Abstract
Cerebral metabolism of ketones is a normal part of the process of brain development. While the mature brain relies on glucose as a primary fuel source, metabolism of ketone bodies remains an alternative energy source under conditions of starvation. The neuroprotective properties of brain ketone metabolism make this alternative substrate a viable therapeutic option for various pathologies. Since the ability to revert to utilizing ketones as an alternative substrate is greatest in the younger post-weaned brain, this particular therapeutic approach remains an untapped resource particularly for pediatric pathological conditions.
Keywords: Brain, Development, Ketones, Injury, Metabolism, Neuroprotection
1. KETONES AND BRAIN DEVELOPMENT
1.1 The Normal Brain and Substrate Metabolism
The brain’s use of metabolic substrates changes with maturation. There is an early transient reliance on lactate shortly after birth, followed by a period of dependence on both ketones and glucose during suckling. After weaning, the brain relies exclusively on glucose metabolism under normal conditions. These changes in cerebral substrate metabolism are accompanied by alterations in systemic substrate availability, substrate transport and enzyme activities for substrate metabolism. These parameters change dramatically for ketone metabolism during brain development. The suckling animal has higher circulating ketone levels, greater number of blood brain barrier transporters and greater enzymatic activities of ketone metabolizing enzymes (Booth et al., 1980; Vannucci et al., 2003). Non-vascular monocarboxylate transporter (MCT)1 and neuronal MCT2 expression remains constant throughout development (Vannucci and Simpson, 2003). In contrast, microvascular MCT1 peaks during suckling and decreases to low levels in the adult brain (Vannucci and Simpson, 2003). During the period of peak ketone utilization, the brain’s capacity to take up ß-hydroxybutyrate (ßOHB) is 6 times greater than the adult rat brain (Cremer, et al. 1976; Hawkins et al. 1971). Upon weaning, there is a decrease in arterial ketone concentrations, followed by a drop in cerebral uptake and finally a down regulation of the monocarboxylate transporters (MCT).
In contrast, changes in glucose metabolism during development are more gradual. At birth, circulating concentrations of glucose are half that of the adult with concentrations gradually increasing to achieve adult levels at postnatal day (PND)10. Changes in substrate availability occur before both the increased expression in cerebral glucose transporters (Glut1 and Glut 3; Vannucci and Simpson, 2003) and the increased activity of glycolytic enzymes (Leong and Clark, 1984). These parameters do not reach maturation until PND30 when adult levels of glucose metabolic rates are achieved (Nehlig et al., 1987).
1.2 The Substrate Metabolism During Starvation
The greatest contribution to the understanding of cerebral metabolic adaptation has come from the early studies of starvation. Prolonged starvation in humans was shown to increase plasma ßOHB levels and decrease brain glucose uptake (Owen et al., 1967). While it has been estimated that cerebral ketone metabolism can provide 60% of the human brain’s metabolic needs, ketones can only provide 15-25% of the rat brain’s energy (Dahlquist and Persson, 1976; Ruderman et al, 1974). It is possible that this reflects a species difference in capacity for ketone metabolism, but may equally be explained by the duration of the starvation or magnitude of ketosis achieved.
Conditions of starvation induce changes in plasma levels of substrate and in transporter expression and the magnitudes vary with age. In normally fed adult mammals, ßOHB metabolism comprises < 3% of total cerebral metabolism and is present in low circulating concentrations (0.1 mmol/ L) with negligible uptake into the brain (Hawkins et al, 1971). However, plasma ketone levels can be increased four- to fivefold within 2 days via ketogenesis associated with starvation or administration of a ketogenic diet resulting in 4.9-fold and 1.5-fold increase in cerebral uptake of ketones in PND20 and adult rats, respectively (Dahlquist and Persson 1976; Hawkins et al, 1971). Ketogenesis after 48 h of starvation among PND57 rats showed a 95% increase in arterial ßOHB levels versus only an 81% increase among PND85 rats (Dahlquist and Persson, 1976). Similarly, there is a significantly greater production of ketones among 5-year-old children (28.6%) versus 10- year olds after 24 h of fasting (Saudubray et al, 1981). Starvation studies have revealed that the ability to generate ketones endogenously is inversely proportional to age even after weaning.
