as a distance between anterior and posterior at pressure level of 1 kPa which, as we found, defines the location of the vaginal wall. Anterior and posterior POP conditions were combined together. We observe on Fig. 6a a spacing increase for the apical aspect of the vagina from 18±6 mm under normal conditions to 32±12 mm under POP stage III. The results of ANOVA support this observation (p<0.01). For the mid-vagina (Fig. 6b), despite of the visible difference in median values, ANOVA cannot guarantee that we have statistically significant result in spacing change between normal condition versus POP groups (p<0.23).
Fig. 6.
Anterior/posterior spacing vs conditions defined by physical examination. a Apical vagina, b Mid-vagina
The patients were asked to assess comfort level of VTI examination relative to manual palpation: 77% said that VTI procedure was the same; 20%, less comfortable; and 3%, more comfortable than manual palpation. No adverse events were reported.
Discussion
While there has been an increasing interest and amount of research dedicated to the evaluation of biomechanical properties of the pelvic floor, the focus of current imaging modalities is largely limited to the pelvic floor muscles and their surrounding connective tissue. It has been much more difficult to evaluate the biomechanical properties of the vaginal wall and its immediate surrounding connective tissue. The specific goal of the VTI is to provide a reproducible and quantifiable means to visualize and measure vaginal tissue elasticity, to improve our understanding of POP and improve our clinical approach to restorative surgery.
A critical review of clinical approaches demonstrates a lack of standardized approaches for assessing the biomechanical conditions of the pelvic floor and vaginal tissue elasticity. Published studies measuring vaginal tissue elasticity properties involve testing of vaginal biopsies obtained at the time of pelvic surgery [17], use of animal models [18], or use of cadaveric tissue [4]. There are several obvious disadvantages of these laboratory measurements including relatively small sample sizes, testing of only local areas of the vagina with an inability to correlate with overall vaginal properties, and removal of the specimen from the living body and the underlying tissues. Other measurements that have been reported include use of suction pressure to deform the vaginal tissue inward and measuring the amount of stretch obtained with a specified pressure application, balloon distention pressure measurements, and subjective physician examiner assessment of elasticity based on a scaled scoring system [19].
More comprehensive imaging techniques, including 3-D or 4-D ultrasound and MRI have provided a great insight into the anatomical abnormalities of pelvic floor support when they are clearly present (cystocele, uterine prolapse, rectocele, or enterocele) [20]. Ultrasonography and MRI also enable evaluation of the function of the pelvic floor with various dynamic maneuvers [21]. Unfortunately, neither imaging technique has been able to demonstrate 3-D elasticity imaging of the vagina. In addition, current imaging findings do not always correlate with the clinical examination or patient symptoms, nor does anatomical correction always lead to functional correction [22]. For these reasons, the clinical role of functional pelvic floor imaging remains unclear at this time. A further obstacle of ultrasound and MRI imaging is the availability of resources and cost of study completion.
To our knowledge, this is the first time 3-D tactile image of the vagina was recorded. Orthogonal cross-sections of the 3-D tactile image allow visualization of anatomy and elasticity distributions (Fig. 2). Tactile imaging reveals not only the elasticity conditions of vaginal wall itself, but the elasticity distribution of underlying tissue structures. These images may be considered as documentation of the current elasticity state of the vaginal walls and surrounding support tissues. Using VTI may offer several other advantages for pelvic tissue imaging. Vaginal tissue elasticity changes may be observed and detected before and after interventional procedure and in time (months or years) by repetitive VTI examination. Quantification of tissue elasticity may be translated in future research into conditions of specific support structures (muscles, fascias, ligaments) to be used further in assessment of the risk of POP development and in planning a reconstructive surgery. Quantitative elasticity data may help to differentiate tissue transformations and correlate them with other data such as congenital defects, childbirth, age, lifestyle, and obesity. The anticipated costs of the VTI imaging will be substantially lower than an ultrasound or MRI, and testing could be performed in less than 5 min in a standard office setting. This may make the VTI modality more applicable for more extensive testing across various patient populations and allow for longitudinal follow-up of patients.
The VTI accuracy of tissue elasticity measurements within 15–20% is acceptable for tissue characterization in the view of the pathological changes to be detected that are estimated to be in the range of 100–300%. Practical accuracy requirement for soft tissue elasticity measurements was established as 10–20% for the elasticity measurements on excised tissue samples [23]. It corresponds to the level of normal mechanical and structural heterogeneity of tissues.
