Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD or dioxin) is a global environmental contaminant and the prototypical ligand for investigating aryl hydrocarbon receptor (AHR)-mediated toxicity. Environmental exposure to TCDD results in developmental and reproductive toxicity in fish, birds and mammals. To resolve the ecotoxicological relevance and human health risks posed by exposure to dioxin-like AHR agonists, a vertebrate model is needed that allows for toxicity studies at various levels of biological organization, assesses adverse reproductive and developmental effects and establishes appropriate integrative correlations between different levels of effects. Here we describe the reproductive and developmental toxicity of TCDD in feral fish species and summarize how using the zebrafish model to investigate TCDD toxicity has enabled us to characterize the AHR signaling in fish and to better understand how dioxin-like chemicals induce toxicity. We propose that such studies can be used to predict the risks that AHR ligands pose to feral fish populations and provide a platform for integrating risk assessments for both ecologically relevant organisms and humans.
Keywords: TCDD, AHR, development, reproduction, toxicity, zebrafish, trout
Introduction
Halogenated aromatic hydrocarbons (HAHs) constitute a class of global environmental contaminants that include polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). HAHs cause toxicity at environmentally relevant levels; and aquatic ecosystems such as the Great Lakes tend to act as repositories for such compounds, putting fish and wildlife that depend on these ecosystems at risk of exposure. Impacts of HAH exposure on inhabitants of the Great Lakes ecosystem was first documented in avian populations due to the availability of close population monitoring (Gilbertson et al., 1991; Jones et al., 1994; Kubiak et al., 1989). Effects of these chemicals on wild fish populations proved more difficult to evaluate as they are less readily observable and effects can be direct (i.e., adverse effects on survival, growth and reproduction) and indirect (i.e., secondary to habitat destruction).
Initial suspicions that HAHs may be affecting wild fish populations occurred when, despite over four decades of re-stocking efforts, lake trout populations were no longer able to sustain themselves (for review see Tillitt et al., 2008). Several lines of evidence suggested that HAH contamination might be responsible for this recruitment failure. The greatest concentrations of HAHs were found in lake trout compared to other fish species (Clark et al., 1984; Hickey et al., 2006) and areas lacking evidence for natural lake trout reproduction contained the highest HAH contamination levels (Baumann and Whittle, 1988; Maack and Sonzogni, 1988). Also, dioxin-like chemicals (PCDDs, PCDFs and coplanar PCBs) act through the aryl hydrocarbon receptor (AHR) pathway and trout express the AHR and its dimerization partner the aryl hydrocarbon nuclear translocator (ARNT) and are capable of responding to AHR agonist exposure with toxicity (Abnet et al., 1999; Pollenz et al., 1996).
Since 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most potent AHR agonist among HAHs, it has become a “prototype” compound for investigating their toxicity. TCDD has the potential to interfere with several biological processes that impair growth and development of various organs and function of the liver and cardiovascular, immune, skeletal, reproductive and central nervous systems (Peterson et al., 1993). AHR signaling mediates the toxicity of HAHs such as TCDD (Schmidt and Bradfield, 1996). In fish, one of the first biological responses to TCDD activation of the AHR pathway is induction of xenobiotic metabolizing enzymes. These are phase I and phase II enzymes, which are crucial for biotransformation of lipophilic compounds. Of these xenobiotic-metabolizing enzymes, induction of cytochrome P450 1A1 (CYP1A1) has become a hallmark response to AHR activation (Hahn and Stegeman, 1994; Hahn et al., 1998; Stegeman et al., 1995) and was initially thought to play an integral role in TCDD toxicity. Since HAHs such as TCDD are poorly metabolized, it was thought that induction of CYP1 A1 might result in toxicity through the generation of reactive oxygen species or modulation of normal signaling cascades. After five decades of intensive research, the mechanisms by which TCDD induces toxicity are not completely understood. However, we now know that developmental toxicity of TCDD is not likely secondary to CYP1A induction as initially thought and that other genes are involved (Carney et al., 2004; Xiong et al., 2008).
Fish larvae are among the most sensitive vertebrates to the toxic effects of TCDD (Peterson et al., 1993); and exhibit similar signs of toxicity as other vertebrates including decreased food intake, wasting syndrome, delayed mortality, lesions in epithelial and lymphomyeloid tissues, cardiovascular dysfunction, edema, hemorrhages, craniofacial malformations, impaired reproductive success and mortality (Carney et al., 2006b; Peterson et al., 1993; Spitsbergen et al., 1991; Tanguay et al., 2003; Walker and Peterson, 1992, 1994a; Walker et al., 1991, 1994). However, while the endpoints of TCDD toxicity are similar among bony fishes, sensitivity to TCDD toxicity can vary greatly between species and the endpoints of toxicity are age-dependent.
We propose that zebrafish can be used as a model to predict the risks that exposure to dioxin-like compounds pose to feral fish populations. Furthermore, since the zebrafish is now an accepted model for human health and disease, we believe that the zebrafish can provide the framework for integrating assessments of risks to both ecologically relevant organisms and humans. Here we present a brief historical perspective of TCDD toxicity in fishes at different life stages, the potential risks that exposure to HAHs such as TCDD pose to feral fish populations, and document how using the zebrafish as a model to investigate TCDD toxicity and AHR signaling has enabled us to make great strides in better understanding how exposures to dioxin-like chemicals induce certain endpoints of reproductive and developmental toxicity.
TCDD Toxicity in Adult and Juvenile Feral Fish
Adult fish are less susceptible to TCDD-induced toxicity compared to earlier life stages, requiring considerably higher body burdens to elicit adverse effects (Lanham et al., 2011; Peterson et al., 1993; Walker and Peterson, 1992, 1994a; Walker et al., 1991, 1994). Lethal potencies of TCDD range from 3–16 µg/kg, with yellow perch, carp and bullhead being less sensitive to TCDD toxicity than adult rainbow trout, bluegill and largemouth bass (Kleeman et al., 1988; van der Weiden et al., 1994). The hallmark signs of overt TCDD toxicity in adult fish is a wasting syndrome and fin necrosis with loss of body weight being both species- and dose-dependent (Kleeman et al., 1988). Yearling rainbow trout exposed to TCDD became hypophagic, exhibited fin necrosis, developed ascites and showed reduced hematopoiesis (Spitsbergen et al., 1986); while adult female lake trout primarily exhibited liver toxicity and displayed reduced hepatocellular glycogen, nuclear chromatin clumping in the liver along with TCDD-induced lesions in the liver and spleen (Walter et al., 2000). Similarly, adult medaka exhibit fatty liver, mild hepatocellular atrophy and glycogen depletion in the liver (Volz et al., 2005, 2006) following exposure to TCDD. Histopathological lesions in adult fish caused by TCDD exposure are also found in the spleen, gill, heart, thymus and gonad (Kleeman et al., 1988; Spitsbergen et al., 1988a, 1988b; van der Weiden et al., 1994).
While TCDD induces several overt toxicities in adult fishes, it also exerts detrimental effects on reproductive success. Reproductive toxicity of TCDD varies among different fish species, and certain species (e.g., salmonids) are more vulnerable than other fish species (Cook et al., 1993; Peterson et al., 1993; Walker and Peterson, 1992, 1994a; Walker et al., 1994). Acute TCDD exposure impairs female reproduction via reduced steroid hormone secretion, depressed capacity for ovarian steroid biosynthesis, altered ovarian growth and development, fecundity with age, age to maturation, secondary sexual characteristics and egg and larval size, reduced egg production and early life stage toxicity of offspring (Giesy et al., 2002; Hutz et al., 1999; Munkittrick et al., 1991, 1998; Palstra et al., 2006; Peterson et al., 1993; Thomas, 1990; Van der Kraak et al., 1992; Walker and Peterson, 1992; Wu et al., 2001). Less is known regarding TCDD-induced reproductive toxicity in male fishes; however, it has been demonstrated in medaka that TCDD causes lesions in the testis characterized by a disorganization of spermatogenesis at the testis periphery, disruption of the interstitium, Leydig cell swelling and Sertoli cell vacuolation (Volz et al., 2005, 2006). Effects of chronic, sublethal TCDD exposure on reproductive success of fish are a growing concern. While this has not been studied extensively, some studies suggest that sublethal exposure to TCDD impairs gonad development, ovulation and survival of offspring (recruitment) (Giesy et al., 2002; Jones et al., 2001; Tietge et al., 1998; Walker et al., 1994; Walter et al., 2000).
Juvenile fishes are also susceptible to TCDD-induced wasting and fin necrosis; however, it is thought that wasting is not ultimately responsible for lethality (Walker and Peterson 1994a). In juvenile fishes, endpoints of TCDD toxicity include cutaneous hyperpigmentation, hemorrhage and ascites as well as histopathologic lesions in several tissues. As in adult fishes, susceptibility to TCDD toxicity in juveniles varies across species. For example, fingerling rainbow trout fed TCDD for 13 weeks followed by 13 weeks of depuration showed cutaneous hemorrhage, reduced growth rate or lethality; while fingerling yellow perch exposed to similar concentrations of TCDD showed no signs of reduced growth rate, fin necrosis, cutaneous hemorrhage or lethality (Kleeman et al., 1986). However, signs of fin necrosis, petechial cutaneous hemorrhage, loss of body weight and ascites following exposure to greater concentrations of TCDD, as well as histopathologic lesions including thymic atrophy, decreased hematopoiesis, focal myocardial necrosis, fibrinous pericarditis, sub mucosal gastric edema and hyperplasia of gill filaments and lamellae were identified (Spitsbergen et al., 1988a). Juvenile rainbow trout administered TCDD intraperitoneally also demonstrated TCDD-induced histopathological lesions including multifocal necrosis of gastric cardiac glandular mucosa and morphological lesions in epithelial and lymphomyeloid tissue. The lymphomyeloid lesions included splenic lymphoid depletion, thymic involution and hypocellularity of hematopoietic tissues in the head and trunk kidney (Spitsbergen et al., 1988b). Peripheral leucopenia and thrombocytopenia were also observed in the juvenile Shasta strain of rainbow trout (Spitsbergen et al., 1988b).
TCDD Developmental Toxicity in Feral Fish
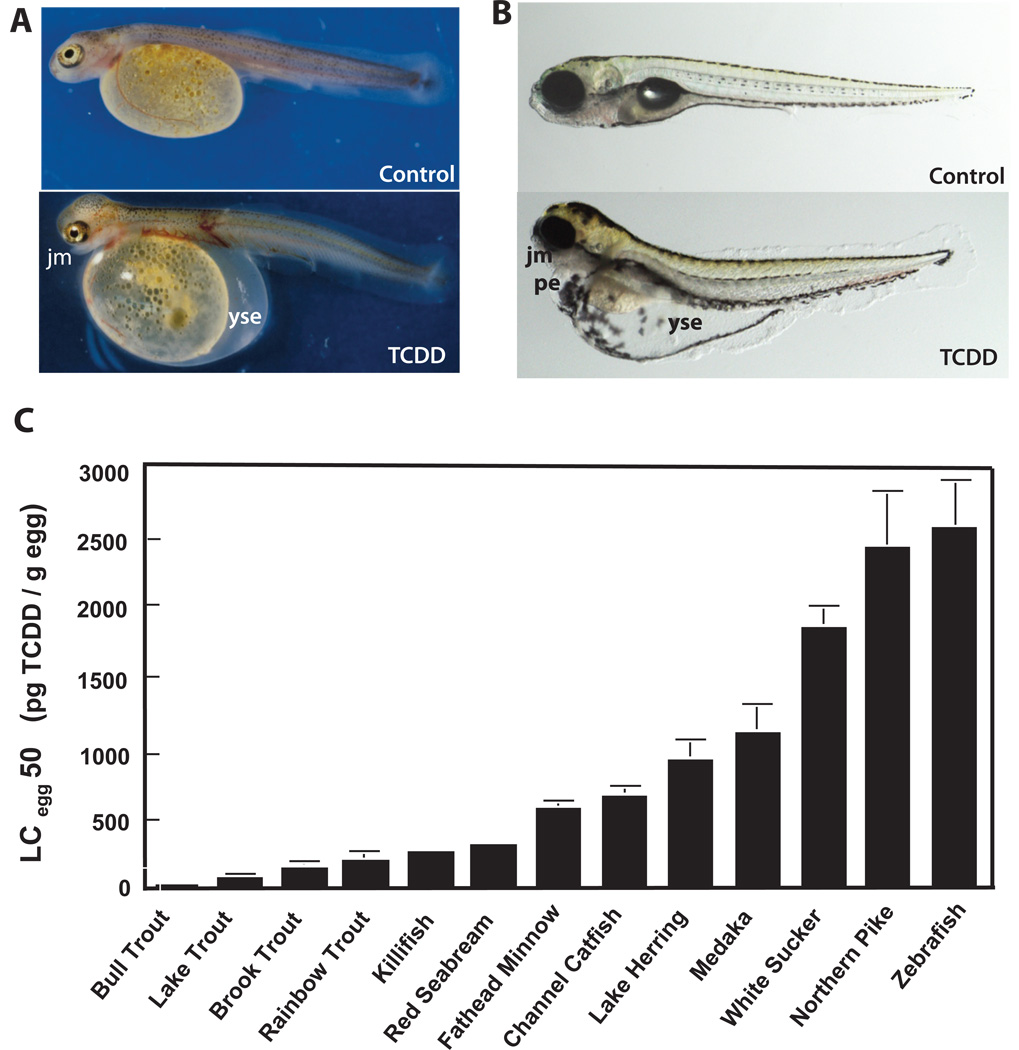
Fish are most sensitive to the toxic effects of TCDD during early stages of development (Peterson et al., 1993). Embryos develop normally through gastrulation and primary organogenesis with most toxic effects manifesting after hatching during larval sac fry development (Spitsbergen et al., 1991; Tillitt et al., 2008; Walker et al., 1991). Early life stage TCDD toxicities include yolk sac edema, hemorrhage, craniofacial malformations, stunted growth and post-hatch mortality (Carney et al., 2006b; Elonen et al., 1998; Johnson et al., 1998; Spitsbergen et al., 1991; Tanguay et al., 2003; Walker and Peterson, 1992, 1994a, 1994b; Walker et al., 1991, 1992, 1994; Yamauchi et al., 2006; Zabel et al., 1995b). These TCDD developmental toxicities (Figure 1A, lake trout larva; 1B, zebrafish larva) closely resemble “blue sac disease,” an edematous syndrome first reported in salmonid hatcheries (Wolf, 1969). The most distinguishing feature of this syndrome is the accumulation of fluid between the membranes surrounding the yolk sac (yolk sac edema) during the sac fry stage (Wolf, 1957, 1969). This edema can manifest as a milky blue color, giving the syndrome its name. In addition to yolk sac edema, other significant features of blue sac disease include pericardial edema, congestion of peripheral blood circulation, hemorrhage and craniofacial malformations (Symula, 1990; Wolf, 1957, 1969). Due to its similarity to blue sac disease, “blue sac syndrome” has since been assigned to describe the developmental defects induced by exposure to dioxin-like chemicals in these fish species during early life stage development (Symula, 1990; Walker et al., 1994).
Figure 1. TCDD potency in causing early life stage mortality by “blue sac” syndrome varies 120 fold across different fish species.
Bull trout and lake trout larvae are the most sensitive to TCDD-induced mortality and zebrafish larvae, the most resistant (C). However, provided a high enough dose of TCDD is administered to cause fish larval mortality, essentially the same signs of TCDD toxicity are seen prior to death in lake trout larvae (A), zebrafish larvae (B) and larvae of all other fish species studied (C). This common toxicity syndrome, that precedes mortality, is referred to as “blue sac.” The hallmark signs of “blue sac” in TCDD-treated lake trout larvae (A) and zebrafish larvae (B) include: yolk sac edema (yse), pericardial edema (pe), jaw malformation (jm), uninflated swim bladder and hyperpigmentation. In lake trout larvae, hemorrhage is also observed, but this is not seen in zebrafish larvae treated with TCDD. These endpoints of TCDD developmental toxicity are accompanied by a smaller heart in both trout and zebrafish larvae (not shown). Heart malformation in TCDD-exposed zebrafish larvae culminates in heart failure and death prior to swim-up. Figure 1A is from Cook et al. (2003) with permission (results were taken from Walker et al., 1991). LCegg50 values in Figure 1C were taken from Elonen et al., 1998; Guiney et al., 1996, 1997; Helder, 1981; Henry et al., 1997; Johnson, et al., 1998; Spitsbergen et al., 1991; Toomey et al., 2001; Walker and Peterson 1994b; Walker et al., 1991; Wright and Tillitt, 2006; Yamauchi et al., 2006; Zabel et al., 1995b).