In addition to the changes in substrate supplies, starvation also has been shown to alter the uptake of ketones in an age-dependent manner. Adult rats on a ketogenic diet for 1 week can achieve 2mmol/L plasma ßOHB levels within 24h, which is sustained for 7 days. Despite the same plasma concentration of ßOHB at these time points, the permeability of 14C-D-ßOHB was two times greater at 7 days than 24h, suggesting that adult cerebral uptake of ßOHB changes with time in the presence of ketones (Moore et al, 1976). PND57 rats show 61% greater cerebral uptake of b-OHB after 48h of starvation compared with PND85 animals (Hawkins et al, 1971; Dahlquist and Persson, 1976). It has also been reported that at the same plasma level of ßOHB, PND35 rats show a 1.7-fold greater brain uptake index than PND50. Some of this age difference in uptake may be attributed to transporter expression or even transporter function. Collectively, these results from both cerebral development and starvation provide insight into the brain’s capacity to adjust to physiologic changes in substrate availability. The age-dependent nature of these changes emphasizes the potential of alternative substrates as therapeutic options for the younger developing brain.
2. KETONES AND BRAIN METABOLISM AFTER INJURY
In the last 10 years the number of publications with the topic of ketones and the brain has almost doubled from the previous decades. There have been increased interest in the effects of ketone metabolism on brain tumors (Stafford et al, 2010; Mukherjee et al., 2002; Seyfried et al., 2003; Zhou et al., 2007), Alzheimer’s disease (Van der Auwers et al., 2005; Reger et al., 2004), Parkinson’s disease (Kweon et al, 2004; Tieu et al., 2003), hypoxia/ischemia (Suzuki et al., 2001,2002; Masuda et al., 2005; Ritter et al., 1996) and traumatic brain injury (TBI; Prins et al., 2004, 2005). Despite the evidence for greater uptake and metabolism of ketones by the younger brain, most of the research focus has been centered on the effects on the adult brain.
2.1 Neuroprotective Properties of Cerebral Ketone Metabolism
The increasing potential utility of cerebral ketone metabolism has directly resulted from the growing evidence for multiple neuroprotective mechanisms. Ketone metabolism has been shown to improve cellular bioenergetics, antioxidant effects, ani-inflammatory traits, and anti-apoptotic properties (Maalouf et al., 2009; Veech et al., 2004). Administration of ketones has been shown to prevent ATP decline following cellular oxidative challenges (Hace et al., 2009), inhibitors of mitochondrial respiration in hippocampal neurons (Kim et al., 2010), and from ischemia in the younger brain (Suzuki et al., 2001, 2002).
The antioxidant effects are achieved through several possible mechanisms including (1) decrease ROS production in juvenile mice (Sullivan et al., 2004) and in dissociated neonatal cortical cells (Maalouf et al., 2007), (2) increased glutathione levels and glutathione peroxidase activity in adult rats (Jarrett et al., 2008; Ziegler et al., 2003), (3) increase uncoupling protein expression in juvenile mice(Sullivan et al., 2004), (4) direct scavenging properties of ketones in cultured hippocampal neurons (Haces et al., 2008), (5) the effects of acetate on neurotransmission (Juge et al., 2010).
Ketones have also been shown to decrease cell death in response to various pathological challenges. Adult rats given the ketogenic diet +TBI were shown to have lower levels of Bax mRNA, Bax protein and cytochrome c release than standard fed TBI animals with consequent 8% less apoptosis at 72hrs post injury (Hu et al., 2009a,b). Similar 3-week ketogenic fed young rats showed increased Bcl-2 expression and 50-70% decrease in infarct volume after focal ischemia (Puchowicz et al., 2008). PND21 rats on the KG diet for 4 days prior to insulin-induced hypoglycemia also showed significantly less neuronal death than standard fed rats (Yamada et al., 2005).
