SUMMARY
The cholesteryl ester transfer protein (CETP) gene plays an essential role in regulating cholesterol homeostasis and is a candidate susceptibility gene for late onset Alzheimer’s disease (AD). Recent finding suggests that the CETP I405V polymorphism (rs5882) is associated with a slower rate of memory decline and a lower risk of incident dementia. Using data from two ongoing epidemiologic clinical-pathologic cohort studies of aging and dementia in the United States, the Religious Order Study and the Memory and Aging Project, we evaluated the association of the CETP I405V polymorphism (rs5882) with cognitive decline and risk of incident AD in more than 1,300 participants of European ancestry. Our results suggest that the CETP I405V polymorphism was associated with a faster rather than a slower rate of decline in cognition over time, and an increased risk of incident AD. This finding is consistent with data showing that the CETP I405V is associated with increased neuritic plaque density at autopsy.
Keywords: association study, cognitive decline, Alzheimer’s disease, CETP
INTRODUCTION
Late onset Alzheimer’s disease (AD) is among the most common and disabling conditions of aging, and is accompanied by severe memory loss and decline in daily living functions. Recent genome-wide association studies have identified a growing number of validated susceptibility loci for late-onset AD, including CR1, CLU, PICALM, and BIN1, in addition to the well-known apolipoprotein E (APOE) locus (Harold et al 2009, Seshadri et al 2010, Lambert et al 2009). Given the central role of APOE in lipid metabolism, other genes in this pathway have also been considered as candidate AD susceptibility genes. Production of amyloid beta, a central piece in the pathological process in AD, may be regulated in part by cholesterol (Michikawa 2003, Sparks et al 1994). Dysregulation of cholesterol homeostasis manifests as abnormal levels of high density lipoprotein (HDL), low density lipoprotein (LDL) and HDL/LDL ratio (Zhu et al 2005). HDL is essential in limiting the amount of cholesterol deposition and removing excess cholesterol load in the cell and reduced levels of HDL have been associated with the risk of AD (Brewer 2004, Merched et al 2000).
Cholesteryl ester transfer protein (CETP) regulates cholesterol homeostasis via the transfer of cholesteryl esters from high density lipoprotein (HDL) to low density lipoprotein (LDL) in exchange for triacylglycerols (TG) (Tall 1993, Barter and Kastelein 2006). Several single nucleotide polymorphisms (SNPs) within CETP have been suggested to influence enzymatic activity or gene expression level. In particular, the Taq1B polymorphism (rs708272) is reported to be associated with lower plasma CETP concentrations and higher HDL cholesterol levels (Fidani et al 2004); C629A (rs1800775) within the gene promoter is associated with decreased expression (Dachet et al 2000); and I405V (rs5882) is associated with reduced CETP, higher HDL levels, and increased lipoprotein particle sizes (Blankenberg et al 2003). Studies of the link between CETP polymorphisms and susceptibility for late onset AD have been equivocal. Among these, the Taq1B polymorphism was found to have no association with clinically diagnosed AD in several studies (Fidani et al 2004, Zhu et al 2005, Chen et al 2008). Alternatively, one study in the Spanish population found a lower risk of AD in subjects homozygous for C629A, but no association with I405V (Rodriguez et al 2006). Another study in subjects of European ancestry found that I405V homozygozity was associated with increased susceptibility for AD, but only in the absence of the APOE ε4 allele (Arias-Vasquez et al 2007). In other reports, neither C629A nor I405V had significant effect on the risk of AD or age-related cognitive change (Qureischie et al 2008, Johnson et al 2007, Chen et al 2008).
In many of these studies, the sample sizes were relatively small and the results relied on cross-sectional case-control analyses. Recently, it has been proposed that more power may be gained through the use of endophenotypes such as level of cognition, change in cognitive function, and markers of neuropathology (Kennedy et al 2003, McQueen et al 2007, Bennett et al 2009, Shulman et al 2010). A recent study on the relation of CETP I405V to longitudinal memory decline and incidence of AD dementia found that valine homozygozity was protective (Sanders et al 2010).
In order to follow up this finding, we used data from two ongoing cohort studies of aging and dementia, the Religious Orders Studies (ROS) and the Memory and Aging Project (MAP), to assess whether CETP I405V is related to change in cognition over time and risk of incident AD, as well as AD neuropathology at autopsy.
