Abstract
Atherosclerosis of conduit epicardial arteries is the principal culprit behind the complications of coronary heart disease, but a growing body of literature indicates that the coronary microcirculation also contributes substantially to the pathophysiology of cardiovascular disease. An understanding of mechanisms regulating microvascular function in humans is an essential foundation for understanding the role in disease, especially since these regulatory mechanisms vary substantially across species and vascular bed. In fact all subjects whose coronary tissue was used in the studies described have medical conditions that warrant cardiac surgery, thus relevance to the normal human must be inferential and is based on tissue from subjects without known arteriosclerotic disease. This review will focus on recent advances in the physiological and pathological mechanisms of coronary microcirculatory control, describing a robust plasticity in maintaining endothelial control over dilation, including mechanisms that are most relevant to the human heart.
Keywords: coronary, microcirculation, flow-mediated vasodilation, endothelium, endothelium-derived hyperpolarization factor, hydrogen peroxide, redox biology
Introduction
Based on direct measurements of intravascular pressure in animal models, coronary resistance is considered to be primarily regulated by arterioles between the diameters of 50-200 microns [41]. Vessels in this size range are most responsive to metabolic, myogenic and humoral stimuli [48]. At baseline, substantial vasomotor tone exists to match oxygen delivery to metabolic need. With elevated metabolic demand, coronary flow can increase 5-fold primarily through arteriolar vasodilatation. This vasodilator reserve can also be utilized to maintain flow when perfusion pressure changes[22]. For example inserting a 50% stenosis in a coronary conduit artery has little effect on flow due to autoregulatory reductions in arteriolar resistance [19]. However with exercise or tighter stenosis (~80%), flow may be compromised when arteriolar dilation is exhausted. Thus conduit and resistance vessels work in harmony to defend myocardial perfusion. The presence of microvascular disease and the concomitant inability to match flow with metabolism and pressure will accentuate the adverse effect of a larger artery stenosis. The inability of the microcirculation to compensate for changes in metabolism and perfusion pressure is one reason that examination of coronary arteriolar function is so important. Another important factor is that arteriolar responses may serve as an indicator large vessel function. It is hypothesized that changes in microvessel phenotype (e.g. endothelial function and release of vasoactive agents) parallels changes that occur in larger vessels, but without the development of atherosclerosis. Epicardial and microvascular coronary endothelial dysfunction predicts future cardiovascular events in the presence or absence of angiographically detectable coronary lesions [20;63].
Evaluation of microvascular function in humans – in vivo approaches
Coronary catheterization is one of the most widely used techniques to assess human coronary arteriolar function. Pharmacological stimuli are used to modulate coronary flow as assessed by a Doppler wire placed in the target vessel. Acetylcholine and substance P are used to assess endothelium-mediated dilation while adenosine, dipyridamole, papaverine or nitroprusside are used to measure endothelium-independent dilator responses. The response to acetylcholine is complex, yielding dose-dependent vasodilation in most cases, but in the presence of risk factors or frank disease, it causes vasoconstriction indicative of endothelial dysfunction [39] and predicts future cardiovascular events [58].
Echocardiography can be used to assess coronary flow velocity non-invasively [43]. It too relies on Doppler interrogation of the proximal coronary arteries for calculation of flow velocity. Both catheter-based and non-invasive echo approaches to measuring microvascular responsiveness by investigating large coronary flow have limitations. Indirectness of the measurement (interrogating conduit vessel rather than microvessel blood flow) is the most obvious limitation. Presence of collateral vessels for example could obscure flow in the microcirculation. Inability to assess the Doppler angle with respect to the vessel and difficulty in accounting for possible changes in epicardial artery diameter at the site and time where the flow measurement is made, also reduce reliability of these techniques.
Myocardial contrast echocardiography (MCE) is another sonographic technique that provides non-invasive assessment of regional myocardial perfusion [26]. MCE was developed in the 1990s by Sanjiv Kaul using sonar pulses to destroy insonified microbubbles which lose their echo-opacification upon release of trapped gases. Timing the reintroduction of tissue contrast from arterial blood outside the heart provides an assessment of regional myocardial perfusion [49]. Advantages of the MCE technique are the ability for repeated non-invasive assessments of cardiovascular function, assessment of regional flow, and the ability to measure blood flow and myocardial blood volume. This allows for quantitative assessment of flow.