Evidence for ketogenic anti-inflammatory effects has emerged in both models of traumatic brain injury and ischemia. Adult head injured rats on the ketogenic diet showed decreased in wet weight/dry weight ratio at 3 days post injury (Hu et al., 2009a,b). PND56 mice infused with ßOHB after middle cerebral artery occlusion showed significant decrease in edema (Suzuki et al., 2002). The anti-inflammatory effects of ketone metabolism do not appear to be restricted to the brain as hindpaw swelling after thermal nociception testing was also decreased in juveniles and adults on the KG diet (Ruskin et al., 2009). There are important age related differences in cerebral maturation of bioenergetics, free radical defenses, cell death mechanisms and swelling that can contribute to differential responses to potential ketogenic therapy. These changes emphasize the continued need to examine the numerous effects of ketone metabolism within different chronological age groups.
2.2 Age and Ketone Use After Traumatic Brain Injury
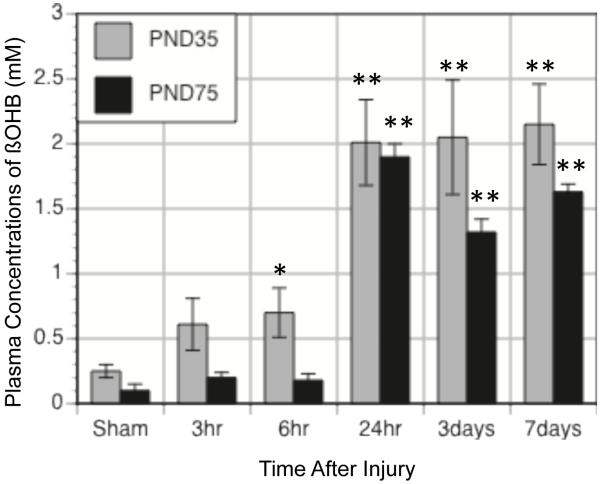
While the normal developmental changes associated with cerebral substrate metabolism are established after weaning, the response of the brain to injury continues to show relevant age differences. There are age-related differences in ketogenesis, induction of transporter expression and cerebral uptake of ketones after brain injury. The same age differences in the rates of ketosis that have been observed following starvation are also seen among traumatic brain injured (TBI) animals on the KG diet (Figure 1). PND35 rat placed on the KG diet immediately after injury show 3-fold increases in plasma ßOHB within 6 hrs. PND75 rats do not show significant increases in plasma ßOHB until 24hrs. The age differences in the rates of ketogenesis have significant impact on approaches for therapeutic interventions. While enteral delivery of ketones to the younger brain after injury maybe sufficiently raise plasma ketones within an appropriate therapeutic window, more rapid forms of administration are likely required for the adult brain after injury.
Figure 1.
Changes in plasma concentrations of ßOHB with time after TBI in PND35 (grey bars) and PND75 (black bars) rats maintained on the KG diet after injury. PND35 animals achieve higher levels of ßOHB earlier than PND75 animals. Data is expressed as mean ±sem. *, ** p<0.05, 0.01 relative to age-matched sham
Similar to ketogenesis, the normal age differences in substrate transporters and metabolism are exacerbated by brain injury. While the expression of primarily neuronal ketone transporter (MCT2) has been shown to be elevated in PND35 rats compared to PND75 rats, TBI induces further 60% increase of MCT2 in the endothelia of PND35 rats and only 25% in adult rat brains (Prins and Giza, 2006). The expression of the primary endothelial ketone transporter (MCT1) was also increased 2.3-fold in PND35 rats at 24hrs post-injury, but not in PND75 rats (Prins et al., 2007). The mechanism for the tranporter induction is currently unknown, but the greater expression of the transporters is likely to contribute to the age differences in ketone uptake after injury.