RESULTS
Genotype data
Genotype data were available on 1,709 study participants. Three hundred and twenty five were excluded from the analysis due to the following criteria: 111 were dementia at baseline, 8 self-reported non-European ancestry, 133 did not have information on APOE, and 73 had no follow-up evaluations (34 died prior to the first follow-up, 14 were yet to have the first follow-up and 25 had only 1 valid cognitive measure). This resulted in a sample of 1,384 participants (Table-1). The rs5882 allele frequencies satisfied Hardy-Weinberg equilibrium in the cohorts, both separately and combined. These frequencies are comparable to those reported in previous studies (Rodriguez et al 2006). The genotypic frequencies of the two cohorts did not differ (X2=0.55, dF=2, p=0.76).
Table-1.
CETP I450V allele and genotype frequencies, n (%)
| ROS and MAP (n=1384) |
ROS (n=641) |
MAP (n=743) |
|
|---|---|---|---|
| Allele | |||
| I (isoleucine) | 2004 (72.4) | 920 (71.8) | 1084 (72.9) |
| V (valine) | 764 (27.6) | 362 (28.2) | 402 (27.1) |
| Genotype | |||
| I/I | 710 (51.3) | 322 (50.2) | 388 (52.2) |
| I/V | 584 (42.2) | 276 (43.1) | 308 (41.5) |
| V/V | 90 (6.5) | 43 (6.7) | 47 (6.3) |
| HWE | X2=4.31, p=0.04 | X2=2.50, p=0.11 | X2=1.88, p=0.17 |
ROS: the Religious Order Study; MAP: the Memory and Aging Project
Demographic characteristic of the cohorts
Although the two cohorts differ in some demographic characteristics, the percentage of APOE ε4 carriers in ROS and MAP did not differ (p= 0.48). The desire to maximize power together with the absence of significant difference in genotypic frequencies led us to combine data from the two cohorts for analyses, similar to what was done in previous studies (Chibnik et al 2011). The mean (±SD) age at enrollment was 78.3 (±7.3) years. The average length of follow-up was 7.5 years. The mean (±SD) level of education was 16.3 (±3.5) years, and 971 (70.1%) of the participants were female. 329 (23.8%) of the participants had at least one ε4 allele (Table 2).
Table-2.
Demographics of participants in the ROS and MAP cohorts
| ROS/MAP | |
|---|---|
| n=1384 | |
| Age at enrollment | 78.3 ± 7.3 |
| Female, n (%) | 971(70.1%) |
| Education | 16.3 ± 3.5 |
| Years of follow-up | 7.5 ± 4.0 |
| Any APOE ε4, n (%) | 329 (23.8%) |
ROS: the Religious Order Study; MAP: the Memory and Aging Project
Association of CETP I405V to change in global cognition
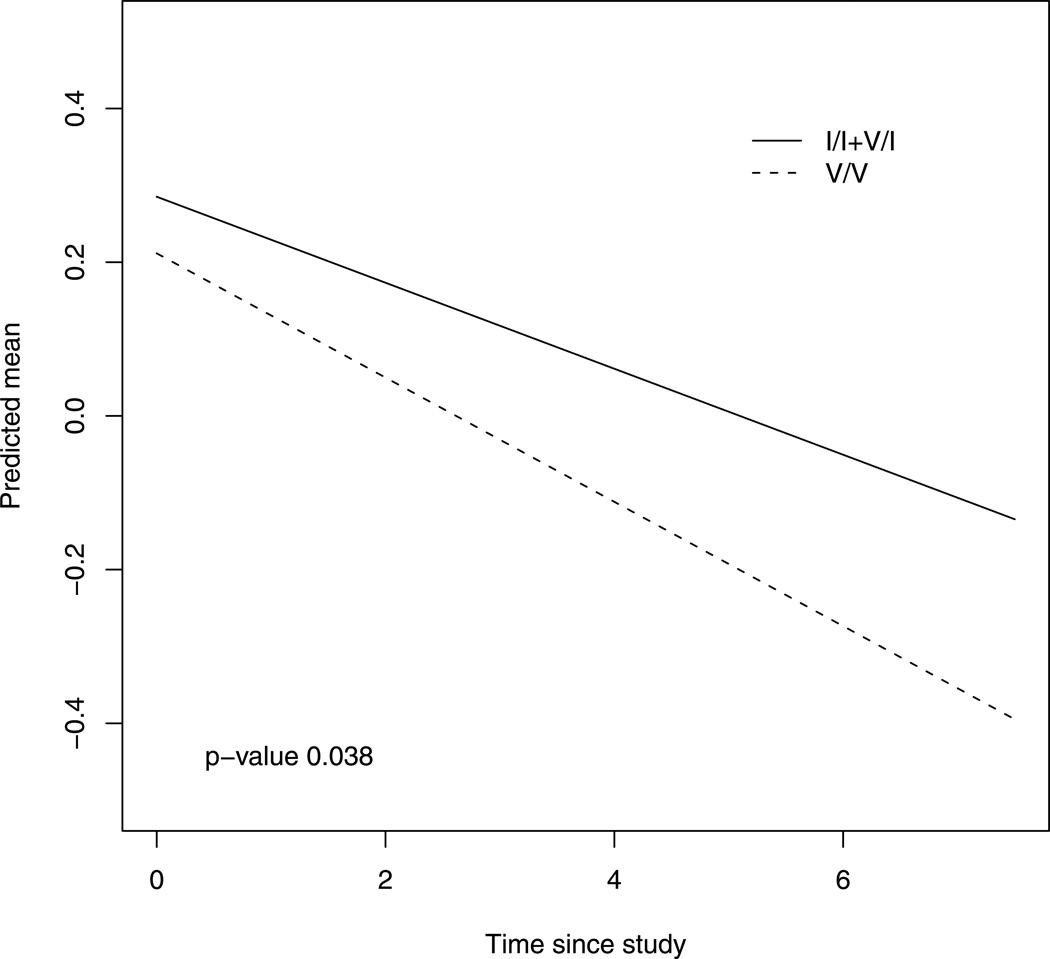