Although the endpoints of TCDD toxicity in larvae are largely identical across various species of bony fish, there are major differences in TCDD potency (Figure 1C). Bull trout and lake trout are the most sensitive fish species to the developmental toxic effects of TCDD (Elonen et al., 1998; Tanguay et al., 2003) with bull trout larvae being three times more sensitive than lake trout (Cook et al., 2000). In laboratory studies, lake trout exposed to TCDD at the fertilized egg stage and observed through the fry stage exhibited the greatest mortality at the sac fry stage (Walker et al., 1991). TCDD-exposed lake trout sac fry exhibited signs of blue sac disease, necrosis of the brain, retina and spinal cord, craniofacial malformations, growth retardation, meningeal and subcutaneous hemorrhage and the severity of edema correlated with cumulative mortality (Guiney et al., 1997, 2000; Spitsbergen et al., 1991; Walker et al., 1991). TCDD exposure elicits similar responses in brook trout and rainbow trout larvae including signs of blue sac disease, craniofacial malformations that include reduced cranial length and shortened maxillas as well as a reduction in eye diameter, opercular defects, multifocal hemorrhages, liver vacuolation and hepatocellular necrosis, pericardial edema, reduced heart size, and retarded development that preceded lethality (Carvalho et al., 2004; Helder, 1981; Hornung et al., 1996a, 1996b, 1999; Walker and Peterson, 1994a, 1994b; Walker et al., 1994). Retinal ganglion cell densities also appeared to be decreased in swim-up rainbow trout resulting in a reduction in visual and motor function, and at high doses reduced prey capture ability (Carvalho and Tillitt, 2004). Reduced circulation, pericardial edema, vascular hemorrhage, blood clots and bone malformations including dorsoventrally and laterally curved spines, deformed neural and haemal spines and caudal fins and alterations in spine calcification in the posterior region were prominent endpoints of TCDD toxicity in the medaka embryo (Cantrell et al., 1996, 1998; Kawamura and Yamashita, 2002). Thus, in fish early life stages the cardiovascular system and developing cartilaginous structures in the craniofacial region are particularly vulnerable to TCDD developmental toxicity.
Impact of TCDD-Like Chemicals on Great Lakes Lake Trout
In the Great Lakes Ecosystem, lake trout have historically been the top predator fish species (Balon, 1980) and constitute an important part of the region’s economy. While populations of lake trout were self-sustaining in the Great Lakes since the late 1800s, populations crashed in the 1950s in all of the Great Lakes except Lake Superior (Tillitt et al., 2008). Overharvesting by commercial fishermen in conjunction with the introduction of the sea lamprey into the Great Lakes has long been thought to be the cause of this population decline.
However, it later became apparent that sac fry mortality caused by exposure to TCDD-like chemicals contributed to the failure of population recruitment of lake trout in the Great Lakes (Cook et al., 2003). Blue sac disease had been identified in feral fish populations, most notably among lake trout of the Great Lakes. Since laboratory exposure of lake trout embryos to waterborne TCDD or PCB 126 resulted in endpoints consistent with blue sac disease (Spitsbergen et al., 1991; Walker et al., 1991; Zabel et al., 1995a), exposure to such environmental toxicants was suspected as a potential cause of the disease, but no association was found between blue sac disease and contamination with chemicals such as 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene (DDE) or total PCBs (Simonin, 1990). However, it was noted that lake trout from Lake Ontario had a higher incidence of blue sac disease than trout from the other Great Lakes, which coincided with higher concentrations of TCDD in Lake Ontario (DeVault et al., 1989; Zacharewski et al., 1989).
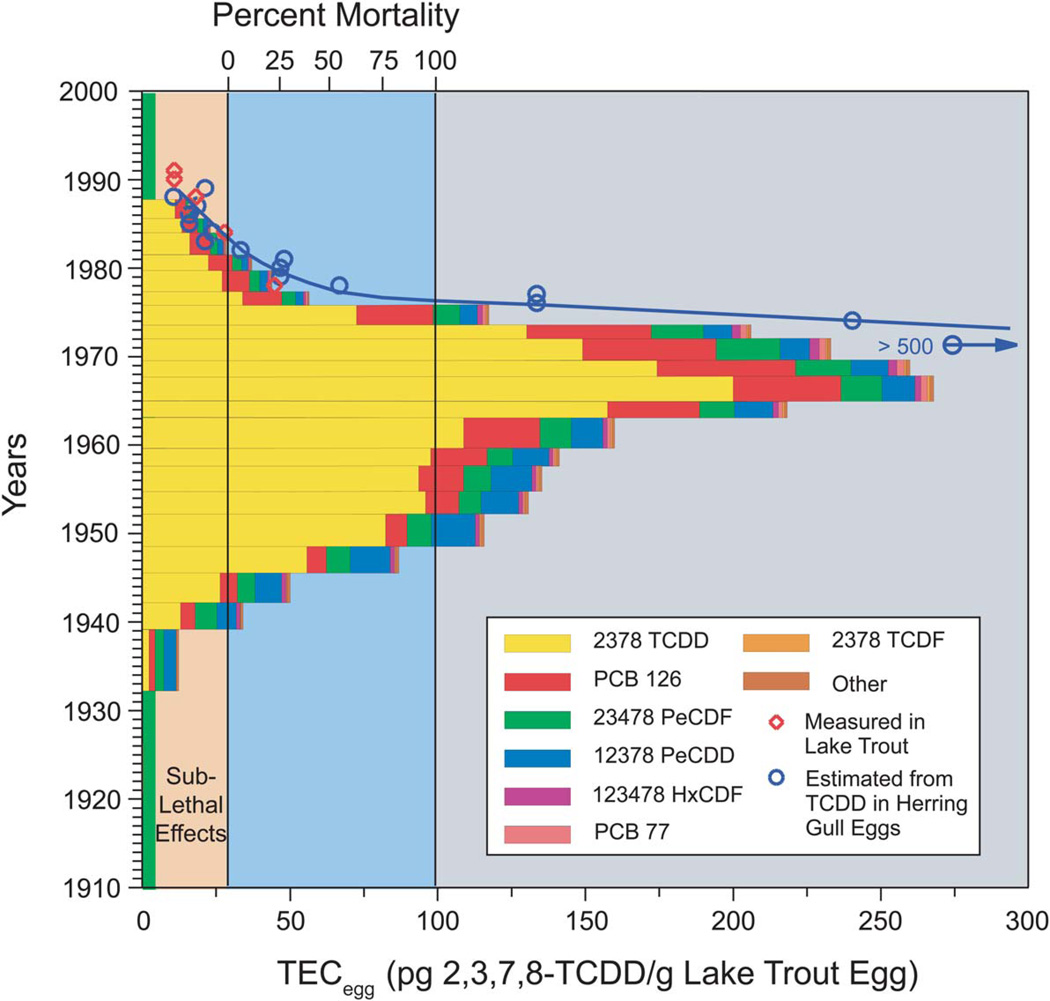
Concern about reproductive success and growth of wild lake trout populations combined with a greater appreciation for the high sensitivity of lake trout larvae to TCDD-induced blue sac syndrome mortality (Cook et al., 2003; Spitsbergen et al., 1991; Tillitt et al., 2008; Walker et al., 1991; Zabel et al., 1995a) led to increased interest in determining the molecular basis for the developmental toxicity of TCDD in aquatic species. Lake trout larvae are among the most sensitive of all fish species to the toxic effects of dioxin-like compounds. Accordingly, it was hypothesized that while the adult populations appear less affected, exposure of lake trout larvae to dioxin-like chemicals, from environmental exposure and maternal transfer, was causing sac fry mortality and impairing natural recruitment such that lake trout populations could no longer sustain themselves in the most contaminated Great Lakes (Cook et al., 2003; Tillitt et al., 2008). In support of this notion, it was subsequently determined that levels of dioxin-like chemicals were high enough in Lake Ontario to kill essentially 100% of lake trout larvae by blue sac syndrome that hatched there from the late 1940s to the late 1970s (Figure 2) (Cook et al., 2003). While environmental levels of AHR agonists have been reduced in recent years, these persistent organic pollutants still constitute a major risk to wild fish populations including lake trout that are highly sensitive to dioxin developmental toxicity. Furthermore, to integrate assessment risks to wildlife and humans, it is important to demonstrate common mechanisms of toxicity across species.
Figure 2. Retrospective prediction of early life stage mortality in Lake Ontario lake trout exposed as eggs to TCDD and related compounds.
Estimates of percent mortality predict a 40 year period, 1940 to 1980, when early life stage mortality of lake trout caused by the exposure of lake trout eggs to dioxin-like chemicals would adversely impact the wild lake trout population in Lake Ontario, regardless of effects caused by other chemical or nonchemical stressors. The vertical, light blue area goes from 0% mortality, at a lake trout egg TCDD toxicity equivalence concentration (TEC) of 29 pg TCDD equivalence/g lake trout egg to 100% mortality, at a TEC of 100 pg TCDD equivalence/g lake trout egg. The vertical, light brown area shows the TEC range in lake trout eggs predicted to be associated with sublethal effects. The vertical, gray area represents 100% mortality. The length of each horizontal bar indicates the total TEC contributed by the various dioxin-like chemicals (box in figure) in lake trout eggs, determined from sediment core analysis for the year that the sediment was deposited on the bottom of the lake. More specifically, the TECs contributed by individual dioxin-like chemicals in lake trout eggs for a particular year were determined from the analysis of these dioxin-like chemicals in radionuclide-dated 1-cm sections of a sediment core (LO87-20) taken from the bottom of eastern Lake Ontario. The concentration of each dioxin-like chemical in each 1-cm sediment section was multiplied by the appropriate biota-sediment accumulation factor (BSAF) for dioxin-like chemicals in lake trout eggs, BSAFs were adjusted for the effect of temporal changes in the chemical distributions between water and sediment, and multiplied by the toxicity equivalence factor (TEF) of that particular dioxin-like chemical based on trout early life stage mortality (Walker and Peterson, 1991; Zabel et al., 1995b) to predict the percent mortality of lake trout sac fry in the year the sediment was deposited on the bottom surface of Lake Ontario (Cook et al., 2003). A red open diamond indicates the TEC in lake trout eggs for a particular year estimated from measurements of dioxin-like chemicals in lake trout tissues that were collected in that year. A blue open circle indicates the TEC in lake trout eggs for a particular year estimated from measurements of the TCDD concentration in herring gull eggs in the same year. Figure 2 is modified from Cook et al. (2003) with permission.
Initial work used rainbow trout and lake trout embryos to study the early life stage developmental effects produced by TCDD (Peterson et al., 1993). However, lake trout proved not to be an amenable model to extensively study TCDD developmental toxicity because the opaque chorions made evaluation of early development difficult, and lake trout embryos and larvae develop slowly taking as much as 3–6 months for hatching and sexual differentiation. Trout species also require large facilities for general fish care and maintenance. Furthermore, as tetraploids lake trout have a higher incidence of duplicated genes, which when combined with slow advances in genome annotation and molecular techniques, limited this model for use in identifying genes and signaling pathways that mediate TCDD toxicity. In order to delineate the molecular mechanism(s) of developmental TCDD toxicity in fish, a different species, whose embryonic and larval development was easily observed, rapid, amendable to genetic manipulation, had available genome annotation and whose maintenance was more manageable than trout was required.
Zebrafish as a Model for Assessing TCDD Reproductive and Developmental Toxicity
Zebrafish have been used extensively as a model for studying development and genetics, and its use has recently expanded to the fields of pharmacology and toxicology (Grunwald and Eisen, 2002; Hill et al., 2005; Peterson et al., 2000; Tanguay et al., 2003). Zebrafish embryogenesis is complete within 72 hours post fertilization (hpf) and zebrafish reach sexual maturity within 3 months (Westerfield, 1993). Adult females readily produce hundreds of eggs on a regular basis, and embryos are transparent which allows for microscopic observation during early development. Most significant are the continuing advances in genetic and molecular tools that have allowed for the understanding of genes and their functions in early developmental processes. For instance, production of mutant and transgenic zebrafish and transient knockdown of gene translation or splicing with targeted morpholino oligonucleotides have resulted in better understanding of gene function during embryonic development (Kawakami, 2005; Mullins and Nusslein-Volhard, 1993; Nasevicius and Ekker, 2000). In addition, advances in the annotation of the zebrafish genome suggested high conservation of genes across zebrafish and mammals (Curwen et al., 2004). Exposure of zebrafish embryos to TCDD also induces the hallmark signs of blue sac disease, establishing this organism as a useful laboratory model for the analysis of TCDD toxicity in fishes (Henry et al., 1997; Walker et al., 1991).
Many of these same attributes make the zebrafish an excellent model organism for examining reproductive toxicity following exposure to TCDD-like chemicals in juveniles and adults (Carvan et al., 2005; Spitsbergen and Kent, 2003). Much is known about the processes of sexual differentiation, anatomy and function of the reproductive system, breeding behaviors, and environmental conditions that affect sexual differentiation in zebrafish (Ankley and Johnson, 2004; Ankley et al., 2009; Coe et al., 2008; Laan et al., 2002). Established protocols for evaluating histopathology in juveniles and adults are readily available (Leal et al., 2009), and several commercial assays exist for measuring serum steroid hormone concentrations. The zebrafish is commonly used for life-cycle toxicity assays, and there is a wealth of data regarding effects of reproductive toxicants in this species (Ankley and Johnson, 2004).
Zebrafish (Figure 1B) exhibit essentially the same profile of TCDD developmental toxicity responses as other larval fishes (Figure 1A), although zebrafish are significantly less sensitive to TCDD-induced early life stage mortality than other fish species (Figure 1C). This is reflected in about a 120 fold difference in the lethal potency of TCDD between the most sensitive fish species, bull trout and least sensitive, zebrafish. Henry et al. (1997) were the first to characterize TCDD developmental toxicity in larval zebrafish, and Elonen et al. (1998) reported similar findings. Reproductive toxicity of TCDD is also similar in adult zebrafish compared with other fish species (King-Heiden et al., 2005, 2006, 2009; Wannemacher et al., 1992). Due to the extensive genetic characterization and rigorous examination of embryonic and larval development in zebrafish, this model is ideal for investigating the cellular and molecular basis of TCDD developmental and reproductive toxicity.