Other techniques are available for assessing human microvascular function in vivo, including traditional nuclear imaging studies, magnetic resonance imaging [25;51], electron beam computed tomography (EBT) [2] and positron emission tomography (PET) scanning [6;21]. For further details on these and other methods also refer to Knaapen et al.[27]
Evaluation of microvascular function in humans – in vitro approaches
A limitation to all in vivo approaches for assessing arteriolar function is the indirectness of the measurement and the influence of extravascular factors including metabolic, neural, humoral, paracrine, and myocardial compressive forces. Myocardial metabolism is the primary regulator of arteriolar tone, and most stimuli that are used to modulate coronary tone in vivo also influence contractility, obscuring direct vascular responses to the stimulus. For example cardiac sympathetic activation elicits a reduction in coronary resistance under vagotomized conditions in the dog, but this is converted to a frank constriction in the presence of beta-adrenergic blockade[16] or in the presence of severe coronary stenosis[23], which attenuates the myocardial metabolic stimulus for dilation. Another, albeit modest, shortcoming of most in vivo measurements is that global changes in resistance are measured. While most flow regulation is governed by arterioles 50-200 micrometers in diameter, a significant portion of resistance remains in other vascular segments downstream from the conduit coronary vessels. Only total resistance is assessed with in vivo techniques, masking the heterogeneity in vessel size-specific responses that are well-described in the coronary bed [32].
For these reasons, in vitro assessment of coronary microvascular function [31] was adapted from approaches used to study other vascular beds [55]. This is especially useful for studies of human tissue where more specific pharmacological, molecular, and physiological approaches can be used. Vessels are isolated from fresh cardiac tissue removed at the time the patient’s heart is cannulated for cardiopulmonary bypass. From the atrial appendage, arterioles are dissected away from surrounding pectinate muscles, yielding 3-5 vessels of 50-200 micrometers in diameter and 2-3 mm in length (reviewed in [35]). The isolated vessel is transferred to a miniaturized chamber containing physiological saline solution (PSS), mounted between two micropipettes, and pressurized with PSS to approximate in vivo conditions [10]. Changes in vessel diameter to pharmacological or mechanical stimuli are measured using electronic calipers connected to a video monitor that projects the image from a video camera connected to a light microscope. Both commercial and home-made systems have been successfully utilized to quantify arteriolar reactivity in this way. An alternative method for slightly larger vessels is wire myography in which stirrups are used to stretch the artery and measure changes in tension generated to stimuli under isometric conditions. This method is faster, but less physiological and often results may differ between techniques (unpublished observations).
In Vitro Isolated Vessels Studies
There are several caveats surrounding in vitro studies of the human coronary circulation. Use of human tissue provides clinical relevance. No animal models can mimic the chronicity and complexity of human coronary atherosclerosis. Furthermore there is substantial variability in mechanisms of dilation from species to species, making it unclear which is most relevant to the human condition. However two important limitations exist.
First, it is not ethically possible to obtain cardiac tissue from truly healthy individuals. Most human coronary microvessels are derived from patients with coronary disease. To address this issue responses are compared using tissue from subjects without coronary artery disease (CAD), or other risk factors, who undergo valve replacement or repair of congenital heart defects.
Second, human subjects are much more heterogeneous than most animal models, risking a type II statistical error when dependent variables are influenced by confounding factors such as gender, age, medications, genetics, or presence of diseases.
Endothelium-dependent human coronary microvascular relaxation
A variety of endothelial derived substances arising from different signaling pathways mediate arteriolar vasodilatation. The responsible agent in a given vascular bed differs from one species to another and from one vascular bed to another within the same species. Conduit vessel responses do not always parallel those in the contiguously connected microvessels. Gender, presence of disease, medications, and age may determine which mediator elicits dilation. For example, in male Wistar rat cremaster muscle, prostacyclin is almost exclusively responsible for flow-induced dilation [28] and participates in other vessels as well [14;66]. In skeletal muscle arterioles from female eNOS KO mice, epoxyeicosatrienoic acid (EET) is the mediator of flow- induced [24]. EETs play a role in other vessels as well, including human coronary vessels [7;33;54]. In pig coronary arterioles, only nitric oxide seems to serve this role [30]. This heterogeneity implies physiological importance to arteriolar endothelium-dependent dilation and is further supported by the powerful compensatory mechanisms that allow substitution of one dilator for another in the presence of disease [46].
Because of the diversity in endothelial mediators of dilation, the remainder of this review will focus on results from in vitro studies of the human resistance vasculature. Attention will be directed toward flow-mediated dilation (FMD) since it occurs ubiquitously throughout the circulation, is endothelium-dependent, and is particularly prominent in arteriolar vessels [48].