We first examined the association of the CETP I405V variant to the rate of cognitive decline using the global measure of cognition. Based on prior work (Sanders et al 2010), our primary analyses tested the recessive genetic model comparing homozygotes for the minor allele (V/V) to the heterozygous (I/V) and homozygous major allele (I/I) classes. By examining the coefficients of the interaction for the genotype and time since baseline, we were able to test the SNP effect on change in cognition (Table 3). Participants with V/V had a steeper decline in global cognition as compared to those with I/V or I/I (β=−0.025, se=0.012, p = 0.038). Figure-1 displays the mean trajectories of global cognition for the groups with different I405V genotype, controlling for age, gender, years of education and APOE.
Table-3.
CETP I405V genotype on the decline in cognition
| Effect on change of cognition | ||
|---|---|---|
| rs5882V/V* | Estimates (SE) | p |
| Global Cognition | −0.025 (0.012) | 0.038 |
| Episodic memory | −0.024 (0.015) | 0.099 |
| Semantic memory | −0.020 (0.013) | 0.141 |
| Working memory | −0.024 (0.010) | 0.011 |
| Perceptual speed | −0.020 (0.013) | 0.143 |
| Visuospatial abilities | −0.012 (0.011) | 0.268 |
rs5882I/I+I/V were used as reference.
Figure 1.
Decline of global cognition for groups with different I405V genotype. Estimated mean trajectories of global cognition over time for groups with different I405V genotype, adjusted for baseline age, sex, education and APOE. The dotted line represents a typical subject with valine homozygote (rs5882V/V), and the solid line refers to the one who had the other genotypes.
Association of CETP I405V with change in cognitive domains
Because prior publications suggest that the I405V variant may have a selective effect on change in episodic memory, we examined its relation to domain-specific summary measures of cognition. Analyses of five separate cognitive domains indicate that whereas the polymorphism was not associated with rate of decline in episodic memory, it was associated with a greater rate of decline in working memory (Table 3). Subjects with V/V declined 50% faster compared to the other genotypes (β=−0.024, se=0.010, p = 0.011).
Association of CETP I405V to incident AD
We next examined the association of I405V and incident AD. Among the 1,384 study participants non-demented at baseline and with at least one follow-up evaluation, 335 (24.2 %) developed incident AD (Table 4). A chi-square test did not indicate an association between the SNP genotype (I/I, I/V, V/V) and incident AD (X2=0.62, dF=2, p =0.73).
Table-4.