Understanding Mechanisms of TCDD Toxicity - AHR Signaling in Zebrafish
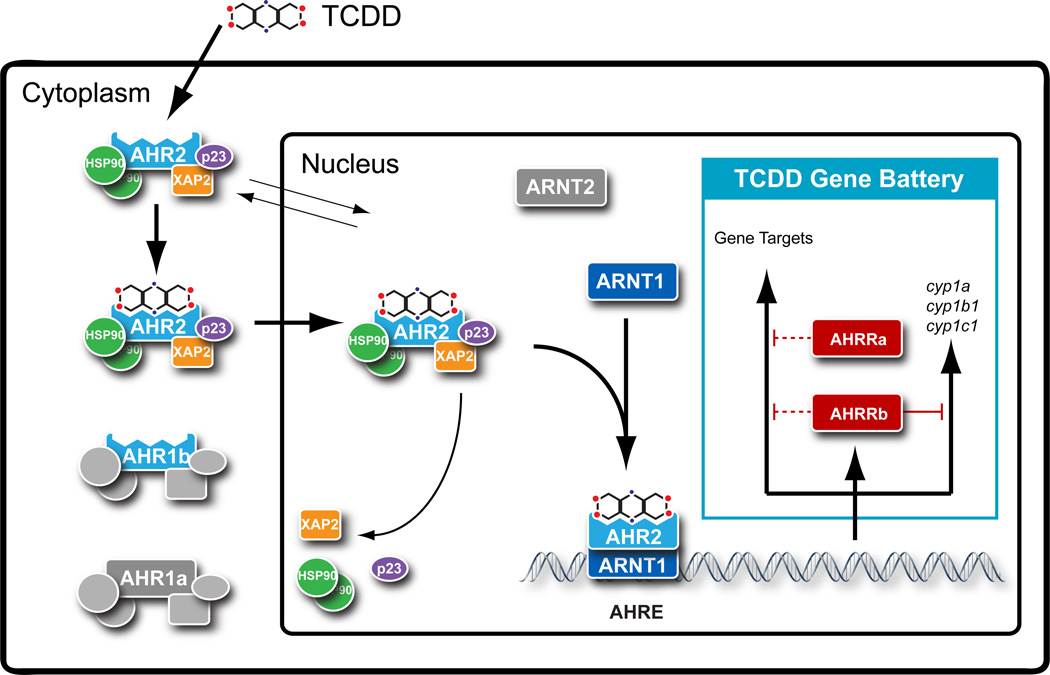
In order to fully utilize the zebrafish as a model to investigate the mechanisms of TCDD toxicity, it was essential to identify and characterize the function of the different AHR signaling pathway components. As previously mentioned, TCDD and other dioxin-like chemicals induce toxicity by binding to the AHR (Figure 3). The AHR protein is highly conserved across vertebrate species and is a basic helix-loop-helix, Per-Arnt-Sim (PAS) domain-containing, ligand-activated transcription factor. In the absence of ligand, the AHR resides in the cytoplasm where it is found in a complex with chaperone proteins including heat shock protein 90 (HSP90) and the AHR interacting protein (AIP, ARA9 or XAP2). Upon activation by a ligand such as TCDD, the AHR complex translocates into the nucleus where it binds to its heterodimer partner, the aryl hydrocarbon receptor nuclear translocator (ARNT). This heterodimer subsequently binds to DNA at AHR response elements (AHREs), also referred to as dioxin response elements (DREs) or xenobiotic response elements (XREs), and induces the transcription of target genes.
Figure 3. The aryl hydrocarbon receptor 2 (AHR2) signaling pathway mediates TCDD toxicity in zebrafish.
As with many fish species, zebrafish have multiple copies of the key proteins, AHR and ARNT that mediate TCDD toxicity. Studies in zebrafish larvae have shown that AHR2 and ARNT1 dimers mediate gene transcription, following activation of AHR2 by TCDD, to cause developmental toxicity (see text for references).
Members of the PAS domain-containing family are typically involved in responding to environmental stimuli such as light or oxygen tension (Taylor and Zhulin, 1999), and the AHR is no exception. Its most well-defined role is as an environmental sensor that mounts an adaptive response to xenobiotic exposure by up-regulating Class I and Class II xenobiotic metabolizing enzymes in a ligand-dependent manner, thereby facilitating detoxification of noxious compounds. However, multiple studies have suggested additional roles for the AHR in development (Fernandez-Salguero et al., 1995; Schmidt et al., 1996), cell-cell interactions (Cho et al., 2004; Ikuta et al., 2004), cell cycle control (Ge and Elferink, 1998; Kolluri et al., 1999; Puga et al., 2000a), circadian rhythms (Garrett and Gasiewicz, 2006; Mukai and Tischkau, 2007; Sato et al., 2008), and endocrine signaling (Ohtake et al., 2003). In addition, some studies indicate that AHR may not only be functioning as a mediator of transcription, but also as a modulator of transcription by interacting with and amplifying or repressing the activity of other transcription factors at non-AHRE sites [e.g., E2F (Marlowe et al., 2004), ER (Ohtake et al., 2003) and BRCA1 (Kang et al., 2008)]. It is thought that AHR-mediated transcriptional regulation underlies the mechanisms by which AHR agonists such as TCDD induce toxicity; however, some actions of TCDD may be AHR-independent and/or result from downstream transcriptional changes (King-Heiden et al., 2008; Pande et al., 2005; Puga et al., 2000b; Volz et al., 2005).
Fish express both AHR and ARNT, in addition to another protein known as the AHR repressor (AHRR) (Karchner et al., 2002), which can antagonize AHR function; however, fish possess multiple copies of AHR genes (Hahn, 2002). Initially cloned from Fundulus heteroclitus and Mustelus canis (Hahn et al., 1997; Karchner et al., 1999), the duplicated AHR genes were found to have diverged and were placed into separate clades denoted as AHR1 and AHR2. Multiple AHR genes were soon identified in other fish species, including zebrafish, Danio rerio (Andreasen et al., 2002; Karchner et al., 2005; Tanguay et al., 1999), Atlantic salmon, Salmo salar (Hansson et al., 2004), and rainbow trout, Oncorhynchus mykiss (Abnet et al., 1999). Zebrafish have three known AHR genes, one from the AHR2 clade (Tanguay et al., 1999) and two from the AHR1 clade (AHR1a and AHR1b) (Andreasen et al., 2002; Karchner et al., 2005); however, the zfAHR1a cannot bind TCDD and appears to be non-functional as a mediator of transcription. The zebrafish also has two ARNT genes (ARNT1 and ARNT2) (Prasch et al., 2006; Tanguay et al., 2000) and two AHRR genes (AHRR1 and AHRR2) (Evans et al., 2005). Both ARNT1 and ARNT2 can produce multiple splice variants (ARNT1a, b, c and ARNT2a, b, c), and three of these, ARNT2b, ARNT1b and ARNT1c, can functionally heterodimerize with zfAHR2 in vitro (Prasch et al., 2006; Tanguay et al., 2000).
Once components of the AHR signaling pathway had been characterized, a series of studies using morpholino technology demonstrated, by knocking down the expression of several components of the AHR pathway, that TCDD toxicity is mediated by zfAHR2 and zfARNT1 dimerization partners but not by the zfCYP1 A in embryonic zebrafish (Antkiewicz et al., 2006; Carney et al., 2004; Prasch et al., 2003, 2004, 2006). Following these milestones, the zebrafish model was poised to investigate the more sensitive endpoints of TCDD developmental toxicity, namely, adverse effects on cardiovascular, craniofacial and ovarian development.
Using Zebrafish to Understand TCDD-Induced Cardiovascular Toxicity
While toxic effects on some organ systems may not result in mortality, the cardiovascular system is perhaps the most evident example of a close association between organ dysfunction and mortality (Heideman et al., 2005). Studies of HAH toxicity in mammals and birds had identified the cardiovascular system as a key target of TCDD toxicity. Also the first published study of TCDD developmental toxicity in lake trout in 1991, identified the cardiovascular system as the initial tissue affected in both the TCDD toxicity syndrome and in blue sac disease of developing lake trout (Spitsbergen et al., 1991). Six years later, Henry et al. (1997) demonstrated that TCDD exposure also adversely affected the cardiovascular system of developing zebrafish (Henry et al., 1997). Zebrafish embryos treated with TCDD shortly after fertilization developed malformed hearts and pericardial edema at 72 hpf (Figure 4A), followed by the onset of yolk sac edema (96 hpf) and mortality (132 hpf). Reduced blood flow in vascular beds of the trunk, head and gills and slowed heart rate also occurred in TCDD-treated larvae prior to or coincident with the onset of other signs of toxicity (Henry et al., 1997). This was the first report in zebrafish to demonstrate that the cardiovascular system was a target of TCDD developmental toxicity. This conclusion was reinforced by a subsequent study on TCDD developmental toxicity in rainbow trout, where arrested heart development and reduced perfusion of tissues with blood were identified as playing a primary role in the developmental toxicity of TCDD (Hornung et al., 1999). Thus, several lines of evidence, in three fish species, lake trout, rainbow trout and zebrafish, demonstrated that the developmental toxicity of TCDD in fish is characterized by cardiovascular toxicity.
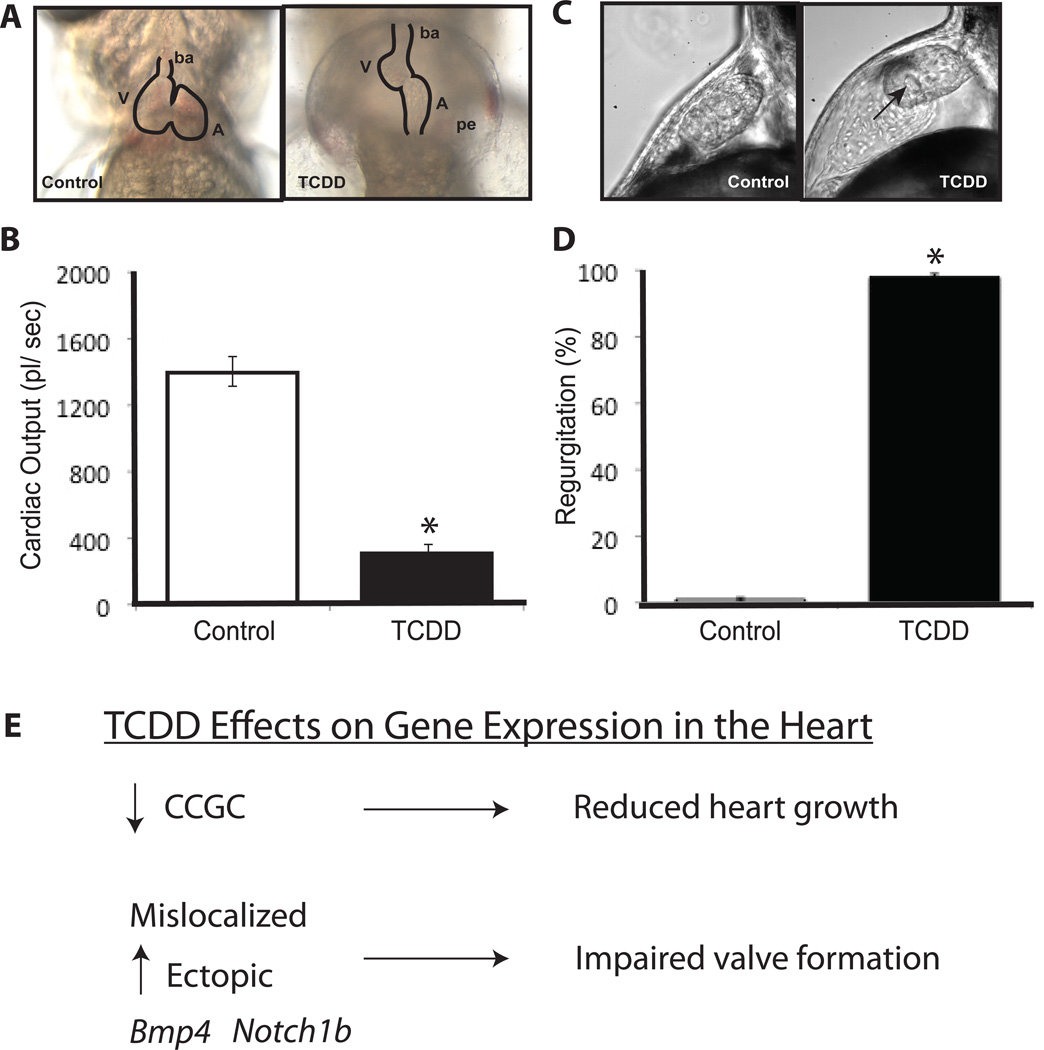
Figure 4. TCDD-induced heart toxicity in zebrafish larvae.
Representative photograph of a gross heart malformation in a TCDD-treated zebrafish larva at 96 hpf (A). Abbreviations are: V = ventricle, A = atrium, ba = bulbus arteriosis and pe = pericardial edema. Reduced cardiac output caused by TCDD exposure (B). Representative photograph is shown of an impaired AV valve closure during systole in a TCDD-treated zebrafish larva heart (C). Arrow points to the open AV valve during systole which is abnormal. Increased percent of TCDD-treated larvae with regurgitation of blood at the AV valve (D). Effects of TCDD on gene expression in the heart (E) includes downregulation of a cell cycle gene cluster (CCGC) that leads to a reduced number of cardiomyocytes and increased and mislocalized ectopic expression of Bmp4 and Notch 1b which impairs heart valve formation. * Denotes significant differences (p<0.05). (A) and (B) are modified from King-Heiden et al. (2009) with permission; (C) and (D) are modified from Mehta et al. (2008) with permission.
TCDD Toxicity in the Vasculature
Since then several studies using TCDD as a prototypical AHR agonist reported toxicity in fish early life stages that could be attributed to adverse effects on the vascular system. Dioxin-treated lake trout embryos develop yolk sac, pericardial and meningeal edema as well as hemorrhage (Spitsbergen et al., 1991). These effects were associated with ultrastructural changes in the endothelium of affected vascular beds of lake trout fry (Guiney et al., 2000). Similar effects were found in medaka embryos where TCDD-induced DNA damage, apoptosis and loss of functional integrity of the vasculature (Cantrell et al., 1996, 1998). Atlantic killifish embryos exposed to TCDD developed edema and hemorrhage accompanied by increased apoptosis in the heart and vasculature (Toomey et al., 2001). In another marine fish, seabream, early embryonic exposure resulted in several toxic effects including subcutaneous, yolk sac, pericardial and meningeal edema (Ortiz-Delgado and Sarasquete, 2004). Induction of CYP1A activity in the vascular endothelium of lake trout embryos was associated with increased vacuolation and cytoplasmic blebbing of the tissue (Guiney et al., 1997, 2000) and in medaka CYP1A induction was correlated with effects on the heart (Cantrell et al., 1998).
More recent studies are shedding additional light on TCDD vascular toxicity in zebrafish larvae. Malformation of certain blood vessels and reduced regional blood flow have been observed in TCDD exposed zebrafish larvae and been shown to be AHR2 and ARNT1 dependent (Dong et al., 2004; Kubota et al., 2011; Teraoka et al., 2009, 2010). Delayed regression of the common cardinal vein was found in TCDD exposed zebrafish and red seabream larvae (Bello et al., 2004; Yamauchi et al., 2006) and malformation of the mesencephalic vein and prosencephalic artery, was discovered in the head of zebrafish larvae exposed to dioxin (Teraoka et al., 2010). The mechanism of the TCDD-induced decrease in midbrain regional blood flow in zebrafish larvae has been the focus of recent research. It was discovered that a TCDD-induced increase in cyclooxygenase 2 (COX-2) is involved in the mesencephalic vein circulation failure and can be prevented by selective COX-2 inhibitors and rescued by knocking down COX-2 activity (Teraoka et al., 2009). TCDD induction of COX-2 has also been observed medaka embryos (Dong et al., 2010). The inhibitory effect of TCDD exposure on midbrain blood flow can also be blocked by selective antagonists of the thromboxane receptor and by suppression of thromboxane A synthetase 1 activity (Teraoka et al., 2009). Thus, COX-2, thromboxane A synthetase, and the thromboxane receptor axis are involved in the mesencephalic vein circulation failure in TCDD-exposed zebrafish larvae (Teraoka et al., 2009). A novel cyp1 subfamily, the cyp1cs, was discovered a few years ago in fish and cyp1c1 and cyp1c2 were cloned and characterized in zebrafish (Godard et al., 2005; Jönsson et al., 2007). Zebrafish adults and embryos showed basal and TCDD-induced expression of both Cyp1c transcripts in the endothelial cells of blood vessels and they appear to play a role in the TCDD-induced decrease in midbrain blood flow (Kubota et al., 2011). This was suggested by gene knock-down of CYP1C1 and CYP1C2 blocking reduced mesencephalic vein blood flow in zebrafish larvae caused by TCDD (Kubota et al., 2011).