It is well known that the soft tissues are anisotropic, nonlinear, and viscoelastic [24–26]. Multiple parameters need to be used for accurate representation of such properties of tissue, such as time-dependent strain, nonlinearity, hysteresis in the stress/strain curve, strain hardening, etc. [27]. But, in most applications, an accurate and comprehensive characterization of tissue mechanical properties is impractical, and simple means provide reproducible tissue characterization [9–11, 23]. Therefore, we are using a simplest non-linear tissue model for vaginal tissue, which allows practical comparison of tissue elasticity in terms of a single parameter such as Young’s modulus. We calculate the average Young’s modulus for selected tissue volume (e.g., see Fig. 2). During this calculation, possible nonlinearity in stress/strain relationship is taken into account since we calculate Young’s modulus as ratio of average pressure gradient to selected distance (frame size) within 3-D tactile image.
We observed variability in tissue elasticity under normal conditions. The entire elasticity range for the apical anterior vagina (Fig. 3a) varied from E=2.5 to 15.8 kPa (variance ±4.3 kPa) and for the apical posterior vagina (Fig. 4a) varied from E=2.5 to 13.5 kPa (variance ±3.1 kPa). This variability substantially exceeds the measurement accuracy. That means we have patient specific variability and pre-POP conditions that have potentially not yet transformed into anatomic changes to classify the cases as POP during physical examination.
Figures 3 and 4 demonstrate significant differences in anterior and posterior vaginal tissue elasticity with POP development stage. This difference is statistically significant as demonstrated by both the visual comparison of the confidence intervals for the presented sample median values and ANOVA testing (p<0.0001). The most affected locations are the mid and apical aspects of the anterior vaginal walls, where elasticity is decreasing up to 340% from normal to POP stage III. The lesser affected is the mid-posterior part where elasticity decreased up to 220%, the apical side walls of the vagina (decrease of approximately 100%), and the mid side walls of the vagina where we did not detect statistically significant tissue elasticity decrease (Fig. 5b). That means the horizontal (anterior, posterior) support structures weaken the most under POP conditions. The results of anterior/posterior tissue elasticity relative changes with POP development are in agreement with our earlier findings [13] and the data published by other investigators [4, 17, 28], although the values of elasticity moduli are different due to variations in used experimental technique.
The VTI allows also anatomical characterization of POP development. Specifically, we found that the anterior/posterior spacing increases for the apical part of vagina from 18±6 mm under normal conditions to 32±12 mm under POP stage III (Fig. 6a). For the mid-vagina, this spacing behaves more conservatively (Fig. 6b), and we have found no statistically significant change among four patient groups. The comfort level of VTI examination was found very close to manual palpation by 77% of patients.
Among methodological limitations, there is a risk related to the patient motion during the VTI examination which may produce image artifacts and an elasticity measurement error. Another limitation could be associated with the use of the one transvaginal probe for all anatomical varieties of female pelvic floor. Yet, another possible limitation could be related to a necessity to use standard probe manipulation technique for visualizing unusual finding.
Conclusions
VTI may serve as a means for 3-D imaging of the vagina and a quantitative assessment of vaginal tissue elasticity, providing important information for furthering our understanding of pelvic organ prolapse and surgical treatment.
Acknowledgements
The authors would like to thank Noune Sarvazyan, PhD, and Armen Sarvazyan, PhD, DSc, for editing assistant and support of this research; Randee Weed, MS, RDMS, for clinical research documentation and data management; Robin Haff, RN, BSN, for study coordination; and Milind Patel for technical assistance with the device. The work was supported by the National Institute on Aging, USA, grant AG034714.
Abbreviations
- ANOVA
One-way analysis of variance
- E
Young’s modulus
- POP
Pelvic organ prolapse
- POP-Q
Pelvic organ prolapse quantification system
- MRI
Magnetic resonance imaging
- VTI
Vaginal tactile imager
Footnotes
Conflicts of interest None.
Contributor Information
Vladimir Egorov, Artann Laboratories, 1459 Lower Ferry Rd., Trenton, NJ 08618, USA, vegorov@artannlabs.com.
Heather van Raalte, Princeton Urogynecology, 601 Ewing Street, Suite B-19, Princeton, NJ 08540, USA.
Vincent Lucente, The Institute for Female Pelvic Medicine & Reconstructive Surgery, 3050 Hamilton Blvd, Suite 200, Allentown, PA 18104, USA.