Distribution of incident AD by CETP I405V genotype, n (column %)
| Incident AD subjects | Control subjects | |
|---|---|---|
| rs5882 I/I | 175 (52.24) | 535 (51.05) |
| rs5882 I/V | 136 (40.60) | 447 (42.65) |
| rs5882 V/V | 24 (7.16) | 66 (6.30) |
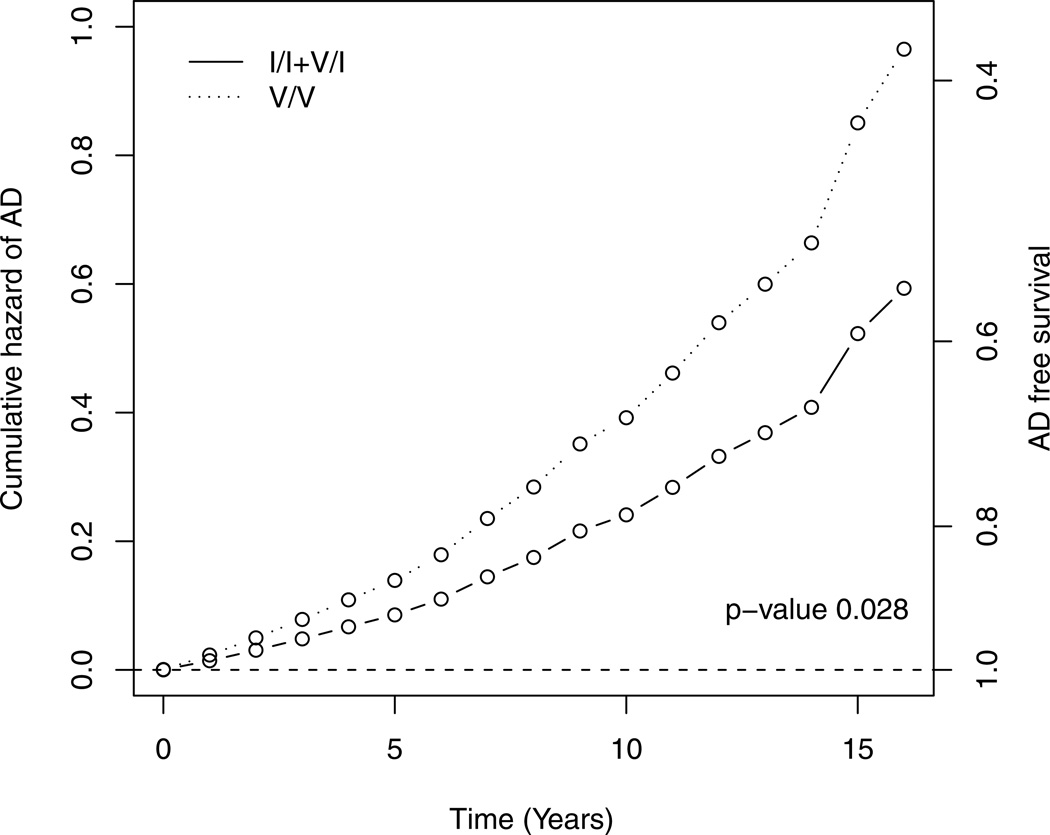
Cox proportional hazard models were used to examine time to incident AD as the outcome of interest. Time was right censored at the last evaluation for those who did not receive the corresponding diagnoses. We again used a recessive model comparing the risk of rs5882V/V to that of the other genotypes, and controlling for age, sex, education, and APOE. The result shows that subjects with V/V genotype had higher risk of incident AD (HR=1.63, 95% CI=1.05–2.61, p =0.028). This is illustrated in Figure-2 which shows the cumulative hazard of AD between V/V (dotted) and the reference genotypes (solid).
Figure 2.
Risk of incident AD for groups with different I405V genotype. Estimated cumulative hazard of AD between rs5882V/V (dotted) and the reference genotypes (solid), adjusted for baseline age, sex, education and APOE. Additional scale for AD free survival was shown on the right axis.
Association of CETP I405V and AD pathology
The final set of analyses examined the relationship between CETP and measures of AD pathology obtained at autopsy. At time of this analysis, we have almost 590 deceased participants with post-mortem data. Depending on the distribution of the pathologic outcomes, a series of models were performed to investigate the association between pathologic outcomes and I405V genotype, controlling for age at death, sex, level of education and APOE. We evaluated the association of the polymorphism to each of the following four pathologic outcomes separately, namely the global measure of AD pathology, and separate measures of neuritic and diffuse plaques and neurofibrillary tangles. As shown in Table-5, the result indicates that for subjects with neuritic plaques, those with V/V had a higher density (p = 0.038). This result provides additional support for the CETP associations with rate of cognitive decline and risk of incident AD, and further suggests a potential mechanism.