There are particularly interesting age and species differences in the effects of ketone uptake on brain glucose uptake. In adult rodents, increases in plasma ketones have no effect on cerebral uptake of glucose. However, younger rats infused with ßOHB show a significant reduction in brain glucose uptake in PND20 (Miller et al., 1986) and PND45- 55 (LaManna et al., 2009). Higher order animals and humans all show decreases in cerebral metabolic rates of glucose in response to ketosis regardless of age (Kammula, 1976; Redies et al., 1989, Hassselbalch et al., 1994,1996). Consistent with the age differences in rodents, glucose uptake decreased by an additional 13% only among PND35 TBI animals on the KG diet (Prins and Hovda, 2009). The metabolism of ketones under these circumstances may flood the acetyl-CoA pool, inhibit pyruvate oxidation and thereby decrease glucose uptake via glycolytic inhibition (Randle et al., 1966; Ruderman et al., 1974).
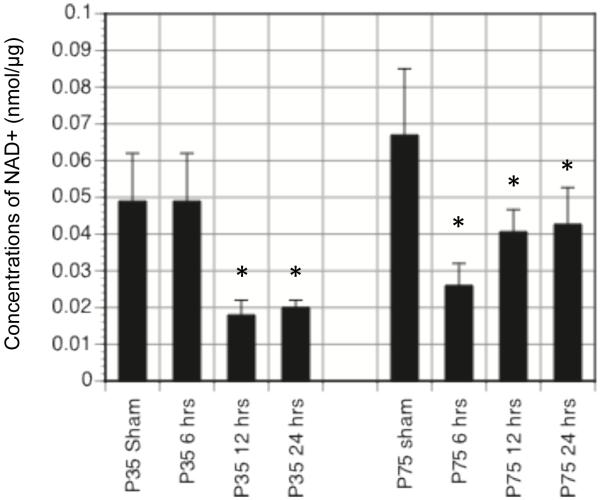
Substitution of cerebral metabolic fuels may be exactly what the younger brain requires after TBI. The rapid brain deformation from TBI initiates a rapid cascade of metabolic changes that ultimately results in a period of glucose metabolic depression in the brain (Yoshino et al., 1991; Thomas et al., 2000; Prins & Hovda, 2009). During this decrease in cerebral glucose uptake, glucose is also diverted to other biochemical pathways (pentose phosphate pathway) (Bartnik et al., 2005) and glycolytic metabolism of glucose is further impaired by low levels of cytosolic NAD+ (nicotinimide adenine dinucleotide; Figure 2). Given the obstructions and diversion of the brain’s primary fuel source, metabolism of ketones should improve cellular energy production, cell survival and functional outcome.
Figure 2.
Concentration of cytosolic NAD+ (nmol/μg) in PND35 and PND75 rats at 6, 12, 24hrs post injury. Animals were given sham or CCI injury and at 6,12,or 24 hrs after injury brains were rapidly removed, the cortical tissue dissected and homogenized ice cold homogenization media (0.25 sucrose, 3mM Tris, pH 7.4,1mM EGTA) with a Dounce homogenizer. The homogenate was centrifuged at 17,000g at 4°C for 10min. The cytoplasmic fraction was removed and analyzed for NAD+ concentrations (Nisselbaum and Green,1969). Cytosolic NAD+ decreases significantly after TBI in both age groups and will likely contribute to glycolytic inhibition via GAPDH.
Administration of ketones via the ketogenic diet after TBI has been shown to improve cellular ATP (Ying et al., 2009), decrease contusion volume (Prins et al.,2005; Prins and Hovda, 2009), and improve motor and cognitive performance (Appelberg et al., 2009) in the PND35 rats. More recently, PND35-40 rats on the KG diet after TBI showed decreased edema at 3 days post-injury, decreased cytochrome c release and less apoptosis than standard fed animals (Hu et al., 2009). Collectively, these studies emphasize the promising effects of brain ketone metabolism in the younger brain after traumatic brain injury.