TCDD Toxicity in the Heart
The discovery of TCDD-induced vascular toxicity in fish by itself, however, does not provide a full understanding of the scale and character of the cardiovascular toxicity of TCDD in developing fish, nor does it strengthen the premise that the heart is a direct target of TCDD toxicity as suggested by Henry et al. (1997). As zebrafish became established as a model vertebrate for investigating TCDD toxicity, the zebrafish embryo and larva were selected for a detailed analysis of the effects of TCDD exposure on heart development and function (Carney et al., 2006a). Early studies of zebrafish embryos treated with a lethal dose of TCDD shortly after fertilization reported development of pericardial edema, reduced cardiac output (Figure 4B) and decreased peripheral blood flow at 72 hpf (Antkiewicz et al., 2005; Belair et al., 2001; Henry et al., 1997; Prasch et al., 2003). Antkiewicz et al. (2005) reported for the first time that the zebrafish heart was affected prior to the onset of both edema and a decrease in peripheral blood flow, with a TCDD-induced reduction in cardiac myocyte number occurring as early as 48 hpf. This demonstrated that the heart is particularly sensitive to dioxin; and sublethal exposure to TCDD was later shown to result in reduced cardiac output, even when pericardial edema was absent and gross morphology of the heart was unaffected (King-Heiden et al., 2009). Furthermore, exposure to 50 pg TCDD/ml from 0–7 weeks post fertilization resulted in persistent cardiovascular toxicity in adults (King-Heiden et al., 2009). Thus, adverse effects on the heart are among the earliest occurring and most sensitive signs of TCDD developmental toxicity in zebrafish.
Results from two different TCDD dosing paradigms suggest that the TCDD-induced decrease in cardiac output is the cause of reduced peripheral blood flow in zebrafish rather than a consequence of it. First, in embryos treated shortly after fertilization, a decrease in cardiac output was detected at 60 hpf, prior to or concomitant with the onset of pericardial edema and decrease in peripheral blood flow (Antkiewicz et al., 2006). Second, larvae treated at 72 hpf exhibited a reduction in stroke volume and cardiac output at 80 hpf, prior to the decline in peripheral blood flow at 84 hpf (Antkiewicz et al., 2005; Carney et al., 2006a). Other TCDD-induced effects on the zebrafish heart included reduction in heart size at 72 hpf and altered heart morphology (Antkiewicz et al., 2005). At 96 hpf, the heart of TCDD-treated embryos was no longer looped, with the ventricle small and compacted and the atrium thin and elongated. These effects were AHR-dependent (Antkiewicz et al., 2006). TCDD also inhibited heart valve development (Figure 4C). This was evidenced by a failure of valve cushion and subsequent valve leaflet formation at the atrio-ventricular (AV) and bulbo-ventricular (BV) valve junctions, resulting in blood regurgitation between heart chambers (Figure 4D) (Mehta et al., 2008). TCDD exposed larvae also exhibited abnormal development of the bulbus arteriosus (Mehta et al., 2008). Finally, the same endpoints of cardiac toxicity observed in zebrafish larvae after exposure to TCDD (Antkiewicz et al., 2005; Carney et al., 2006a; Mehta et al., 2008) were seen after exposure to another potent AHR agonist, 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (Grimes et al., 2008).
The cardiotoxic responses elicited by TCDD exposure suggest that signaling pathways responsible for normal heart development are disrupted (Figure 4E). By evaluating the TCDD-induced gene expression changes underlying the cardiotoxicity in larval zebrafish, we have learned much about its mechanism of action in causing this endpoint of toxicity. Carney et al. (2006a) performed a time course analysis of the transcriptional response to TCDD exposure that began at 72 hpf in the hearts of zebrafish larvae 1, 2, 4 and 12 h later. TCDD induced rapid expression changes of 42 genes (within 1 h), the majority were involved in xenobiotic metabolism, proliferation, contractility and regulation of heart development (Carney et al., 2006a). These rapidly induced gene expression changes preceded signs of TCDD cardiovascular toxicity, making them strong candidates as downstream targets of AHR signaling that contribute and/or mediate TCDD-induced cardiovascular toxicity. This immediate transcriptional response in the heart of zebrafish larvae suggests that the heart may be direct target of TCDD toxicity. Specifically, a cluster of genes that includes genes that promote cell division and growth by functioning in DNA replication, DNA repair, regulation of the cell cycle, cell division, transcription and chromosome assembly and maintenance are down regulated preceding TCDD-induced attenuation of heart function (Carney et al., 2006a; Chen et al., 2008). Taken together, downregulation of this cluster of genes, referred to as the cell cycle gene cluster (CCGC), is considered responsible for the reduction in number of cardiomyocytes in the TCDD-exposed zebrafish embryo heart (Figure 4E). TCDD also affects the expression of genes in zebrafish embryos that are important for heart function, and suggests altered expression of genes for both sarcomeric components and mitochondrial ROS production may play a role in TCDD-induced cardiac toxicity (Handley-Goldstone et al., 2005). Finally the failure of valve formation in the heart of zebrafish embryos exposed to TCDD is associated with increased ectopic expression, as well as mislocalized expression, of Bmp4 and Notch1b in areas of the heart where valves would normally form (Mehta et al, 2008; Figure 4E).
Using Zebrafish to Understand TCDD-Induced Craniofacial Malformation
The skeleton is another example where organ dysfunction in fish negatively impacts survival. Maximal growth rate in fish larvae through the transition period from endogenous to exogenous feeding is necessary for survival with a depression in growth rate associated with high mortality (Cowan and Shaw, 2002). Exposure to TCDD has adverse effects on skeletal development and body growth in several species of fish, demonstrating that the skeleton is another target of TCDD toxicity (Carvalho et al., 2004; Cook et al. 2003; Helder, 1980, 1981; Henry et al., 1997; Hill et al., 2004; Hornung et al., 1999; Teraoka et al., 2002; Walker et al., 1991). Craniofacial cartilage development is particularly vulnerable to TCDD exposure, and much has been learned about the mechanisms by which this occurs using the zebrafish model. TCDD-induced jaw malformation appears to be independent of cardiovascular effects as this malformation occurs prior to decreases in peripheral blood flow (Henry et al., 1997; Teraoka et al., 2002).
Craniofacial cartilage malformations induced by TCDD are observed in zebrafish as early as 60 hpf (Teraoka et al., 2002) and are characterized by stunted upper and lower jaw cartilage growth that prevents anterior extension of the lower jaw beyond the eyes, resulting in a shortened jaw in TCDD-exposed larvae (Henry et al., 1997; Figure 5A). The most striking effects of TCDD on the lower jaw cartilages are the malformation of Meckel’s, palatoquadrate and ceratohyal cartilages (Figure 5B). Meckel’s cartilage fails to extend anteriorly as far as the ethmoid plate, and palatoquadrate cartilages are shorter. Furthermore, the acute angle formed at the intersection of the ceratohyal cartilages in vehicle control larvae is lost in TCDD-treated larvae (Teraoka et al., 2002, 2006; Xiong et al., 2008). Similar to the ceratohyal cartilages, the acute angles where branchial arches intersect are also disrupted. Closer examination of the upper jaw also identified a smaller and shorter ethmoid plate in TCDD-exposed larvae. Histological analysis of the head in zebrafish larvae exposed to TCDD revealed intact, albeit smaller, cartilage, bone and muscle (Henry et al., 1997). Overall, TCDD alters the shape and reduces the size of the head skeletal elements. This is illustrated by the reduction in lower jaw length in TCDD-exposed larvae (Figure 5C).
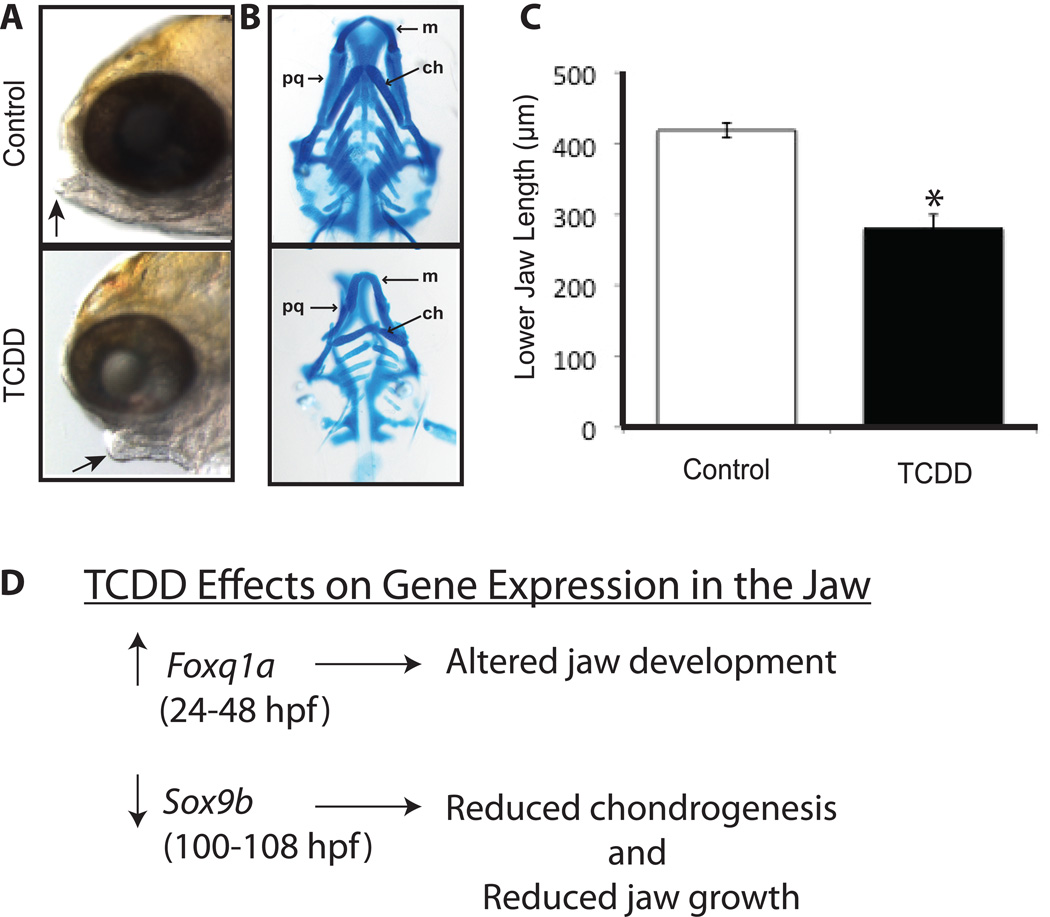
Figure 5. TCDD-induced jaw malformation in zebrafish.
Representative photograph of impaired jaw formation in a TCDD-treated zebrafish larva at 120 hpf (A). The arrow points to the end of a shorter lower jaw in a representative TCDD-exposed larva (A). A representative photograph shows reduced size and altered shape of Alcian blue stained jaw cartilages in a TCDD-exposed larva (B). Decreased lower jaw length is shown for the TCDD treatment group at 120 hpf (C). Effects of TCDD on gene expression in the zebrafish larva jaw (D) reveals early upregulation of Foxq1b and later downregulation of Sox9b mRNAs. Jaw cartilage abbreviations are: m = Meckel’s, pq = palatoquadrate, and ch = ceratohyal. * Denotes significant differences (p<0.05). Photographs in (B) were taken from Xiong et al. (2008) with permission.
Development of craniofacial structures involves establishing lineages of skeletal precursors, re-arrangements and proliferation of cartilage condensations (chondrocyte differentiation) and cartilage organogenesis followed by endochondral ossification, all of which is tightly coordinated with the development of the vascular and nervous system (Yelick and Schilling, 2002). Alcian blue staining of cartilages in zebrafish embryos exposed to TCDD demonstrate that chondrocyte differentiation was not inhibited as many of the cartilages developed; however, the shape and size of the cartilages were altered (Teraoka et al., 2002). Subtle malformations of the lower jaw occur even when cardiovascular toxicity is not present (Hill et al., 2004; King-Heiden et al., 2009), and exposure to 25 pg TCDD/ml from 0–7 weeks post fertilization resulted in persistent craniofacial malformations in adults (King-Heiden et al., 2009). Together, these results suggest that altered blood flow does not result in craniofacial toxicity and that TCDD does not affect differentiation of the cranial components (i.e., chondrocytes, osteocytes and myocytes), but instead disrupts proliferation and/or expedites apoptosis of these cranial cell types resulting in jaw malformation.
As expected, TCDD-induced jaw malformation is AHR2-dependent in developing zebrafish embryos (Prasch et al., 2003). In addition, it was also determined that ARNT1, not ARNT2, was the critical dimerization binding partner with AHR2 that mediated TCDD-induced jaw toxicity in zebrafish embryos (Prasch et al., 2006). While initial chondrocyte differentiation is not affected by TCDD, it is possible that TCDD could disrupt the expression of genes important for regulating the initial patterning of craniofacial structures or the process of endochondrial ossification. Since hedgehog signaling plays such a prominent role in craniofacial development (Eberhart et al., 2006), alterations in this signaling cascade may underlie TCDD-induced malformation of skeletal elements (Teraoka et al., 2006). Exposure to TCDD decreases Sonic hedgehog a and b transcript expression in an AHR2-dependent manner; however, subsequent downregulation of hedgehog receptors Patched 1 and 2, which are dependent upon hedgehog signaling, suggests that other pathways in addition to hedgehog impair jaw growth (Teraoka et al., 2006). Therefore, identification of an AHR-induced transcriptional response was necessary to elucidate the molecular mechanism(s) of TCDD-induced jaw malformation.
To identify other pathways important for jaw development disrupted by TCDD, Xiong et al. (2008) used microarray techniques to identify transcripts dysregulated in isolated jaw tissue 1, 2, 3 and 12 h, after 96 hpf larvae were exposed to TCDD for 1 h. As expected, TCDD increased expression of AHR-regulated genes (i.e., cyp1a, cyp1b1, and sulfotransferase), in addition to causing dysregulation of 24 genes involved with cartilage or bone development. Genes activated by TCDD included those important for promoting terminal chondrocyte differentiation and cartilage morphogenesis; while those suppressed included transcription factors important for chondrogenic cell specification, osteocyte adhesion molecules, and key components in cartilage extracellular matrix. Most notably, TCDD-induced a 15 fold decrease in the expression of Sox9b (Xiong et al., 2008), a transcription factor essential for regulating proper differentiation and proliferation of chondrocytes (Yan et al., 2002, 2005). It was subsequently demonstrated that heterozygous sox9b mutant zebrafish larvae were more susceptible to TCDD-induced jaw malformation than wild type larvae and that over expression of Sox9b in TCDD-treated larvae ameliorated TCDD effects on jaw development (Xiong et al., 2008). Downregulation of Sox9b by TCDD was first detected in the regenerating fin of zebrafish (Andreasen et al., 2006; Mathew et al., 2008), a process that also involves chondrogenesis. Xiong et al. (2008) hypothesize that disruption of Sox9b expression may expedite terminal chondrocyte differentiation, causing proliferation in chondrocytes to cease (Figure 5D). These findings strongly implicate Sox9b as a downstream effector of TCDD-induced jaw malformation.
Planchart and Mattingly (2010) uncovered a different developmental pathway important for regulating the formation of the lower jaw that also is sensitive to disruption by TCDD. Foxq1a (previously named foxq1b) is rapidly induced in zebrafish embryos exposed to TCDD during the earliest stages of development (0–24 hpf) and also is associated with disruption of Meckel’s cartilage (Planchart and Mattingly, 2010; Figure 5D). Foxq1a is a gene in the family of forkhead box transcription factors important for the development of craniofacial structures. Foxq1a was identified to be upregulated prior to cyp1a, an AHR target gene, suggesting Foxq1a is likely a direct target of TCDD-activated AHR signaling. Supporting data suggests Foxq1a upregulation was dependent on AHR2 signaling, and Foxq1a expression was observed in the jaw primordium showing localization in Meckel’s cartilage and other posterior arches affected by TCDD exposure.
Thus, exposure to TCDD during early embryonic and larval development disrupts two pathways that regulate both the development (Foxq1a) and growth (Sox9b) of lower jaw structures (Figure 5D). Importantly, these studies illustrate the usefulness of zebrafish in identifying multiple genes and signaling pathways that when disrupted by TCDD lead to a specific endpoint of developmental toxicity, jaw malformation.