Table-5.
CETP I405V genotype on brain pathology
| Pathologic outcomes | Model | Difference by genotype (se) | p-value | |
|---|---|---|---|---|
| Global pathology | ANCOVA | 0.094 (0.062) | 0.129 | |
| Neuritic plaque | Zero inflated regression | absolute | 0.124 (0.102) | 0.224 |
| conditional | 0.156 (0.075) | 0.038 | ||
| Diffuse plaque | Zero inflated regression | absolute | 0.055 (0.091) | 0.546 |
| conditional | 0.065 (0.079) | 0.413 | ||
| Neurofibrillary tangles | ANCOVA | 0.082 (0.64) | 0.203 | |
ANCOVA: analysis of covariance
Conditional refers to mean estimates conditional on response Y > 0
Absolute is the product of conditional estimates and estimated prob(Y>0)
DISCUSSION
The CETP I405V polymorphism has recently been suggested to have a protective effect on memory decline and risk of incident dementia (Sanders et al 2010), although prior studies were equivocal (Rodriguez et al 2006, Arias-Vasquez et al 2007). Using data from two ongoing epidemiologic clinical-pathologic cohort studies, we investigated the association of I405V with cognitive decline, risk of incident AD, as well as AD pathology. Contrary to Sanders et al., our results suggest that I450V was associated with a steeper decline in global cognition, an association that appears to be primarily related to decline in working memory. Moreover, valine homozygotes showed a higher risk of incident AD, and among deceased subjects, a greater density of neuritic plaques. Together, these data suggest that the CETP I405V polymorphism is associated with an increased risk of AD.
Several plausible factors might have contributed to the lack of agreement between our findings and those of Sanders et al. First, the discordant direction of effect could be due to population stratification and distortions in allele frequencies associated with a mixed race/ethnicity cohort. In contrast to Sanders et al, our study was restricted to analyses of a homogenous population, including only participants of European ancestry in order to minimize potential confounding due to population stratification. This could be important since, based on HapMap data, the allele frequency of the I405V polymorphism differs substantially across race and ethnicity (International HapMap Consortium 2007). The minor allele frequency of I405V in our cohort (27.6%) was lower than that reported in the Sanders et al. study (43.5%). Notably, that study included subjects of African-American ancestry, in whom the valine allele is in fact the predominant variant (66%)--i.e. the minor allele rs5882V in Europeans becomes the major allele in the African American population. A second possible explanation for the difference in the direction of the allelic association is the potential presence of genetic modifiers that vary by race/ethnicity. In this model, the putative valine risk allele that we observe in European ancestry subjects, might behave instead as a protective allele in a predominantly African American cohort, due to variation at additional unknown genes. Ultimately, it will be important to evaluate CETP I405V in a variety of homogeneous cohorts to differentiate amongst these potential explanations. Finally, it remains possible that the discordant findings reflect statistical fluctuation around the null hypothesis, and that CETP I405V is in fact not a true AD risk allele. While an intriguing candidate gene, the CETP locus has not been found in the top results of recently reported AD genome-wide scans (Naj et al 2011; Hollingworth et al 2011); future analysis of CETP in these studies and in larger ongoing genetic meta-analyses of AD will be important to understand the full impact of this locus on cognition and AD.
One strength of our analyses is that both study cohorts have relatively long follow-up. The average follow-up times are six years for MAP and ten years for the ROS. More than 300 participants have been diagnosed with incident AD and more than 500 brains have been autopsied. This allows us to investigate the full spectrum of disease progression, ranging from trajectory of cognitive decline over time, risk of disease onset, to the neuropathology after death. The full mechanism for the association of CETP I405V with AD susceptibility remains to be determined. The CETP I405V polymorphism has previously been shown to cause decreased CETP protein levels and increased HDL levels (Arias-Vasquez et al 2007); however, as for APOE, the relationship between these genes role in lipid homeostasis and promotion of AD pathology and associated cognitive impairment remains to be elaborated. Our data suggests that the polymorphism may lead to clinical AD in part through an association with neuritic plaques. This is similar to what we have reported for the APOE and CR1 susceptibility loci, and is consistent with the central role of amyloid pathology in the clinical manifestation of AD (Bennett et al 2003, Chibnik et al 2011). Importantly, recent evidence supports a role for APOE in regulating the clearance of amyloid-Beta peptide from the brain (Castellano et al 2011), and it is possible that CETP also participates in this process.