2.3 Age and Ketone Use After Ischemia
In addition to TBI, The beneficial effects of ketone metabolism have also been documented in juvenile models of hypoxia/ischemia. PND35 mice infused intravenously with ßOHB before or after bilateral carotid artery occlusion showed less edema, improved ATP and lower levels of lactate (Suzuki et al., 2001). In a subsequent study, Suzuki et al., (2002) showed infusion of ßOHB after transient middle cerebral artery occlusion to decrease infarct volume, lipid peroxidation and cognitive deficits. Even 1hr delay of ßOHB infusion provided neuroprotection from transient occlusion. A more recent study has focused on understanding the protective mechanisms of the ketogenic diet for ischemia. PND28 rats were fed the KG diet for 3 weeks prior to or infused intraventricularly for 4 days prior to middle cerebral artery occlusion (Puchowicz et al., 2008). Infusion and diet both resulted in 55-70% reduction in infarct volume, 3-fold increase in HIF1alpha and Bcl-2 proteins and 55% increase in succinate. The authors propose that the elevation in succinate inhibits the enzyme that degrades HIF1alpha thereby increasing neuroprotection.
2.4 Age and Ketone Use After Other Challenges
The effects of ketone metabolism on conditions of hypoglycemia have also been studied in the younger brain. PND21 rats maintained on the KG diet for 4 days prior to insulin-induced hypoglycemia showed significantly less neuronal cell death than standard fed rats (Yamada et a., 2005). The KG fed rats were also able to maintain upright posture, walk and show protective reflexes during tail suspension.
Ketone metabolism has been shown to reduce cerebral inflammation in the younger brains after TBI and ischemia, and more recently shown to reduce peripheral swelling following a model of pain. PND21 and adult rats were given KG diet for 3 weeks before hindpaw thermal nociception testing (Ruskin et al., 2009). Both age groups on the diet showed increased tolerance to pain and reduced peripheral swelling, though the anti- inflammatory response was greater in the juveniles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Booth RFG, Patel TB, Clark JB. The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem. 1980;34:17–25. doi: 10.1111/j.1471-4159.1980.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Braun LD, Oldendorf WH. Changes during development in transport processes of the blood-brain barrier. Biochim et Biophy Acta. 1976;448:633–637. doi: 10.1016/0005-2736(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Dahlquist G, Persson B. The rat of cerebral utilization of glucose, ketone bodies, and oxygen: a comparative in vivo study of infant and adult rats. Pediatr Res. 1976;10:910–917. doi: 10.1203/00006450-197611000-00002. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Hovda DA, Prins ML, Harris NG. Metabolic and bioenergetic changes with ketogenic diet vs standard diet after controlled cortical impact in juvenile rat brains measured by in vitro magnetic resonance spectroscopy. Society for Neurotrauma. 2009;26:A80. [Google Scholar]
- Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Jakobsen J, Hagemen LP, Holm S, Paulson OB. Brain metabolism during short-term starvation in humans. J Cereb Blood Flow and Metab. 1994;14:125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Pauls OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Willianson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Qiao L, Yan W, Tan QF, Yin HX. The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj. 2009a;23:459–465. doi: 10.1080/02699050902788469. [DOI] [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Jin W, Yin HX. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann Clin Lab Sci. 2009b;39:s76–83. [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammula RG. Metabolism of ketone bodies by ovine brain in vivo. Am J Physiol. 1976;231:1490–1494. doi: 10.1152/ajplegacy.1976.231.5.1490. [DOI] [PubMed] [Google Scholar]
- Kweon G, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K, Kang U. Distince mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem. 2004;279:51783–51792. doi: 10.1074/jbc.M407336200. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, Lee Z. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301–306. doi: 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S, Clark J. Regional enzyme development in rat brain. Enzymes associated with glucose utilization. Biochem J. 1984;218:131–138. doi: 10.1042/bj2180131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Research Reviews. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda R, Monahan JW, Kashiwaya Y. D-beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res. 2005;80:501–509. doi: 10.1002/jnr.20464. [DOI] [PubMed] [Google Scholar]