Using Zebrafish to Understand TCDD-Induced Reproductive Toxicity
While most TCDD research in zebrafish has focused on developmental toxicity, another important endpoint of toxicity is impaired reproduction. The reproductive toxicity of TCDD in fish is of interest because TCDD is an endocrine disrupting compound (EDC). It inhibits estrogen biosynthesis in fish most likely by inhibiting steroidogenic enzyme activity (Hutz et al., 2006). In contrast, in the rat TCDD inhibits the rate-limiting step in testicular steroidogenesis, mobilization of cholesterol to the inner mitochondria (Kleeman et al., 1990); but this possible mechanism of action has not yet been investigated in fish. Estrogens coordinate processes that are vital for population viability including regulation of embryonic development and all aspects of reproductive development from sex differentiation to gonad maturation, reproductive cycling and behaviors. In fish, periods of gonad differentiation and reproduction in mature adults offer two “windows” of enhanced susceptibility to disruption by TCDD. Since endocrine signaling is important for the regulation of early development and gonad differentiation, exposure to TCDD during critical ontogenetic periods may cause permanent functional changes that result in reduced fitness and reproductive capacity later in life (Bigsby et al., 1999; Guillette et al., 1995; Segner, 2006). This may occur by TCDD exposure altering reproductive behavior, population sex ratio and/or sexual selection in addition to decreasing reproductive capacity. Such alterations can affect community structure and genetic diversity and lower long-term fitness of feral fish populations (Guinand et al., 2003; Keller and Waller, 2002).
Reproductive toxicity of TCDD in zebrafish is similar to that reported in other fish species and consists of reduced fitness as both reproductive capacity and recruitment of offspring is reduced. Most research on TCDD reproductive toxicity in zebrafish has evaluated effects on female reproduction and demonstrated that ovarian development and egg release is impaired. In adults, dietary exposure to acutely toxic levels of TCDD induce overt toxicity associated with dose-dependent reductions in egg production and a complete suppression of spawning activity, corresponding with arrested gonad development and oocyte atresia (Wannemacher et al., 1992). Exposure to sublethal concentrations of TCDD also impairs female reproduction. Adults exposed via the diet show reduced egg release; however, even when ovarian development is not significantly impacted, subtle physiological changes induced by TCDD lead to altered follicular development and decreased serum 17β estradiol and vitellogenin concentrations that correlate with reduced reproductive capacity (King-Heiden et al., 2005, 2006).
The mechanisms by which TCDD modulates follicular development within a mature ovary are not understood. One study shows that exposure of adult female zebrafish to TCDD downregulates enzymes involved in estrogen biosynthesis and attenuates estrogen signaling pathways (King-Heiden et al., 2008). Histopathological analyses of the ovary also show that adverse effects of TCDD on follicular development and vitellogenesis probably result from a direct effect on the ovary by modulating follicular development and inducing follicular atresia (King-Heiden et al., 2006, 2009).
While TCDD has known effects on the adult ovary, less is known about its impacts on the developing gonad. Since reproductive hormones regulate gonad development, there is potential for TCDD to impact sexual differentiation and sex ratios as well as cause organizational effects that alter reproductive success later in life. Waterborne exposure of zebrafish to TCDD from the embryo stage of development through sex differentiation was discovered to impair reproductive capacity of not only the TCDD-exposed zebrafish when they reached adulthood (Figure 6A), but also their offspring (King-Heiden et al., 2009). Some TCDD exposed females had ovarian lesions (Figure 6B) while TCDD exposed males showed no lesions in the testis. Not all of the TCDD-exposed females showed reduced egg production. About half of the TCDD-exposed females produced only slightly fewer eggs per spawn compared to control females, while the other half exhibited complete reproductive failure (< 20 eggs/spawn) (King-Heiden et al., 2009). While King-Heiden et al. (2009) did not directly correlate ovary histopathology to reduced reproductive capacity of the TCDD-exposed females, the results show that ovaries from these TCDD-exposed females contained significantly more atretic follicles, smaller vitellogenic follicles and approximately half of the TCDD-exposed females had malformed ovaries (Figure 6C). Since other aspects of reproduction (i.e., fertility, gamete quality and recruitment) were also impaired, it suggests that exposure to low concentrations of dioxin-like chemicals during early life stage development and sexual differentiation in fish could pose a threat to the sustainability of TCDD-exposed feral fish populations (King-Heiden et al., 2009).
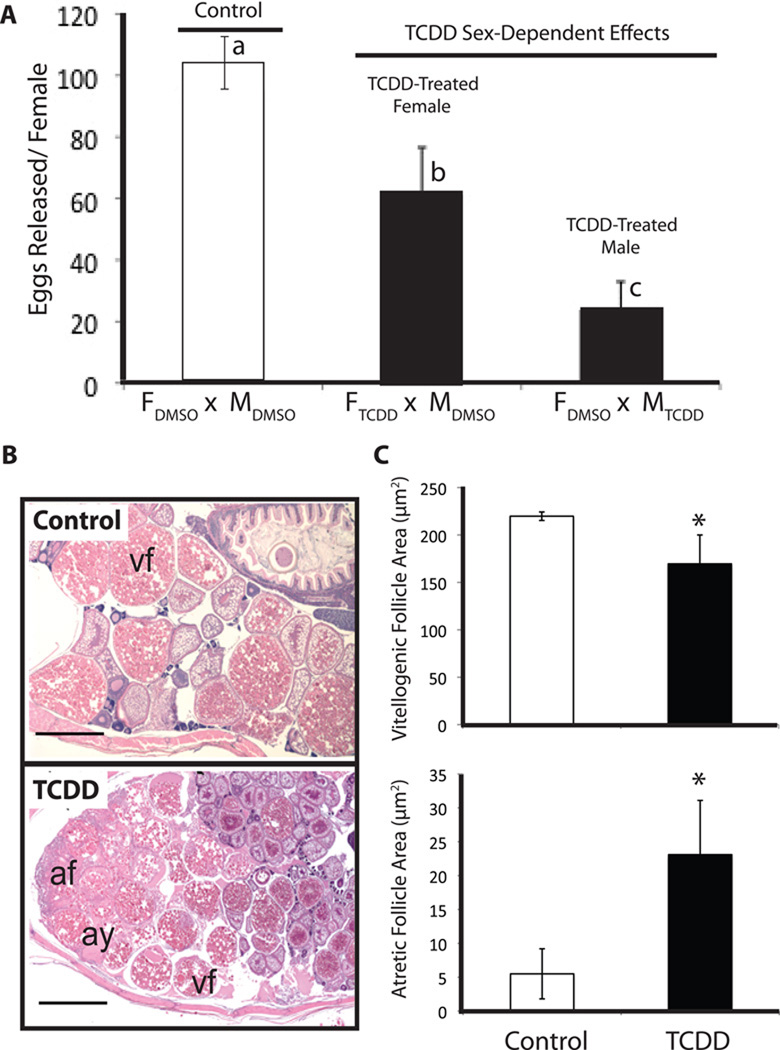
Figure 6. Reproductive toxicity in adult zebrafish following TCDD exposure from embryonic development through gonad differentiation.
TCDD exposure reduces the number of eggs released by a female zebrafish during spawning (A) with TCDD-exposed males contributing more to the effect than TCDD-exposed females. Abbreviations: FDMSO = control females; FTCDD = TCDD-exposed females; MDMSO = control males; MTCDD = TCDD-exposed males. Representative photomicrographs demonstrating ovarian lesions in TCDD-exposed females (B) where vf = vitellogenic follicle, af = atretic follicle, and ay = amorphous yolk material. TCDD exposure alters follicular development (C). In female zebrafish exposed to TCDD, vitellogenic follicles were about 23% smaller and area of the ovary (normalized to area observed) containing atretic follicles and amorphous yolk material was increased (C). Either a lowercase letter or an * denote a significant difference (p<0.05). Length of the horizontal line at the bottom of photomicrographs in panel B = 1000 um. Results in (A) and (B) are modified from King-Heiden et al. (2009) with permission.
Little is known regarding the effects of TCDD exposure on male fish reproduction. Despite a lack of observed pathology in the testis, male zebrafish exposed to TCDD during early stages of development appear to be a larger contributor to reduced female egg release during spawning than TCDD-exposed female zebrafish (Figure 6A) (King-Heiden et al., 2009). This finding highlights the need for a more careful evaluation of the impacts of TCDD exposure on the testis, sperm count, sperm motility and male spawning behavior.
While direct impacts of TCDD on gonad development, egg production and fertilization success have obvious impacts on reproductive capacity, it is also important to consider the effects of TCDD exposure on recruitment of offspring. Not surprisingly, maternal transfer of TCDD to eggs reduces offspring survival, induces the typical signs of larval toxicity (e.g., blue sac syndrome) and reduces recruitment (Cook et al., 2003; King-Heiden et al., 2005; Peterson et al., 1993; Tillitt et al., 2008). However, some effects on offspring recruitment may not be solely related to maternal transfer of TCDD to the eggs and subsequent uptake of TCDD from the eggs by the developing embryos and larvae. This point is illustrated by F1 generation offspring, derived from F0 zebrafish exposed to TCDD during early stages of development, also showing reduced reproductive success and ovarian malformations (King-Heiden et al., 2009). The F1 generation zebrafish were only exposed to TCDD as a germ cell. That is, germ cells giving rise to the F1 generation were directly exposed to TCDD during early development of the parental F0 generation. There was no other exposure of the F1 generation to TCDD. Yet the F1 generation still exhibited reproductive toxicity which demonstrates that their germ cell-only exposure to TCDD was sufficient to cause reproductive toxicity (King-Heiden et al., 2009). The significance of this finding is that it raises the possibility for TCDD reproductive toxicity in fish to be transgenerational in nature. If this hypothesis is supported by future studies, it would mean that the reproductive toxicity caused by transient exposure to TCDD during early development and sexual differentiation might span multiple generations, and if great enough, reduce the size of exposed wild fish populations.
Translating TCDD Toxicity in Zebrafish to Wild Fish Populations
High bioaccumulation of dioxin-like compounds by fish has the potential, if their larvae are sufficiently sensitive to TCDD-induced mortality, to prevent recruitment of offspring into the population (Cook et al., 2003; Tillitt et al., 2008). At lower levels of TCDD exposure than are required to produce blue sac syndrome associated mortality there is the potential for TCDD also to impair survival of adults, by decreasing feeding, predation avoidance and reproductive capacity or by shifting the population dynamics due to a change in sex ratio or effects on parentage (Coe et al., 2008; Kidd et al., 2007; Lange et al., 2008; Nash et al., 2004; Tyler et al., 1998). Evaluating population-level consequences requires careful examination of the effects of sublethal TCDD exposure on development, reproductive capacity and successful recruitment. Such studies are difficult to address in wild fish populations due to complexities of the ecosystem and inability to control multiple and concurrent influences that act upon feral fish populations.
Since multi-generation studies in wild fish populations are costly and pose numerous technical difficulties, laboratory studies using a small fish model such as TCDD-exposed zebrafish can provide the framework for predicting risk to TCDD-exposed feral fish populations. Dioxin developmental toxicity in zebrafish larvae has been well characterized and is consistent with endpoints of toxicity observed in several wild fish species, substantiating its use as a model organism for assessing the impact of environmental toxicant exposures on feral fish populations. Recent work describing the territorial and competitive breeding behavior of zebrafish in relation to reproductive success, along with zebrafish being group spawners which is the most common reproductive strategy in fish, makes them ideal for laboratory-based reproductive and developmental toxicity studies. The use of TCDD-exposed zebrafish for life-cycle and transgenerational studies has recently been demonstrated (King-Heiden et al., 2009). Those results support the use of zebrafish for evaluating population relevant effects of TCDD-like chemical exposures in wild fish and their offspring, as has been done for estrogenic compounds (Coe et al., 2008; Kidd et al., 2007).
While zebrafish allow us to gain insight into endpoints and mechanisms of TCDD toxicity in fish, as with any laboratory fish model, it also has limitations when it comes to translating risk to feral fish populations. Population responses to pollution are highly dependent upon life history traits. Iteroparous fish that have short life spans and generation times tend to be less sensitive to chronic stressors than semelparous or seasonal iteroparous fish with longer life spans and moderate to low fecundity (Schaaf et al., 1987; Spromberg and Birge, 2005). Furthermore, while zebrafish are useful for predicting risk to many fish faunas such as the Cyprinidaae and Percidae families, because they share similar “opportunistic” life history strategies, many of the more TCDD sensitive species like the Salmonids tend to be “equilibrium strategists” (Mims et al., 2010), making comparisons and predictions from TCDD toxicity findings in zebrafish more challenging. Standard toxicity assays with zebrafish would benefit from incorporating toxicity effects into a modeling framework such as dynamic energy budget models (Muller et al., 2010) or other individually based population models (Miller et al., 2007; Van Winkle et al., 1993). This would allow toxicologists to go beyond determining the no observable effect concentration (NOEC) for TCDD in a particular fish species to identifying more ecologically-relevant measures that predict population level risks (Congdon et al., 2001; Muller et al., 2010; Spromberg and Birge, 2005). Additionally, cross species comparisons of TCDD reproductive toxicity in zebrafish and other fish species with different modes of reproduction and life history traits will help to identify target organs within the male and female reproductive system that are most susceptible to disruption by TCDD. This should further elevate utility of the zebrafish model in predicting reproductive risk to dioxin-like compounds for feral fish populations with different modes of reproduction.
Evolved Resistance in Wild Fish Populations to the Toxicity of Dioxin-Like Compounds
Long-term exposure to environmental contaminants over multiple generations has the potential to drive local adaptation (Wiens and Graham, 2005). Therefore it is not surprising that resistance to dioxin toxicity has been observed in Atlantic killifish, Fundulus heteroclitus, from three Atlantic coast estuaries highly contaminated with PCBs (VanVeld and Nacci, 2008) and in Atlantic tomcod from a region of the Hudson River where there is extensive PCB pollution (Wirgin et al., 2011). In most, but not all, of the PCB-exposed killifish populations resistance to PCB126-induced embryo toxicity was heritable between the F1 and F2 generation and was unrelated to altered expression of AHR signaling pathway components (Nacci et al., 2010; Powell et al., 2000). Greater insight into the mechanistic basis of resistance to the dioxin-like PCBs was obtained in Hudson River tomcod where variants in AHR2 were observed that were nearly absent in tomcod elsewhere (Wirgin et al., 2011). AHR2-1, common in resistant Hudson River tomcod, was impaired in both TCDD binding ability and in driving expression in reporter gene assays (compared to the more prevalent AHR2-2). Since affinity of AHR2-1 for TCDD in Hudson River tomcod was five times lower than that of AHR2-2, it is considered the primary mechanism for evolved resistance to dioxin-like chemicals in this highly PCB-contaminated species. The reduced AHR2-1 binding affinity for TCDD in resistant tomcod was associated with a two-amino acid deletion outside of the receptor’s ligand binding domain at the site of interaction with the chaperone protein, AIP/ARA9/XAP2, which stabilizes AHR enhancing its ability to bind ligand and activate transcription (Wirgin et al., 2011). Accordingly it is possible that the two-amino acid deletion altered the conformation of AHR2-1 so dioxin-like AHR agonists had reduced access to the ligand binding domain and/or stability of AHR2-1 may have been altered. Either effect could contribute to the resistant phenotype. Thus, from the killifish and tomcod examples above, it appears that multiple mechanisms of resistance to dioxin-like compounds exist in fish, both AHR-dependent and AHR-independent. Further work is needed to identify key molecular targets, in addition to AHR2, that mediate the multiple mechanisms of resistance to AHR agonist toxicity in fish (Whitehead et al., 2010; 2011).