EXPERIMENTAL PROCEDURES
Study participants
Subjects are participants enrolled in two ongoing longitudinal clinical-pathologic cohort studies, the Religious Order Studies (ROS) and the Memory and Aging Project (MAP).
Participants from ROS are older Catholic nuns, priests, and brothers who agreed to annual clinical evaluations including a medical history, cognitive function testing, neurologic examination, blood specimen collection, and brain donation at time-of-death. They come from about 40 groups across the United States. Since January 1994, over 1100 persons completed the baseline clinical evaluation and the follow-up rate of survivors exceeds 95%. Detailed information on the ROS study has been published elsewhere (Wilson et al 2004, Bennett et al 2006a).
Participants from MAP are residents of approximately 40 senior housing facilities in the Chicago metropolitan area, including subsidized housing facilities, retirement communities, and retirement homes. Similar to ROS, participants from MAP have consented to undergo annual uniform, structured, clinical evaluations, including a medical history, cognitive function testing, neurologic examination, and blood specimen collection, and brain donation at time-of-death. Since October 1997, MAP study has enrolled over 1400 participants with follow-up rate of 90% among the survivors. Further information on the MAP study has been previously published Bennett et al 2006a, Bennett et al 2005a).
Both studies were approved by the Institutional Review Board of Rush University Medical Center. Informed consent and an anatomical gift act were obtained from each participant following a detailed presentation of the risks and benefits associated with studies participation.
Genotyping
DNA was extracted from whole blood, lymphocytes or frozen post-mortem brain tissue. Genotype data for the CETP I405V polymorphism (rs5882) were extracted from an imputed genome-wide dataset generated on the Affymetrix Genechip 6.0 platform at the Broad Institute’s Center for Genotyping (n=1204) or the Translational Genomics Research Institute (n=674). These two data sets underwent the same quality control (QC) analysis in parallel. At the conclusion of the QC pipeline, 1709 individuals were available for analysis. The imputation was performed using MACH (version 1.0.16a) and HapMap release 22 CEU (build 36). Further details on genotyping have been previously published (Chibnik et al 2011). The imputed I405V polymorphism (rs5882) has an O/E ratio of 0.61 (INFO score), indicating satisfactory imputation quality.
Cognitive decline, clinical AD, and neuropathologic outcomes
Annual cognitive function data has been collected from the participants of the two cohorts. Briefly, cognitive function was assessed via a battery of 21 tests in each study, of which 19 were in common, including the MMSE which was only used to describe the cohort, and complex ideational material which was only used for diagnostic classification. The remaining 17 tests were combined into a composite measure of cognition, and also separate summary measures of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability as previously described (Wilson et al 2007).
To compute the composite measure of global cognitive function, raw scores on each of the individual tests were converted to z-scores using the baseline mean and standard deviation of the entire cohort, and the z-scores of all 17 tests were averaged. Higher scores indicate better performance in cognitive functions. Summary scores for the five cognitive domains were derived similarly. Psychometric information on these summary scores, including factor analytic support for the five domains, is contained in previous publications (Wilson et al 2002, Wilson et al 2005).
The follow-up clinical evaluations on ROS and MAP participants provided data on incident dementia/AD. Clinicians review all clinical data, blinded to data from previous years, and conduct an in-person examination of each subject when feasible. The clinical diagnosis of dementia/AD follows the NINCDS/ADRDA criteria as previously described (Bennett et al 2006a). Dementia criteria require evidence of loss of cognition from a previous level of performance with impairment in multiple areas of cognition. The NINCDS/ADRDA criteria for "probable" Alzheimer’s disease require a loss of memory and other cognitive abilities, with impairment in two or more cognitive domains, one of which must be memory, as documented by neuropsychological performance testing.