- Miller AL. Regional glucose and beta-hydroxybutyrate use by developing rat brain. Metab Brain Dis. 1986;1:53–61. doi: 10.1007/BF00998477. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Lione AP, Sugden MC, Regen DM. ß-hydroxybutyrate transport in rat brain: developmental and dietary modulations. Am J Physiol. 1976;230:619–630. doi: 10.1152/ajplegacy.1976.230.3.619. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer. 2002;86:1615–1621. doi: 10.1038/sj.bjc.6600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, de Vasconcelos AP, Boyet S. Quantitative autoradiographic measurement of local cerebral glucose utilization in freely moving rats during postnatal development. J Neurosci. 1987;8:2321–2333. doi: 10.1523/JNEUROSCI.08-07-02321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clinic Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M, Hovda D. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Deng-Bryant Y, Appelberg S, Hovda DA. Changes in Cerebral Microvessel Expression of MCT1 and GLUT1 following Controlled cortical impact in juvenile and adult rats. Society for Neurotrauma. 2007;24:1267. [Google Scholar]
- Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev Neurosci. 2006;28:447–456. doi: 10.1159/000094170. [DOI] [PubMed] [Google Scholar]
- Prins ML, Lee SM, Fujima LS, Hovda DA. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J Neurochem. 2004;90:666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA, Denton RM, Pogson CI. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Redies C, Hoffer LJ, Beil C, Marliss EB, Evans AC, Lariviere F, Marrett S, Meyer E, Diksic M, Gjedde A. Generalized decrease in brain glucose metabolism during fasting in humans studied by PET. Am J Physiol. 1989;256:E805–810. doi: 10.1152/ajpendo.1989.256.6.E805. [DOI] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of ß-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Robertson CS, Goodman JC, Contant CF, Grossman RG. Evaluation of carbohydrate-free diet for patients with severe head injury. J Neurotrauma. 1996;13:473–485. doi: 10.1089/neu.1996.13.473. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Ross PS, Berger M, Goodman MN. Regulation of glucose and ketone-body metabolism in brain of anesthetized rats. Biochem J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Kawamura M, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS ONE. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudubray JM, Marsac C, Limal JM, Dumurgier E, Charpentier C, Ogier H, Coude FX. Variation in plasma ketone bodies during a 24-hour fast in normal and in hypoglycemic children: Relationship to age. J Pediatr. 1981;98:904–908. doi: 10.1016/s0022-3476(81)80583-5. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89:1375–1382. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Sheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 2010;11(10):710–718. doi: 10.1186/1743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Beta-hydroxybutyrate, a cerebral function improving agent, protects rate brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpm J Pharmacol. 2002;89:36–43. doi: 10.1254/jjp.89.36. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Effect of beta-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn J Pharmacol. 2001;87:143–150. doi: 10.1254/jjp.87.143. [DOI] [PubMed] [Google Scholar]
- Thomas S, Prins ML, Samii M, Hovda DA. Cerebral metabolic response to traumatic brain injury sustained early in development: a 2-deoxy-D-glucose autoradiographic study. J Neurotrauma. 2000;17:649–665. doi: 10.1089/089771500415409. [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta- hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab. 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rensing N, Thio LL. Ketogenic diet reduces hypoglycemia- induced neuronal death in young rats. Neuroscience Letters. 2005;385:210–214. doi: 10.1016/j.neulet.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Hovda D, Kawamata T, Katayama Y, Becker D. Dynamic changes in local cerebral glucose utilization following cerebral concussive in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Ribeiro LC, Hagnn M, Siqueira IR, Araujo E, Torres ILS, Gottfried C, Netto CA, Goncalves CA. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem Res. 2003;28:1793–1797. doi: 10.1023/a:1026107405399. [DOI] [PubMed] [Google Scholar]