TCDD Effects on the Zebrafish Transcriptome
As appreciation of the biological complexity of dioxin toxicity has grown one is forced to acknowledge the futility of trying to understand the AHR signaling pathway as a linear process. In truth, the AHR is just one node in a vast, interconnected and self-supporting network whose interactions at any given time define the state of the cell. How AHR activation by dioxin-like compounds perturbs this state is dependent upon the initial conditions at the time of exposure, which is to say that AHR signaling and toxicity can vary depending upon developmental time and the specific cell or tissue being examined. Fortunately, technology is allowing us to delve more broadly and deeply into this complexity. Much of the recent knowledge that has been discovered in zebrafish linking endpoints of TCDD toxicity to specific gene targets has been obtained using a transcriptomic approach, which provides a snapshot of the transcriptional changes following TCDD exposure. The first use of this approach to explore TCDD inducible target genes in zebrafish was by Stegeman and coworkers (Handley-Goldstone et al., 2005). At the time, methods to extract embryonic zebrafish hearts (Burns et al., 2005) were not available. To circumvent this limitation and still probe for cardiac specific effects, arrays were synthesized using a heart specific cDNA library. This method identified several categories of transcripts disrupted by TCDD consistent with the cardiac phenotype, providing targets for further exploration that would not have been achievable otherwise. Since then, a number of labs have utilized the transcriptomics approach to interrogate multiple tissues at different times to explore various endpoints of TCDD toxicity in zebrafish including heart malformations (Carney et al., 2006), jaw defects (Planchart et al., 2010; Xiong et al., 2008) and regeneration defects (Andreasen et al., 2006). The work on the TCDD inducible jaw and regeneration defects identified gene targets that can be directly manipulated to rescue their respective toxic endpoints (Mathew et al., 2008; Xiong et al., 2008).
The commercial availability of zebrafish microarrays have made the technique more accessible and practical for labs to perform; however the approach has been hampered by differences and limitations in coverage of the zebrafish genome, which means that important AHR gene targets have likely been missed along the way. With the advent of deep sequencing technology, isolated RNA can be converted into cDNA and directly sequenced then mapped to the genome to provide a transcriptional readout in a process known as RNA-Seq (Wang et al., 2009). This approach does away with the need for specific probe based arrays and has already been applied to zebrafish embryos (Zheng et al., 2011). Work in other systems have applied the technique to single cells (Tang et al., 2009), which may prove useful in dissecting cell specific interactions and mechanisms of TCDD toxicity in complex multi-cellular tissues, or homogenous tissues exposed to morphogenetic gradients.
Given the inherent complexity of developmental processes within particular organs and across the organism as a whole, some endpoints of dioxin toxicity may not be traceable to a specific gene and may instead involve the interplay of several factors. (Carney et al., 2006; Waits and Nebert, 2011). However, as transcriptomic approaches have matured so have the tools for data analysis. These tools can give added dimension to transcriptomic data and place the results in context with gene and protein interaction networks, which may provide insight into potential epistatic effects. In addition, these interactome maps might provide insight into similarities and differences in endpoints of TCDD toxicity between tissues and across species. Several of these tools were utilized in a recent analysis of transcriptomic data following dioxin exposure in zebrafish with interesting results that demonstrate the power of this approach (Alexeyenko et al., 2010).
Relevance of TCDD Toxicity in Zebrafish to Human Health
Several reviews highlight the usefulness of zebrafish as a model for developmental biology and toxicology research (Ankley and Johnson, 2004; Carney et al., 2006b; Carvan et al., 2005; Hill et al., 2005; McGonnell and Fowkes, 2006; Spitsbergen and Kent, 2003; Teraoka et al., 2003). The zebrafish has also been used as a model for human diseases such as DiGeorge syndrome (Piotrowski et al., 2003), hepatoerythropoietic porphyria (Wang et al., 1998), erythropoietic protoporphyria (Childs et al., 2000), cardiovascular disease (Chan and Mably, 2011) and diabetes (Kinkel and Prince, 2009) and has emerged as the premier model for screening chemical libraries to identify compounds that suppress a particular disease phenotype associated with a known human disease (Burns et al., 2005; Margolis and Plowman, 2004; Peterson et al., 2000).
By using zebrafish, great strides have been made in understanding TCDD reproductive and developmental toxicity (Tanguay et al., 2003; Carney et al., 2006b; Hill et al., 2005). Some of the endpoints of TCDD toxicity such as alterations in heart and craniofacial development also have been observed in mammals (Allen and Leamy, 2001; Ikeda et al., 2000; Ivnitski et al., 2001; Keller et al., 2007; Thackaberry et al., 2005a, 2005b;) and may have implications for human health. The adverse effects of TCDD and PCB 126 on zebrafish larval heart development resemble hypoplastic left heart failure in human newborns (Grimes et al., 2008). Humans living near inland waters contaminated with HAHs and other contaminants exhibit a greater risk for heart related defects such as valvular stenosis along with other cardiac malformations (Goldberg et al., 1990). More recently a study identified a region of Baltimore where an increased frequency of hypoplastic left heart syndrome is correlated with the industrial release of solvents, dioxins and PCBs (Kuehl and Loffredo, 2006). Further analysis of TCDD-induced cardiotoxicity in zebrafish is certain to yield more insight into the genes and signaling pathways underlying this developmental defect. And, while further research is required, the association of downregulation of Sox9b with jaw malformation in TCDD-exposed zebrafish may also prove useful in understanding the skeletal abnormalities associated with campomelic dysplasia in humans with sox9 deficiency (Foster et al., 1994; Wagner et al., 1994).
Conclusion
Although there is still much to be learned, our knowledge of dioxin toxicity and AHR signaling pathways has been enriched by the examination of small fish species, particularly zebrafish. In addition to addressing questions concerning the toxic effects of TCDD, the zebrafish model promises to offer insights into the endogenous function of the AHR protein that are relevant to feral fish and human health. Demonstrating and understanding mechanisms of AHR-mediated toxicity that are common between humans and fish and wildlife are necessary if we are to integrate findings from laboratory and ecotoxicology studies with human health risk assessment (Carvan et al., 2008; Cook et al., 2003; Hinton et al., 2005; Tillitt et al., 2008).
By understanding the mode of action of dioxin-like chemicals in greater depth, we may be able to develop biomarkers for specific adverse effects that can be used for both ecotoxicological and human health risk assessment because of the high degree of evolutionary conservation among vertebrates. Fortunately, transcriptome analysis and interactome mapping (Alexeyenko et al., 2010) along with new methodologies in zebrafish such as ChIP-chip (Wardle et al., 2006), more rapid transgenic construction techniques (Kwan et al., 2007), RNA-Seq (Wang et al., 2009), genome-wide quantitative trait loci (QTL) analysis (Waits and Nebert, 2011) and advances in morpholino technology (Shestopalov et al., 2007) can be expected to further accelerate knowledge of AHR biology, including the identification of new transcriptional targets and mechanisms underlying the diverse endpoints of TCDD toxicity (Alexeyenko et al., 2010; Waits and Nebert, 2011; Yoshioka et al., 2011).
Highlights.
TCDD causes “blue sac” toxicity in fish larvae that culminates in death
“Blue sac” consists of yolk sac edema, jaw and heart malformation and heart failure
TCDD toxicity is mediated by AHR signaling-induced changes in gene expression
Downregulation of Sox9b in the jaw is required for TCDD-induced jaw malformation
Developmental exposure causes reproductive toxicity in adults and the next generation
Acknowledgements
This work was supported by NIH grant R01-ES-012716 from the NIEHS (W. Heideman and R.E. Peterson); University of Wisconsin Sea Grant Institute, National Sea Grant College Program, NOAA, Sea Grant Projects: R/BT-12, Award NA86RG00477; R/BT-14, −16 and −17, Award NA16RG2257; R/BT-20, −22 and −25 Award NA100AR4170070 (W. Heideman and R.E. Peterson); Molecular and Environmental Toxicology Predoctoral and Postdoctoral Training Grant (T32 ES007015) from NIEHS (K. Lanham and T. King-Heiden); NRSA (F32 ES016714-01) from NIEHS (T. King-Heiden) and University of Wisconsin-La Crosse River Studies Center (T. King-Heiden and A. Ganser). Portions of this review were presented by R.E. Peterson at an African Conference, “Reproduction and the Environment,” Nairobi, Kenya, East Africa, March 13–20, 2011. We thank Ms. Dorothy Nesbit for expert technical assistance.
Abbreviations
- HAHs
halogenated aromatic hydrocarbons
- PCBs
polychlorinated biphenyls
- PCDDs
polychlorinated dibenzo-p-dioxins
- PCDFs
polychlorinated dibenzofurans
- DDE
1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene
- AHR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- TCDD or dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin
- CYP1A1
cytochrome P450 1A1
- PAS
Per-Arnt-Sim
- HSP90
heat shock protein 90
- AIP, ARA9 or XAP2
AHR interacting protein
- AHRE
AHR response element
- XRE
xenobiotic response element
- DRE
dioxin response element
- AHRR
AHR repressor
- CCGC
cell cycle gene cluster
- EDC
endocrine disrupting compound
- AV
atrio-ventricular
- BV
bulbo-ventricular
- hpf
hours post fertilization
- TEC
TCDD toxicity equivalence concentration
- TEF
toxicity equivalence factor
- BSAF
biota-sediment accumulation factor
- COX-2
cyclooxygenase 2
- PCB 126
3,3’,4,4’,5-pentachlorobiphenyl
- NOEC
no observable effect concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In this review the same nomenclature is used for both fish and mammals in naming genes, mRNAs, and proteins (ahr, gene; Ahr, mRNA; and AHR, protein).
References
- Abnet CC, Tanguay RL, Hahn ME, Heideman W, Peterson RE. Two forms of aryl hydrocarbon receptor type 2 in rainbow trout (Oncorhynchus mykiss). Evidence for differential expression and enhancer specificity. J. Biol. Chem. 1999;274:15159–15166. doi: 10.1074/jbc.274.21.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeyenko A, Wassenberg DM, Lobenhofer EK, Yen J, Linney E, Sonnhammer EL, Meyer JN. Dynamic zebrafish interactome reveals transcriptional mechanisms of dioxin toxicity. PLoS One. 2010;5:e10465. doi: 10.1371/journal.pone.0010465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DE, Leamy LJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects size and shape, but not asymmetry, of mandibles in mice. Ecotoxicol. 2001;10:167–176. doi: 10.1023/a:1016693911300. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. Thezebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol. Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Mathew LK, Tanguay RL. Regenerative growth is impacted by TCDD: gene expression analysis reveals extracellular matrix modulation. Toxicol. Sci. 2006;92:254–269. doi: 10.1093/toxsci/kfj118. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bencic DC, Breen MS, Collette TW, Conolly RB, Denslow ND, Edwards SW, Ekman DR, Garcia-Reyero N, Jensen KM, Lazorchak JM, Martinovic D, Miller DH, Perkins EJ, Orlando EF, Villeneuve DL, Wang RL, Watanabe KH. Endocrine disrupting chemicals in fish: developing exposure indicators and predictive models of effects based on mechanism of action. Aquat. Toxicol. 2009;92:168–178. doi: 10.1016/j.aquatox.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Johnson RD. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004;45:469–483. doi: 10.1093/ilar.45.4.469. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Balon EK. Early ontogeny of the lake charr, Salvelinus (Christivomer) namaycush. In: Balon EK, editor. Charrs: Salmonid Fishes of the Genus Salvelinus. The Hague, The Netherlands: Dr. W. Junk Publishers; 1980. pp. 485–562. [Google Scholar]
- Baumann PC, Whittle DM. The status of selected organics in the Laurentian Great Lakes: an overview of DDT, PCBs, dioxins, furans, and aromatic hydrocarbons. Aquat. Toxicol. 1988;11:241–257. [Google Scholar]
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- Bigsby R, Chapin RE, Daston GP, Davis BJ, Gorski J, Gray LE, Howdeshell KL, Zoeller RT, vom Saal FS. Evaluating the effects of endocrine disruptors on endocrine function during development. Environ. Health Perspect. 1999;107:613–618. doi: 10.1289/ehp.99107s4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CG, MacRae CA. Purification of hearts from zebrafish embryos.. [published erratum appears in Biotechniques 40, 596, 2006] Biotechniques. 2006;40 274, 276, 278. [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Joy-Schlezinger J, Stegeman JJ, Tillitt DE, Hannink M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol. Appl. Pharmacol. 1998;148:24–34. doi: 10.1006/taap.1997.8309. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD): the embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in Medaka (Orizias latipes) Toxicol. Appl. Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006a;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A–independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A. Clin. Mol. Teratol. 2006b;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Carvalho PS, Tillitt DE. 2,3,7,8-TCDD effects on visual structure and function in swim-up rainbow trout. Environ. Sci. Technol. 2004;38:6300–6306. doi: 10.1021/es034857i. [DOI] [PubMed] [Google Scholar]
- Carvalho PS, Noltie DB, Tillitt DE. Intra-strain dioxin sensitivity and morphometric effects in swim-up rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2004;137:133–142. doi: 10.1016/j.cca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, Incardona JP, Rise ML. Meeting the challenges of aquatic vertebrate ecotoxicology. Biosci. 2008;58:1015–1025. [Google Scholar]
- Carvan MJ, King Heiden TC, Tomasiawicz H, Mommsen TP, Moon TW. The utility of zebrafish as a model for toxicological research. In: Mommsen TP, Moon TP, editors. Biochemistry and Molecular Biology of Fishes. vol. 6. The Netherlands: Elsevier; 2005. pp. 3–41. [Google Scholar]
- Chan J, Mably JD. Dissection of cardiovascular development and disease pathways in zebrafish. Prog. Mol. Biol. Transl. Sci. 2011;100:111–1153. doi: 10.1016/B978-0-12-384878-9.00004-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Carney SA, Peterson RE, Heideman W. Comparative genomics identifies genes mediating cardiotoxicity in the embryonic zebrafish heart. Physiol. Genomics. 2008;33:148–158. doi: 10.1152/physiolgenomics.00214.2007. [DOI] [PubMed] [Google Scholar]
- Childs S, Weinstein BM, Mohideen MA, Donohue S, Bonkovsky H, Fishman MC. Zebrafish dracula encodes ferrochelatase and its mutation provides a model for erythropoietic protoporphyria. Curr. Biol. 2000;10:1001–1004. doi: 10.1016/s0960-9822(00)00653-9. [DOI] [PubMed] [Google Scholar]
- Cho YC, Zheng W, Jefcoate CR. Disruption of cell-cell contact maximally but transiently activates AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol. Appl. Pharmacol. 2004;199:220–238. doi: 10.1016/j.taap.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Clark JR, Devault D, Weishaar JA, Bowden RJ. Contaminant analysis of fillets from Great Lakes Coho Salmon. J. Great Lakes Res. 1984;10:38–47. [Google Scholar]
- Coe TS, Hamilton PB, Hodgson D, Paull GC, Stevens JR, Sumner K, Tyler CR. An environmental estrogen alters reproductive hierarchies, disrupting sexual selection in group-spawning fish. Environ. Sci. Technol. 2008;42:5020–5025. doi: 10.1021/es800277q. [DOI] [PubMed] [Google Scholar]
- Congdon JD, Dunham AE, Hopkins WA, Rowe CL, Hinton TG. Resource allocation-based life histories: a conceptual basis for studies of ecological toxicology. Environ. Toxicol. Chem. 2001;20:1698–1703. [PubMed] [Google Scholar]
- Cook P, Hornung MW, Fredenberg W, Lawonn M, Loeffler I, Peterson R. Vulnerability of bull trout to early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other AhR agonists. SETAC 21st Annual Meeting, Abstract Book, Abstract. 2000;158:38. [Google Scholar]
- Cook PM, Robbins JA, Endicott DD, Lodge KB, Guiney PD, Walker MK, Zabel EW, Peterson RE. Effects of aryl hydrocarbon receptor-mediated early life stage toxicity on lake trout populations in Lake Ontario during the 20th century. Environ. Sci. Technol. 2003;37:3864–3877. doi: 10.1021/es034045m. [DOI] [PubMed] [Google Scholar]
- Cook PM, Erickson RJ, Spehar RL, Bradbury SP, Ankley GT. Interim report on data and methods for assessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin risks to aquatic life and associated wildlife, EPA/600/R-93/055. Duluth, MN: Office of Research and Development; 1993. pp. 1–167. [Google Scholar]