Brain autopsies were performed for nearly all cases at 12 pre-determined sites across the United States including the Rush Alzheimer’s Disease Center. Autopsy procedures have been described in detail (Schneider et al 2004, Bennett et al 2005b, Bennett et al 2006b). A composite AD pathology score was used in the analyses. It was based on counts of neuritic and diffuse plaques and neurofibrillary tangles as previously described (Bennett et al 2003, Bennett et al 2006c). Separate summary measures of neurofibrillary tangles and neuritic and diffuse plaques were created in a similar fashion.
Statistical analysis
To examine the association between genotype and change in cognition, random coefficient models were used to characterize individual trajectories of change in cognition and to test the relation of the SNP to annual rate of change in cognition (Wilson et al 2002), adjusting for age, sex, level of education and APOE. In these models, our outcome of interest is decline over time, and the interaction between time and valine homozygote is used to test the hypothesis that those with rs5882V/V decline at a different rate than rs5882I/V+I/I.
To examine the association of the polymorphism and time to incident AD, we used discrete-time proportional hazards models as our primary analyses. Only those without AD diagnosis at baseline were included. The models included a term for SNP’s genotype, as well as terms to adjust for age, sex, education level and APOE. These models make the assumption that the hazards conferred by the risk factor are proportional over time. The strength is that they allow for any shape of the underlying hazard function.
For the pathologic outcomes, since the data were positively skewed, square root transformation was applied. Analysis of covariance was performed to check for the association between I405V genotype and a global measure of pathology and neurofibrillary tangles. Further examination of the data showed that for the outcomes of neuritic and diffuse plaques there were huge spikes in the distribution at zero, even after the transformation. Therefore, zero inflated regression models were applied in order to account for these excessive zeros, where we simultaneously modeled zeros versus nonzeros with a logistic regression and all nonzero values with a linear regression.
Finally considering this is a replication study to confirm previous findings, we imposed a nominal threshold of p<0.05 for significance in our analyses. All the analyses were implemented using SAS software, version 9.2 (SAS Institute Inc, 2008).
ACKNOWLEDGMENTS
We are indebted to all the participants of the Religious Order Study and the Memory and Aging Project, as well as the staff at the Rush Alzheimer’s Disease Center for this work. We thank Dong Tran, BS, for helping with assembling the genetic dataset and Woojeong Bang, MS, for statistical programming. This research was supported by National Institute on Aging grants R01 AG30146, R01 AG17917, R01 AG15819, P30 AG10161, and K08 AG034290.
REFERENCES
- 1.Arias-Vasquez A, Isaacs A, Aulchenko YS, Hofman A, Oostra BA, Breteler M, van Duijn CM. The cholesteryl ester transfer protein (CETP) gene and the risk of Alzheimers disease. Neurogenetics. 2007;8:189–193. doi: 10.1007/s10048-007-0089-x. [DOI] [PubMed] [Google Scholar]
- 2.Barter PJ, Kastelein JJ. Targeting cholesteryl ester transfer protein for the prevention and management of cardiovascular disease. J Am Coll Cardiol. 2006;47(3):492–499. doi: 10.1016/j.jacc.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005a;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005b;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006a;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006b;27(3):169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet neurology. 2006c;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology. 2009;72(17):1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankenberg S, Rupprecht HJ, Bickel C, Jiang XC, Poirier O, Lackner KJ, Meyer J, Cambien F, Tiret L. Common genetic variation of the cholesteryl ester transfer protein gene strongly predicts future cardiovascular death in patients with coronary artery disease. J Am Coll Cardiol. 2003;41(11):1983–1989. doi: 10.1016/s0735-1097(03)00408-x. [DOI] [PubMed] [Google Scholar]
- 11.Brewer HB. Increasing HDL cholesterol levels. N Engl J Med. 2004;350:1491–1494. doi: 10.1056/NEJMp048023. [DOI] [PubMed] [Google Scholar]