Flow-mediated vasodilation in the human coronary microcirculation
The endothelium constitutes the largest continuous layer of cells in the body. Throughout life virtually all of these cells are continuously exposed to shear forces generated from blood flow. It is not unexpected then that shear stress activates a variety of endothelial signaling pathways with the nature of the shear determining the type of signal. For example laminar shear typically elicits an anti-inflammatory, pro-proliferative set of responses, while turbulent or oscillatory shear does the opposite [65]. Pulsatility further modulates the response to shear [8].
One can postulate a physiological advantage for FMD as a sensitizer of metabolic dilation. As resistance vessels relax in response to vasodilator metabolites, the resulting increase in flow (to match oxygen consumption with oxygen delivery) further dilates the artery/arteriole, facilitating the dilator process. Upstream vessels supplying metabolically active regions would also undergo FMD. This would further improve flow exclusively to the region where the blood is needed. Thus FMD serves as a means for mechanical communication between resistance and upstream vessels in coordinating an optimal and localized increase in flow during metabolic demand.
FMD may also serve to “fine tune” other local vasomotor regulatory factors. In response to a rise in pressure, myogenic constriction defends vascular wall tension through an active reduction in vessel diameter. An increase in pressure with reduced wall diameter results in an increase in flow and thus shear. FMD in this case would modulate the myogenic constriction [30].
Mediators of FMD in human coronary arterioles
We examined FMD in isolated human coronary arterioles (HCA) mounted between two pipettes and pressurized with physiological saline solution to mimic estimated pressure in vivo [11]. By raising the height of one pressure reservoir and reducing the height of the other by similar magnitude, we can effect an increase in flow without a change in intraluminal pressure. It is critical to use pipettes of matched resistance so that changes in diameter during flow reflect FMD.
As reported in most other vessels, FMD of HCA is endothelium-dependent[46]. At flows comparable to those seen during high physiological shear rates (~20 dynes/cm2), dilation approaches nearly 100% of that seen to the potent endothelium-independent dilator, papaverine. These observations are observed independent of age, gender, presence or absence of disease, or medications. However the mediators of dilation do appear to vary with age and disease. The vast majority of subjects we have examined had extensive coronary artery disease (CAD). In these subjects EDHF was responsible for virtually 100% of the FMD. However we previously observed in small numbers of subjects without CAD that nitric oxide (NO) contributes to about one third of FMD [46]. Recent preliminary data have identified that in pediatric subjects (ages 2-16) with congenital heart disease, FMD in atrial arterioles is mediated almost exclusively by prostaglandins (i.e. inhibited by indomethacin) [72]. We have also observed in larger numbers of adults without CAD, that NO is the primary mediator of FMD, even when age-matched for those with CAD [72]. This contrasts with our prior observation of ~30% contribution from NO [46], but in that paper, several of the subjects without CAD were young children, where L-NAME (used to assess the NO component to dilation) would be expected to have minimal effect since prostaglandins are the primary endothelial-derived dilator substances. The data are consistent with a transition in the role of mediators of FMD in the human heart from youth to adulthood (prostaglandins to NO) and again with the onset of disease (NO to endothelial derived relaxation factor (EHDF)).
EDHFs represent a heterogeneous class of dilator substances/mechanisms that result in smooth muscle hyperpolarization and relaxation (from inhibition of voltage-dependent calcium channels). In addition to its dilator capacity, the importance of EDHF lies in its ability to compensate for loss of other mediators, most notably NO, during elevations in reactive oxygen species as occur with inflammation, CAD and its risk factors [52]. Thus certain EDHFs continue to elicit dilation under conditions where NO cannot.
A variety of mediators of endothelial hyperpolarization have been identified, including anandamide [57], C-natriuruetic peptide [9], potassium ions [15], epoxyeicosatrienoic acid EET [7], NO [12], and H2O2 [36]. Identification of a role for EDHF in vasodilatation has often been one of exclusion; namely, observing an endothelium-dependent dilation associated with smooth muscle hyperpolarization in the presence of inhibitors of NOS and cyclooxygenase. Demonstration of frank endothelium-dependent smooth muscle hyperpolarization strongly supports the contention. Clearly FMD of HCA from subjects with CAD meets this definition of EDHF, but this does not indicate which EDHF mediates FMD in the human heart.