- Cowan JJH, Shaw RF. Recruitment. In: Fuiman LA, Werner RG, editors. Fisheries Science: The unique contributions of early life stages. Ames, IA: Blackwell Science, Ltd; 2002. pp. 88–111. [Google Scholar]
- Curwen V, Eyras E, Andrews TD, Clarke L, Mongin E, Searle SM, Clamp M. The Ensembl automatic gene annotation system. Genome Res. 2004;14:942–950. doi: 10.1101/gr.1858004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault D, Dunn W, Bergqvist P, Wiberg K, Rappe C. Polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxins in Great Lakes fish: a baseline and interlake comparison. Environ. Sci. Technol. 1989;8:1013–1022. [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga H. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dong W, Matsumura F, Kullman SW. TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias latipes) embryos. Toxicol. Sci. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Elonen GE, Spehar RL, Holcombe GW, Johnson RD, Fernandez JD, Erickson RJ, Tietge JE, Cook PM. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life stage development. Environ. Toxicol. Chem. 1998;17:472–483. [Google Scholar]
- Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: structure, function, evolution, and AHR-dependent regulation in vivo. Arch. Biochem. Biophys. 2005;441:151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Goodfellow J, David B, Alan JS. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8- tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol. Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 1998;273:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Jones PD, Kannan K, Newsted JL, Tillitt DE, Williams LL. Effects of chronic dietary exposure to environmentally relevant concentrations to 2,3,7,8-tetrachlorodibenzo-p-dioxin on survival, growth, reproduction and biochemical responses of female rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2002;59:35–53. doi: 10.1016/s0166-445x(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Gilbertson M, Kubiak T, Ludwig J, Fox G. Great Lakes embryo mortality, edema, and deformities syndrome (GLEMEDS) in colonial fish-eating birds: similarity to chick-edema disease. J. Toxicol. Environ. Health. 1991;33:455–520. doi: 10.1080/15287399109531538. [DOI] [PubMed] [Google Scholar]
- Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. The new vertebrate CYP1C family: cloning of new subfamily members and phylogenetic analysis. Biochem. Biophys. Res. Commun. 2005;331:1016–1024. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Lebowitz MD, Graver EJ, Hicks S. An association of human congenital cardiac malformations and drinking water contaminants. J. Am. Coll. Cardiol. 1990;16:155–164. doi: 10.1016/0735-1097(90)90473-3. [DOI] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai H-J, Kirby ML. PCB 126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol. Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish - emergence of a new model vertebrate. Nat. Rev. Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Crain DA, Rooney AA, Pickford DB. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect. 1995;103:157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinand B, Scribner KT, Page KS, Burnham-Curtis MK. Genetic variation over space and time: analyses of extinct and remnant lake trout populations in the Upper Great Lakes. Proc. Biol. Sci. 2003;270:425–433. doi: 10.1098/rspb.2002.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol. Appl. Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Guiney PD, Walker MK, Spitsbergen JM, Peterson RE. Hemodynamic dysfunction and cytochrome P4501A mRNA expression induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin during embryonic stages of lake trout development. Toxicol. Appl. Pharmacol. 2000;168:1–14. doi: 10.1006/taap.2000.8999. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem. Biol. Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Stegeman JJ. Regulation of cytochrome P4501A1 in teleosts: sustained induction of CYP1A1 mRNA, protein, and catalytic activity by 2,3,7,8-tetrachlorodibenzofuran in the marine fish Stenotomus chrysops. Toxicol. Appl. Pharmacol. 1994;127:187–198. doi: 10.1006/taap.1994.1153. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Woodin BR, Stegeman JJ, Tillitt DE. Aryl hydrocarbon receptor function in early vertebrates: inducibility of cytochrome P450 1A in agnathan and elasmobranch fish. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1998;120:67–75. doi: 10.1016/s0742-8413(98)00007-3. [DOI] [PubMed] [Google Scholar]
- Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol. Sci. 2005;85:683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- Hansson MC, Wittzell H, Persson K, von Schantz T. Unprecedented genomic diversity of AhR1 and AhR2 genes in Atlantic salmon (Salmo salar L.) Aquat. Toxicol. 2004;68:219–232. doi: 10.1016/j.aquatox.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Heideman W, Antkiewicz DS, Carney SA, Peterson RE. Zebrafish and cardiac toxicology. Cardiovasc. Toxicol. 2005;5:203–214. doi: 10.1385/ct:5:2:203. [DOI] [PubMed] [Google Scholar]
- Helder T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on early life stages of the pike (Esox lucius, L.) Sci. Total Environ. 1980;14:255–264. [Google Scholar]
- Helder T. Effects of 2,3,7,8-tetrachlorodibenzo-dioxin (TCDD) on early life stages of rainbow trout (Salmo gairdneri, Richardson) Toxicol. 1981;19:101–112. doi: 10.1016/0300-483x(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hickey JP, Batterman SA, Chernyak SM. Trends of chlorinated organic contaminants in great lakes trout and walleye from 1970 to 1998. Arch. Environ. Contam. Toxicol. 2006;50:97–110. doi: 10.1007/s00244-005-1007-6. [DOI] [PubMed] [Google Scholar]
- Hill A, Howard V, Cossins A. Characterization of TCDD-induced craniofacial malformations and retardation of zebrafish growth. J. Fish Biol. 2004;64:911–922. [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hinton DE, Kullman SW, Hardman RC, Volz DC, Chen PJ, Carney M, Bencic DC. Resolving mechanisms of toxicity while pursuing ecotoxicological relevance? Mar. Pollut. Bull. 2005;51:635–648. doi: 10.1016/j.marpolbul.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol. Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Zabel EW, Peterson RE. Additive interactions between pairs of polybrominated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners in a rainbow trout early life stage mortality bioassay. Toxicol. Appl. Pharmacol. 1996a;140:345–355. doi: 10.1006/taap.1996.0230. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Zabel EW, Peterson RE. Toxic equivalency factors of polybrominated dibenzo-p-dioxin, dibenzofuran, biphenyl, and polyhalogenated diphenyl ether congeners based on rainbow trout early life stage mortality. Toxicol Appl. Pharmacol. 1996b;140:227–234. doi: 10.1006/taap.1996.0217. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Wimpee BAB, Dasmahapatra A, Weber DN, Heimler I, Chaffin CL. Differential modulation by aromatic hydrocarbon receptor agonist of circulating estradiol-17beta and estrogen-receptor DNA-binding capability in female rainbow trout (Oncorhynchus mykiss) Zool. Sci. 1999;16:161–166. [Google Scholar]
- Hutz RJ, Carvan MJ, Baldridge MG, Conley LK, King-Heiden TC. Environmental toxicants and effects on female reproductive function. Trends Repro. Biol. 2006;2:1–11. doi: 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ishii Y, Kato H, Akazawa D, Hatsumura M, Ishida T, Matsusue K, Yamada H, Oguri K. Suppression of carbonic anhydrase III in rat liver by a dioxin-related toxic compound, coplanar polychlorinated biphenyl, 3, 3’,4,4’,5-pentachlorobiphenyl. Arch. Biochem. Biophys. 2000;380:159–164. doi: 10.1006/abbi.2000.1911. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Kobayashi Y, Kawajiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J. Biol. Chem. 2004;279:19209–19216. doi: 10.1074/jbc.M310492200. [DOI] [PubMed] [Google Scholar]
- Ivnitski I, Elmaoued R, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratol. 2001;64:201–212. doi: 10.1002/tera.1065. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Tietge JE, Jensen KM, Fernandez JD, Linnum AL, Lothenbach DB, Holcombe GW, Cook PM, Christ SA, Lattier DL, Gordon DA. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to early life stage brook trout (Salvelinus fontinalis) following parental dietary exposure. Environ. Toxicol. Chem. 1998;17:2408–2421. [Google Scholar]
- Jones PD, Giesy JP, Newsted JL, Verbrugge DA, Ludwig JP, Ludwig ME, Auman HJ, Crawford R, Tillitt DE, Kubiak TJ, Best DA. Accumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents by double-crested cormorant (Phalacrocorax auritus, Pelicaniformes) chicks in the North American Great Lakes. Ecotoxicol. Environ. S af. 1994;27:192–209. doi: 10.1006/eesa.1994.1016. [DOI] [PubMed] [Google Scholar]
- Jones PD, Kannan K, Newsted JL, Tillitt DE, Williams LL, Giesy JP. Accumulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin by rainbow trout (Onchorhynchus mykiss) at environmentally relevant dietary concentrations. Environ. Toxicol. Chem. 2001;20:344–350. [PubMed] [Google Scholar]
- Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3’,4,4’,5-pentachlorobiphenyl-induced expression of cytochrome P450 1A, 1B and 1C genes in zebrafish. Toxicol. Appl. Pharmacol. 2007;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Cho CH, Hu Y, Li R, Bae I. BRCA1 transcriptional activity is enhanced by interactions between its AD1 domain and AhR. Cancer Chemother. Pharmacol. 2008;62:965–975. doi: 10.1007/s00280-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J. Biol. Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus. Evidence for a novel subfamily of ligand-binding basic helix loop helix-Per-ARNT-Sim (bHLH-PAS) factors. J. Biol. Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transposon tools and methods in zebrafish. Dev. Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Yamashita I. Aryl hydrocarbon receptor is required for prevention of blood clotting and for the development of vasculature and bone in the embryos of medaka fish, Oryzias latipes. Zoolog. Sci. 2002;19:309–319. doi: 10.2108/zsj.19.309. [DOI] [PubMed] [Google Scholar]
- Keller JM, Allen DE, Davis CR, Leamy LJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects fluctuating asymmetry of molar shape in mice, and an epistatic interaction of two genes for molar size. Heredity. 2007;98:259–267. doi: 10.1038/sj.hdy.6800928. [DOI] [PubMed] [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–240. [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden T, Carvan MJ, Hutz RJ., III Inhibition of follicular development, vitellogenesis, and serum 17β-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;90:490–499. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- King-Heiden T, Hutz RJ, Carvan MJ., III Accumulation, tissue distribution, and maternal transfer of dietary 2,3,7,8-tetrachlorodibenzo-p-dioxin: impacts on reproductive success of zebrafish. Toxicol. Sci. 2005;87:497–507. doi: 10.1093/toxsci/kfi201. [DOI] [PubMed] [Google Scholar]
- King-Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol. Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., III Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: Insights into TCDD-induced endocrine disruption and reproductive toxicity. Repro. Toxicol. 2008;25:47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman JM, Olson JR, Peterson RE. Species differences in 2,3,7,8- tetrachlorodibenzo-p-dioxin toxicity and biotransformation in fish. Fundam. Appl. Toxicol. 1988;10:206–213. doi: 10.1016/0272-0590(88)90304-1. [DOI] [PubMed] [Google Scholar]
- Kleeman JM, Olson JR, Chen SM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin metabolism and disposition in yellow perch. Toxicol. Appl. Pharmacol. 1986;83:402–411. doi: 10.1016/0041-008x(86)90222-x. [DOI] [PubMed] [Google Scholar]
- Kleeman JM, Moore RW, Peterson RE. Inhibition of testicular steroidogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: evidence that the key lesion occurs prior to or during pregnenolone formation. Toxicol. Appl. Pharmacol. 1990;106:112–125. doi: 10.1016/0041-008x(90)90111-7. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Weiss C, Koff A, Gottlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA, Sileo L, Docherty DE, Erdman TC. Microcontaminants and reproductive impairment of the Forster’s tern on Green Bay, Lake Michigan--1983. Arch. Environ. Contam. Toxicol. 1989;18:706–727. doi: 10.1007/BF01225009. [DOI] [PubMed] [Google Scholar]
- Kubota A, Stegeman JJ, Woodin BR, Iwanaga T, Harano R, Peterson RE, Hiraga T, Teraoka H. Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl Pharmacol. 2011;253:244–252. doi: 10.1016/j.taap.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl KS, Loffredo CA. A cluster of hypoplastic left heart malformation in Baltimore, Maryland. Pediatr. Cardiol. 2006;27:25–31. doi: 10.1007/s00246-005-0859-x. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Laan M, Richmond H, He C, Campbell RK. Zebrafish as a model for vertebrate reproduction: characterization of the first functional zebrafish (Danio rerio) gonadotropin receptor. Gen. Comp. Endocrinol. 2002;125:349–364. doi: 10.1006/gcen.2001.7738. [DOI] [PubMed] [Google Scholar]
- Lange A, Katsu Y, Ichikawa R, Paull GC, Chidgey LL, Coe TS, Iguchi T, Tyler CR. Altered sexual development in roach (Rutilus rutilus) exposed to environmental concentrations of the pharmaceutical 17alpha-ethinylestradiol and associated expression dynamics of aromatases and estrogen receptors. Toxicol. Sci. 2008;106:113–123. doi: 10.1093/toxsci/kfn151. [DOI] [PubMed] [Google Scholar]
- Lanham KA, Peterson RE, Heideman W. Sensitivity to dioxin decreases as zebrafish mature. Toxicol. Sci. 2011 doi: 10.1093/toxsci/kfs103. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MC, Cardoso ER, Nobrega RH, Batlouni SR, Bogerd J, Franca LR, Schulz RW. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 2009;81:177–187. doi: 10.1095/biolreprod.109.076299. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Maack L, Sonzogni WC. Analysis of polychlorobiphenyl congeners in Wisconsin fish. Arch. Environ. Contam. Toxicol. 1988;17:711–719. doi: 10.1007/BF01061977. [DOI] [PubMed] [Google Scholar]
- Margolis J, Plowman GD. Overcoming the gridlock in discovery research. Nat. Biotechnol. 2004;22:522–524. doi: 10.1038/nbt0504-522. [DOI] [PubMed] [Google Scholar]
- Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F–dependent promoters and represses S phase-specific gene expression. J. Biol. Chem. 2004;279:29013–29022. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- Mathew LK, Sengupta SS, Ladu J, Andreasen EA, Tanguay RL. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 2008;22:3087–3096. doi: 10.1096/fj.08-109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM, Fowkes RC. Fishing for gene function - endocrine modeling in the zebrafish. J. Endocrinol. 2006;189:425–439. doi: 10.1677/joe.1.06683. [DOI] [PubMed] [Google Scholar]
- Mehta V, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicol. Sci. 2008;104:303–311. doi: 10.1093/toxsci/kfn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Jensen KM, Villeneuve DL, Kahl MD, Makynen EA, Durhan EJ, Ankley GT. Linkage of biochemical responses to population-level effects: a case study with vitelogenin in the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 2007;26:521–527. doi: 10.1897/06-318r.1. [DOI] [PubMed] [Google Scholar]
- Mims MC, Olden JD, Shattuck ZR, Poff NL. Life history trait diversity of native freshwater fishes of North America. Ecol. Freshwater Fish. 2010;19:390–400. [Google Scholar]
- Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol. Sci. 2007;95:172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- Muller EB, Nisbet RM, Berkley HA. Sublethal toxicant effects with dynamic energy budget theory: model formation. Ecotoxicol. 2010;19:48–60. doi: 10.1007/s10646-009-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins MC, Nusslein-Volhard C. Mutational approaches to studying embryonic pattern formation in the zebrafish. Curr. Opin. Genet. Dev. 1993;3:648–654. doi: 10.1016/0959-437x(93)90102-u. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, McMaster ME, McCarthy LH, Servos MR, Van der Kraak GJ. An overview of recent studies on the potential of pulp-mill effluents to alter reproductive parameters in fish. J. Toxicol. Environ. Health B Crit. Rev. 1998;1:347–371. doi: 10.1080/10937409809524558. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, Portt CB, Van der Kraak GJ, Smith IR, Rokosh DA. Impact of bleached kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni) population. Can. J. Fish. Aquat. Sci. 1991;48:1371–1380. [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S. Adaptation of the estuarine fish (Atlantic killifish) to polychlorinated biphenyls (PCBs) Estuaries and Coasts. 2010;33:853–864. [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect. 2004;112:1725–1733. doi: 10.1289/ehp.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Ordas A, Hegedus Z, Henkel CV, Stockhammer OW, Butler D, Jansen HJ, Racz P, Mink M, Spaink HP, Meijer AH. Deep sequencing of the innate immune transcriptomic response of zebrafish embryos to Salmonella infection. Fish Shellfish Immunol. 2010 doi: 10.1016/j.fsi.2010.08.022. e-pub ahead of print, [DOI] [PubMed] [Google Scholar]