- 12.Castellano JM, Kim J, Stewart FR, et al. Human apoE Isoforms differentially regulate brain amyloid-beta peptide clearance. Science Translational Medicine. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Yang J, Tang Z, Dong X, Feng Z, Yu S, Chan P. Cholesteryl ester transfer protein polymorphism D442G associated with a potential decreased risk for Alzheimers disease as a modifier for APOE ε4 in Chinese. Brain Research. 2008;1187:52–57. doi: 10.1016/j.brainres.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Chibnik LB, Shulman JM, Leurgans SE, et al. CR1 is associated with amyloid plaque burden and age-related cognitive decline. Ann Neurol. 2011;69(3):560–569. doi: 10.1002/ana.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dachet C, Poirier O, Cambien F, Chapman J, Rouis M. New functional promoter polymorphism, CETP/ -629, in cholesteryl ester transfer protein (CETP) gene related to CETP mass and high density lipoprotein cholesterol levels (role of SP1/Sp3 in transcriptional regulation) Aterioscler. Thromb. Vasc. Biol. 2000;20:507–515. doi: 10.1161/01.atv.20.2.507. [DOI] [PubMed] [Google Scholar]
- 16.Fidani L, Goulas A, Crook R, Peterson RC, Tangalos E, Kotsis A, Hardy J. An association study of the cholesteryl ester transfer protein TaqI B polymorphism with late onset Alzheimers disease. Neuroscience Letters. 2004;357:152–154. doi: 10.1016/j.neulet.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 17.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. Epub 2011 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson W, Harris SE, Collins P, Starr JM, Whalley LJ, Deary IJ. No association of CETP genotype with cognitive function or age-related cognitive change. Neuroscience Letters. 2007;420(2):189–192. doi: 10.1016/j.neulet.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy JL, Farrer LA, Andreasen NC, Mayeux R, St George-Hyslop P. The genetics of adult-onset neuropsychiatric disease: complexities and conundra? Science. 2003;302:822–826. doi: 10.1126/science.1092132. [DOI] [PubMed] [Google Scholar]
- 21.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature genetics. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 22.McQueen MB, Bertram L, Lange C, et al. Exploring candidate gene associations with neuropsychological performance. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:987–991. doi: 10.1002/ajmg.b.30500. [DOI] [PubMed] [Google Scholar]
- 23.Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high density lipoprotein cholesterol and serum apolipprotein AI concentrations are highly correlated with severity of Alzheimer’s disease. Neurobiol. Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 24.Michikawa M. The role of cholesterol in pathogenesis of Alzheimer’s disease. Dual metabolic interaction between amyloid β-protein and cholesterol. Mol Neurobiol. 2003;27:1–12. doi: 10.1385/MN:27:1:1. [DOI] [PubMed] [Google Scholar]
- 25.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. Epub 2011 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureischie H, Heun R, Lütjohann D, Popp J, Jessen F, Ledschbor-Frahnert C, Thiele H, Maier W, Hentschel F, Kelemen P, Kölsch H. CETP polymorphisms influence cholesterol metabolism but not Alzheimer's disease risk. Brain Res. 2008;26:1–6. doi: 10.1016/j.brainres.2008.07.047. 1232. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez E, Mateo I, Infante J, Llorca J, Berciano J, Combarros O. Cholesteryl ester transfer protein (CETP) polymorphism modifies the Alzheimer’s disease risk associated with APOE epsilon 4 allele. Journal of Neurology. 2006;253(2):181–185. doi: 10.1007/s00415-005-0945-2. [DOI] [PubMed] [Google Scholar]
- 28.Sanders AE, Wang G, Katz M, Derby CA, Bazilai N, Ozelius L, Lipton RB. Association of a functional polymorphism in the cholesteryl ester transfer protein (CETP) gene with memory decline and incidence of dementia. JAMA. 2010;303:150–158. doi: 10.1001/jama.2009.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 30.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 31.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman JM, Chibnik LB, Aubin C, Schneider JA, Bennett DA, De Jager PL. Intermediate phenotypes identify divergent pathways to Alzheimer's disease. PLoS One. 2010;5(6):e11244. doi: 10.1371/journal.pone.0011244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 34.Tall AR. Plasma cholesteryl ester transfer protein. J. Lipid Res. 1993;34:1255–1274. [PubMed] [Google Scholar]
- 35.The International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 37.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious order study: overview and change in cognitive and motor speed. Aging Neuropsychology and Cognition. 2004;11:280–303. [Google Scholar]
- 38.Wilson RS, Barnes LL, Krueger KR, et al. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 39.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Psychosomatic medicine. 2007;69(1):47–53. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Gopalraj RK, Kelly JF, Bennett DA, Estus S. Lack of gentic association of cholesteryl ester transfer protein polymorphisms with late onset Alzheimers disease. Neuroscience Letters. 2005;381:36–41. doi: 10.1016/j.neulet.2005.01.078. [DOI] [PubMed] [Google Scholar]