Identifying the EDHF responsible for FMD in the human coronary microcirculation
We have applied a pharmacological/electrophysiological approach to address this question. Both epoxyeicosatrienoic acid (EET; (11,12- regioisomer) [34;46] and H2O2 [36] elicit a dose-dependent dilation of HCA. This dilation is prevented in the presence of elevated bath potassium, and is inhibited by blockers of calcium-activated potassium channels on the smooth muscle [36;45]. Inhibition of either cytochrome P450 epoxygenase [46] or administration of PEG-catalase [45] blocks FMD in human atrial arterioles. This occurs in the presence or absence of inhibitors of nitric oxide synthase (L-NAME) and cyclooxygenase (indomethacin; INDO). These data support a role for both EETs and H2O2 in mediating FMD in the human heart (Figure 2).
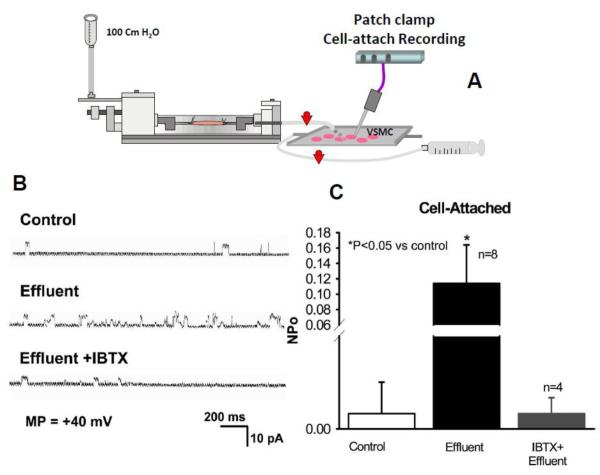
Figure 2. Effect of effluent from donor vessel on K channel activity in cell-attached patches of coronary smooth muscle cells.
Preparation is depicted in (A) showing a cannulated donor vessel perfused by physiological saline with the effluent bathing cultured fresh vascular smooth muscle cells from the same patient. As indicated in sample traces (A) and summarized data (B), effluent from donor vessel greatly enhanced Npo (probability of channel opening) in cell-attached patches of smooth muscle cells. The ehancement was blocked by 100 nmol/L iberiotoxin (IBTX). Adapted from Liu et al [36] and Reproduced with permission from the American Heart Association.
We next sought to determine which of these agents is released from the endothelial cell and acts on the smooth muscle to bring about hyperpolarization. To do this we employed a bioassay system where an upstream arteriole (endothelium intact) and downstream arteriole (endothelium denuded) are connected in series using two bath chambers so that the effluent from the upstream vessel perfuses the downstream arteriole [36]. When a pressure gradient is created from the proximal port of the upstream vessel to the distal port of the downstream vessel, dilation is observed in both vessels only if the direction of flow is from the intact to denuded vessel. Reversal of flow dilates the endothelium-intact vessel only. This indicates that endothelial substances are released from the donor vessel into the lumen and then travel downstream to dilate the detector vessel.
We can also collect the effluent from the donor vessel and assess it for EDHF. For example when the effluent from a perfused vessel is dripped onto primary cultures of vascular smooth muscle cells from the same subject, there is an increase in BKCa channel activity that can be quenched by iberiotoxin, a selective BKCa inhibitor [36] (Figure 3).
Figure 3.
Signaling pathways for flow-mediated dilation (FMD) in the human coronary microcirculation. Major regulators of FMD include nitric oxide (NO), prostacyclin (PGJ2), epoxyeicosatrienoic acid (EET), and hydrogen peroxide (H2O2). Each of these factors can act on the underlying smooth muscle cells to induce vasodilation as illustrated. Dashed lines represent pathways that may be active in other vessels, species, and conditions, but do not play a major role in humans with coronary disease. Abbreviations: ETC = electron transport chain. AA = Arachidonic Acid, SOD = Superoxide Dismutase; CAT = Catalase, O2· = Superoxide, ONOO− = peroxynitrite, eNOS = endothelial Nitric oxide synthase. PLA = Phospholipase A2, TRPV4 = Transient receptor potential cation channel subfamily V member 4. CYP450 = cytochrome P450, EET = Epoxyeicosatrienoic acid, Cox = Cyclooxygenase , TX = Thromboxane. PKG1a = Cyclic GMP-dependent Protein Kinase G1 alpha, NADPH Oxidase = Nicotinamide Adenine Dinucleotide Phosphate-Oxidase.