References
- 1.Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183:277–285. doi: 10.1067/mob.2000.107583. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144:S146–S158. doi: 10.1016/j.ejogrb.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Jean-Charles C, Rubod C, Brieu M, Boukerrou M, Fasel J, Cosson M. Biomechanical properties of prolapsed or non-prolapsed vaginal tissue: impact on genital prolapse surgery. Int Urogynecol J. 2010;21:1535–1538. doi: 10.1007/s00192-010-1208-z. [DOI] [PubMed] [Google Scholar]
- 5.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 6.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 7.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging-a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 8.Elgeti T, Beling M, Hamm B, Braun J, Sack I. Elasticity-based determination of isovolumetric phases in the human heart. J Cardiovasc Magn Reson. 2010;12:1–8. doi: 10.1186/1532-429X-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss RE, Egorov V, Ayrapetyan S, Sarvazyan N, Sarvazyan AP. Prostate mechanical imaging: a new method for prostate assessment. Urology. 2008;71:425–429. doi: 10.1016/j.urology.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egorov V, Sarvazyan AP. Mechanical imaging of the breast. IEEE Trans Med Imaging. 2008;27:1275–1287. doi: 10.1109/TMI.2008.922192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellman PS. Tactile Imaging. Ph.D. Thesis presented to Harvard University Division of Engineering and Applied Sciences. 1999. [Google Scholar]
- 12.Sarvazyan AP. Mechanical imaging: a new technology for medical diagnostics. Int J Med Inf. 1998;49:195–216. doi: 10.1016/s1386-5056(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 13.Egorov V, van Raalte H, Sarvazyan AP. Vaginal tactile imager. IEEE Trans Biomed Eng. 2010;57:1736–1744. doi: 10.1109/TBME.2010.2045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 15.Friedman RM, Hester KD, Green BG, LaMotte RH. Magnitude estimation of softness. Exp Brain Res. 2008;191:133–142. doi: 10.1007/s00221-008-1507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978;32:12–16. [Google Scholar]
- 17.Lei L, Song Y, Chen R. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J. 2007;18:603–607. doi: 10.1007/s00192-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 18.Prantil RL, Jankowski RJ, Kaiho Y, et al. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol. 2007;292:1229–1237. doi: 10.1152/ajprenal.00292.2006. [DOI] [PubMed] [Google Scholar]
- 19.Bo K, Finckenhagen HB. Vaginal palpation of pelvic floor muscle strength: inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta Obstet Gynecol Scand. 2001;80:883–887. doi: 10.1034/j.1600-0412.2001.801003.x. [DOI] [PubMed] [Google Scholar]
- 20.Tunn R, Petri E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction: an imaging panel and practical approach. Ultrasound Obstet Gynecol. 2003;22(2):205–213. doi: 10.1002/uog.189. [DOI] [PubMed] [Google Scholar]
- 21.Constantinou CE. Dynamics of female pelvic floor function using urodynamics, ultrasound and magnetic resonance imaging (MRI) Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S159–S165. doi: 10.1016/j.ejogrb.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoro GA, Wieczorek AP, Dietz HP, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol. 2011;37(4):381–396. doi: 10.1002/uog.8816. [DOI] [PubMed] [Google Scholar]
- 23.Egorov V, Tsyuryupa S, Kanilo S, Kogit M, Sarvazyan A. Soft tissue elastometer. Med Eng Phys. 2008;30(2):206–212. doi: 10.1016/j.medengphy.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krouskop TA, Wheeler TM, Kaller F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20(4):260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 25.Rubod C, Boukerrou M, Brieu M, et al. Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J. 2008;19(811–816):2008. doi: 10.1007/s00192-007-0533-3. [DOI] [PubMed] [Google Scholar]
- 26.Martins PALS, Peña E, Calvo B, Doblaré M, Mascarenhas T, Natal Jorge RM, Ferreira AJM. Prediction of nonlinear elastic behavior of vaginal tissue: experimental results and model formulation. Comp Methods Biomech Biomed Eng. 2010;13:327–337. doi: 10.1080/10255840903208197. [DOI] [PubMed] [Google Scholar]
- 27.Aglyamov SR, Egorov V, Emelianov SY, et al. A nonlinear model for mechanical imaging. Proceedings of the 7th International Conference on the ultrasonic measurement and imaging of tissue elasticity; Oct 27–30; Austin, Texas. 2008. p. 89. [Google Scholar]
- 28.da Silva-Filho AL, Martins PA, Parente MP, et al. Translation of biomechanics research to urogynecology. Arch Gynecol Obstet. 2010;282:149–155. doi: 10.1007/s00404-010-1396-2. [DOI] [PubMed] [Google Scholar]