- Ortiz-Delgado JB, Sarasquete C. Toxicity, histopathological alterations and immunohistochemical CYP1A induction in the early life stages of the seabream, Sparus aurata, following waterborne exposure to B(a)P and TCDD. J. Mol. Histol. 2004;35:29–45. doi: 10.1023/b:hijo.0000020952.20386.16. [DOI] [PubMed] [Google Scholar]
- Palstra AP, van Ginneken VJ, Murk AJ, van den Thillart GE. Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften. 2006;93:145–148. doi: 10.1007/s00114-005-0080-z. [DOI] [PubMed] [Google Scholar]
- Pande K, Moran SM, Bradfield CA. Aspects of dioxin toxicity are mediated by interleukin 1 -like cytokines. Mol. Pharmacol. 2005;67:1393–1398. doi: 10.1124/mol.105.010983. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–5052. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Planchart A, Mattingly CJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin upregulates FoxQ1b in zebrafish jaw primordium. Chem. Res. Toxicol. 2010;23:480–487. doi: 10.1021/tx9003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollenz RS, Sullivan HR, Holmes J, Necela B, Peterson RE. Isolation and expression of cDNAs from rainbow trout (Oncorhynchus mykiss) that encode two novel basic helix-loop-Helix/PER-ARNT-SIM (bHLH/PAS) proteins with distinct functions in the presence of the aryl hydrocarbon receptor. Evidence for alternative mRNA splicing and dominant negative activity in the bHLH/PAS family. J. Biol. Chem. 1996;271:30886–30896. doi: 10.1074/jbc.271.48.30886. [DOI] [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue specific expression of AHR1, AHR2 and ARNT2 in dioxin-sensitive and -resistant populations of the marine fish, Fundulus heteroclitus. Toxicol. Sci. 57:229. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Heideman W, Peterson RE. ARNT2 is not required for TCDD developmental toxicity in zebrafish. Toxicol. Sci. 2004;82:250–258. doi: 10.1093/toxsci/kfh235. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Puga A, Barnes SJ, Chang C, Zhu H, Nephew KP, Khan SA, Shertzer HG. Activation of transcription factors activator protein-1 and nuclear factor-kappaB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem. Pharmacol. 2000a;59:997–1005. doi: 10.1016/s0006-2952(99)00406-2. [DOI] [PubMed] [Google Scholar]
- Puga A, Maier A, Medvedovic M. The transcriptional signature of dioxin in human hepatoma HepG2 cells. Biochem. Pharmacol. 2000b;60:1129–1142. doi: 10.1016/s0006-2952(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ, Komai M. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008;229:10–19. doi: 10.1016/j.taap.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Schaaf WE, Peters DS, Vaugan DS, Coston-Clements L, Krouse CW. Fish population responses to chronic and acute pollution: the influence of life history strategies. Estuaries. 1987;10:267–275. [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Ann. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segner H. Comment on: Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 2006;40:1084–1085. doi: 10.1021/es051791d. [DOI] [PubMed] [Google Scholar]
- Shestopalov IA, Sinha S, Chen JK. Light-controlled gene silencing in zebrafish embryos. Nat. Chem. Biol. 2007;3:650–651. doi: 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- Simonin H. International Joint Commission. Windsor, Ontario, Canada: 1990. Summary of reproductive studies in Lake Ontario salmonids, Roundtable on: Contaminant-caused reproductive problems in salmonids. [Google Scholar]
- Spitsbergen J, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2, 3, 7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1991;19:41–72. [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research - advantages and current limitations. Toxicol. Pathol. 2003;31:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Kleeman JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in yellow perch (Perca flavescens) J. Toxicol. Environ. Health. 1988a;23:359–383. doi: 10.1080/15287398809531120. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kleeman JM, Peterson RE. Morphologic lesions and acute toxicity in rainbow trout (Salmo gairdneri) treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Toxicol. Environ. Health. 1988b;23:333–358. doi: 10.1080/15287398809531119. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Schat KA, Kleeman JM, Peterson RE. Interactions of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with immune responses of rainbow trout. Vet. Immunol. Immunopathol. 1986;12:263–280. doi: 10.1016/0165-2427(86)90130-3. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1991;19:41–72. [Google Scholar]
- Spromberg JA, Birge WJ. Modeling the effects of chronic toxicity on fish populations: the influence of life-history strategies. Environ. Toxicol. Chem. 2005;24:1532–1540. doi: 10.1897/04-160.1. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Hahn ME, Weisbrod R, Woodin BR, Joy JS, Najibi S, Cohen RA. Induction of cytochrome P4501A1 by aryl hydrocarbon receptor agonists in porcine aorta endothelial cells in culture and cytochrome P4501 A1 activity in intact cells. Mol. Pharmacol. 1995;47:296–306. [PubMed] [Google Scholar]
- Symula J, Meade J, Skea JC, Cummings L, Colquhoun JR, Dean HJ, Miccoli J. Blue-sac disease in Lake Ontario lake trout. J. Great Lakes Res. 1990;16:41–52. [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman ELee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole transcriptome analysis of a single cell. Nat Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Abnet CC, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim. Biophys. Acta. 1999;1444:35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Andreasen E, Heideman W, Peterson RE. Identification and expression of alternatively spliced aryl hydrocarbon nuclear translocator 2 (ARNT2) cDNAs from zebrafish with distinct functions. Biochim. Biophys. Acta. 2000;1494:117–128. doi: 10.1016/s0167-4781(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Andreasen E, Walker MK, Peterson RE. Dioxin toxicity and aryl hydrocarbon receptor signaling in fish. In: Schecter A, Gasiewicz TA, editors. Dioxins and Health. New Jersey: John Wiley & Sons; 2003. pp. 603–628. [Google Scholar]
- Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anom. (Kyoto) 2003;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Okuhara Y, Iwasa H, Shindo A, Hill AJ, Kawakami A, Hiraga T. Impairment of lower jaw growth in developing zebrafish exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and reduced hedgehog expression. Aquat. Toxicol. 2006;78:103–113. doi: 10.1016/j.aquatox.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol. Appl. Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Ogawa A, Kubota A, Stegeman JJ, Peterson RE, Hiraga T. Malformation of certain brain blood vessels caused by TCDD activation of Ahr2/Arnt1 signaling in developing zebrafish. Aquat. Toxicol. 2010;99:241–247. doi: 10.1016/j.aquatox.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol. Sci. 2005a;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Nunez BA, Ivnitski-Steele ID, Friggins M, Walker MK. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on murine heart development: alteration in fetal and postnatal cardiac growth, and postnatal cardiac chronotropy. Toxicol. Sci. 2005b;88:242–249. doi: 10.1093/toxsci/kfi302. [DOI] [PubMed] [Google Scholar]
- Thomas P. Teleost model for studying the effects of chemicals on female reproductive endocrine function. J. Exp. Zool. Suppl. 1990;4:126–128. doi: 10.1002/jez.1402560421. [DOI] [PubMed] [Google Scholar]
- Tietge JE, Johnson RD, Jensen KM, Cook PM, Elonen GE, Fernandez JD, Holcombe GW, Lothenbach DB, Nichols JW. Reproductive toxicity and disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin in adult brook trout (Salvelinus fontinalis) following dietary exposure. Environ. Toxicol. Chem. 1998;17:2395–2407. [Google Scholar]
- Tillitt DE, Cook PM, Giesy JP, Heideman W, Peterson RE. Reproductive Impairment of Great Lakes Lake Trout by Dioxin-Like Chemicals. In: Di Giulio RT, Hinton DE, editors. Toxicology of Fishes. Boca Raton, Florida: CRC Press; 2008. pp. 819–875. [Google Scholar]
- Toomey BH, Bello S, Hahn ME, Cantrell S, Wright P, Tillitt DE, Di Giulio RT. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptotic cell death and cytochrome P4501A expression in developing Fundulus heteroclitus embryos. Aquat. Toxicol. 2001;53:127–138. doi: 10.1016/s0166-445x(00)00161-2. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit. Rev. Toxicol. 1998;28:319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- Van der Kraak GJ, Munkittrick KR, McMaster ME, Portt CB, Chang JP. Exposure to bleached kraft pulp mill effluent disrupts the pituitary-gonadal axis of white sucker at multiple sites. Toxicol. Appl. Pharmacol. 1992;115:224–233. doi: 10.1016/0041-008x(92)90327-o. [DOI] [PubMed] [Google Scholar]
- van der Weiden MEJ, Bleumink R, Seinen W, van den Berg M. Concurrence of P4501A induction and toxic effects in the mirror carp (Cyprinus carpio), after administration of a low dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat. Toxicol. 1994;29:147–162. [Google Scholar]
- Van Veld PA, Nacci DF. Toxicity Resistance. In: Di Giulio RT, Hinton DE, editors. Toxicology of Fishes. Boca Raton, Florida: CRC Press; 2008. pp. 597–641. [Google Scholar]
- Van Winkle W, Rose KA, Winemiller KO, DeAngelis DL, Christensen SW. Linking life history theory, environmental setting, and individual-based modeling to compare responses of different species to environmental change. Trans. Amer. Fisheries. Soc. 1993;122:459–466. [Google Scholar]
- Volz DC, Bencic DC, Hinton DE, Law JM, Kullman SW. 2,3,7,8- Tetrachlorodibenzo-p-dioxin (TCDD) induces organ-specific differential gene expression in male Japanese medaka (Oryzias latipes) Toxicol. Sci. 2005;85:572–584. doi: 10.1093/toxsci/kfi109. [DOI] [PubMed] [Google Scholar]
- Volz DC, Hinton DE, Law JM, Kullman SW. Dynamic gene expression changes precede dioxin-induced liver pathogenesis in medaka fish. Toxicol. Sci. 2006;89:524–534. doi: 10.1093/toxsci/kfj033. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Waits ER, Nebert DW. Genetic architecture of susceptibility to PCB126-induced developmental cardiotoxicity in zebrafish. Toxicol. Sci. 2011;122:466–475. doi: 10.1093/toxsci/kfr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Cook PM, Batterman AR, Butterworth BC, Berini C, Libal JJ, Hufnagel LC, Peterson RE. Translocation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) from adult female lake trout (Salvelinus namaycush) to oocytes: effects on early life stage development and sac fry survival. Can. J. Fish. Aquat. Sci. 1994;51:1410–1419. [Google Scholar]
- Walker MK, Hufnagle LC, Clayton MK, Peterson RE. An egg injection method for assessing early life stage mortality of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in rainbow trout (Onchorhynchus mykiss) Aquat. Toxicol. 1992;22:15–38. [Google Scholar]
- Walker MK, Peterson RE. Potencies of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyl congeners, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin, for producing early life stage mortality in rainbow trout, (Oncorhyncus mykiss) Aquatic Toxicol. 1991;21:219–238. [Google Scholar]
- Walker MK, Peterson RE. Toxicity of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls during fish early development. In: Colborn T, Clement C, editors. Chemically Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection, Mehlman, MA. Princeton, New Jersey: Princeton Scientific Publishing, Co., Inc; 1992. pp. 195–202. (Series Ed.) [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. New York: Plenum Press; 1994a. pp. 347–387. [Google Scholar]
- Walker MK, Peterson RE. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to brook trout (Salvelinus fontinalis) during early development. Environ. Toxicol. Chem. 1994b;113:817–820. [Google Scholar]
- Walker MK, Spitsbergen JM, Olson JR, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) toxicity during early life stage development of lake trout (Salvelinus namaycush) Can. J. Fish. Aquat. Sci. 1991;48:875–883. [Google Scholar]
- Walter GL, Jones PD, Giesy JP. Pathologic alterations in adult rainbow trout, Oncorhynchus mykiss, exposed to dietary 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat. Toxicol. 2000;50:287–299. doi: 10.1016/s0166-445x(00)00095-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Long Q, Marty SD, Sassa S, Lin S. A zebrafish model for hepatoerythropoietic porphyria. Nat. Genet. 1998;20:239–243. doi: 10.1038/3041. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R, Rebstock A, Kulzer E, Schrenk D, Bock KW. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproduction and oogenesis in zebrafish (Brachydanio rerio) Chemosphere. 1992;24:1361–1368. [Google Scholar]
- Wardle FC, Odom DT, Bell GW, Yuan B, Danford TW, Wiellette EL, Herbolsheimer E, Sive HL, Young RA, Smith JC. Zebrafish promoter microarrays identify actively transcribed embryonic genes. Genome Biol. 2006;7:R71. doi: 10.1186/gb-2006-7-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene, Oregon: University of Oregon Press; 1993. [Google Scholar]
- Whitehead A, Pilcher W, Champlin D, Nacci D. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc Dbiol. Sci. 2011 doi: 10.1098/rspb.2011.0847. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A, Triant DA, Champlin D, Cacci D. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol. Ecol. 2010;19:5186–5203. doi: 10.1111/j.1365-294X.2010.04829.x. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Graham CH. Niche conservation: integrating evolution, ecology, and conservation biology. Annual. Rev. Ecol. Evol. and Systematics. 2005;36:519–539. [Google Scholar]
- Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn M. Mechanistic basis of resistance to PCBs in Atlantic Tomcod from the Hudson River. Science. 2011;331:1322–1325. doi: 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K. Experimental induction of blue-sac disease. Trans. Am. Fish. Soc. 1957;86:61–70. [Google Scholar]
- Wolf K. Blue-sac disease of fish, U.S. Fish and Wildlife Service, Fish Disease Leaflet. 1969:1–4. No. 15. [Google Scholar]
- Wright PJ, Tillitt DE. Embryotoxicity of Great Lakes lake trout extracts to developing rainbow trout. Aquat. Toxicol. 2006;47:77–92. [Google Scholar]
- Wu WZ, Li W, Xu Y, Wang JW. Long-term toxic impact of 2,3,7,8- tetrachlorodibenzo-p-dioxin on the reproduction, sexual differentiation, and development of different life stages of Gobiocypris rarus and Daphnia magna. Ecotoxicol. Environ. Saf. 2001;48:293–300. doi: 10.1006/eesa.2000.2013. [DOI] [PubMed] [Google Scholar]
- Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor-mediated downregulation of Sox9b causes jaw malformations in zebrafish embryos. Mol. Pharmacol. 2008;74:1544–1553. doi: 10.1124/mol.108.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Kim E-Y, Iwata H, Yasuhiro S, Tanabe S. Toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in developing red seabream (Pagrus major) embryo: an association of morphological deformities with AHR1, AHR2 and CYP1A expressions. Aquat. Toxicol. 2006;80:166–179. doi: 10.1016/j.aquatox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, Chung BC, Westerfield M, Haffter P, Hopkins N, Kimmel C, Postlethwait JH. A zebrafish sox9 gene required for cartilage morphogenesis. Development. 2002;129:5065–5079. doi: 10.1242/dev.129.21.5065. [DOI] [PubMed] [Google Scholar]
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, Postlethwait JH. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- Yelick PC, Schilling TF. Molecular dissection of craniofacial development using zebrafish. Crit. Rev. Oral Biol. Med. 2002;13:308–322. doi: 10.1177/154411130201300402. [DOI] [PubMed] [Google Scholar]
- Yoshioka W, Peterson RE, Tohyama C. Molecular targets that link dioxin exposure and toxicity phenotypes. J. Steroid Biochem. Mol. Biol. 2011 doi: 10.1016/j.jsbmb.2010.12.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel EW, Cook PM, Peterson RE. Potency of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126), alone and in combination with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), to produce lake trout early life stage mortality. Environ. Toxicol. Chem. 1995a;14:2175–2179. [Google Scholar]
- Zabel EW, Cook PM, Peterson RE. Toxic equivalency factors of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners based on early life stage mortality in rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 1995b;13:315–328. [Google Scholar]
- Zacharewski T, Safe L, Safe S, Chittim B, DeVault D. Comparative analysis of polychlorinated dibenzo-p-dioxin and dibenzofuran congeners in Great Lakes fish extracts by gas chromatography-mass spectrometry and in vitro enzyme induction activities. Environ. Sci. Technol. 1989;23:730–735. [Google Scholar]
- Zheng W, Wang Z, Collins JE, Andrews RM, Stemple D, Gong Z. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS One. 2011;6:e24019. doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]