Prior observations using the bioassay system and a sensitive HPLC assay, showed that EETs are released into the donor (endothelium-intact) vessel effluent in response to bradykinin [34]. When put into the detector vessel bath solution, a specific EET antagonist (EEZE) [34] reduced dilation to bradykinin indicating that EETs can serve as the transferrable agent mediating hyperpolarization of smooth muscle cells. Surprisingly, in response to shear stress, we have been unable to detect any regioisomer of EET in the donor vessel effluent, thus it has been difficult to confirm a role for EET as the EDHF during FMD. However we do measure H2O2 emanating from the donor vessel in response to shear [36]. Furthermore the detector vessel dilation during shear is blocked by administration of PEG-catalase. When the bioassay is modified as described above so that the donor vessel effluent bathes freshly isolated HCA smooth muscle cells [36], a catalase-reversible increase in both 2′, 7′- dichlorofluorescin (DCF) fluorescence (a commonly used peroxide detecting assay to detect oxidative stress) and single channel BKCa activity is observed. These data confirm that H2O2 is the factor acting to hyperpolarize smooth muscle cells and elicit dilation.
Proof that H2O2 is the transferrable factor mediating FMD
These data do not however prove the H2O2 is the factor transferred from endothelium to smooth muscle. Perhaps the H2O2 arises from the smooth muscle in response to a distinct agent that is released from the donor vessel endothelium. To test whether the H2O2 that is responsible for the dilation arises from the endothelium, we passed the donor effluent through a column of beads with covalently bound but active catalase [36]. Naked beads inserted between bioassay vessels did not alter the detector dilation to flow through the donor vessel, but when catalase-bound beads were used, the dilation and distal detection of H2O2 formation were abolished. These data eliminated the possibility that some other factor was released from the endothelium to stimulate H2O2 formation in the vascular smooth muscle and confirm that H2O2 is the transferrable factor. Therefore in contrast to bradykinin, where EETs serve as an important EDHF, with FMD H2O2 is the endothelium-derived mediator.
Source of hydrogen peroxide
H2O2 is typically generated by two-electron reduction of oxygen. This can occur in one enzymatic step, or more typically it involves generation of the intermediate ROS, superoxide. Multiple cellular sources are capable of generating superoxide including NADPH oxidase (NOX 1 and NOX4 in the endothelium), xanthine oxidase, cyclooxygenase, uncoupled eNOS, mitochondria, lipoxygenases, and some CYP450 enzymes. Cellular ROS generation can stimulate other intracellular signaling pathways, making it difficult to determine which ROS source is responsible for FMD. Rather than test each potential source separately, we initially sought to measure ROS release from endothelial cells in response to shear. Using electron paramagnetic resonance and the spin adduct BMPO, we detected a strong ubisemiquinone signal in the vessel effluent following shear. This was only present in vessels with endothelium intact and could be elicited multiple times in the same vessel [38]. The ubisemiquinone fingerprint transformed within 1 minute to a pattern suggestive of hydroxyl radical, thereby implicating generation of hydrogen peroxide. Since ubisemiquinone is specifically localized to the mitochondria, these data indicate that mitochondrial sources (likely via the respiratory chain) are responsible for the EDHF released from HCA during flow. This is consistent with the known role of mitochondria as the major source of superoxide generation in most cells. [18;47;50;56;59].
Transfer of an electron to oxygen can occur at several sites along the mitochondrial electron transport chain. FeS++ groups in complex I and the Qo segment of the quinone cycle in complex III are common sources for electrons to escape the transport chain and reduce oxygen. This occurs more frequently when mitochondria are hyperpolarized and ATP/ADP ratios are high. Superoxide cannot easily cross mitochondrial membranes but when its formation is directed toward the matrix, MnSOD rapidly converts it to H2O2 which is freely diffusible to the cytosol. When directed toward the intermembrane space, CuZn SOD and convert superoxide to hydrogen peroxide.
We used pharmacological and fluorescence detection approaches to establish the role of mitochondrial ROS in mediating FMD. First, flow in HCA causes increases in mitoSOX fluorescence (a marker for mitochondrial derived superoxide), an indicator of mitochondrial superoxide formation (unpublished data). This is associated with an elevation in cytosolic peroxides. More importantly specific inhibitors of mitochondrial complexes I and III (rotenone and myxothiazol) but not of complex IV (cyanide) reduced both mitoSOX fluorescence and FMD [38]. This shows that inhibition of mitochondrial respiration is not sufficient to block FMD; rather reduction in mitochondrial ROS formation is required. Dilation to papaverine was not affected by any inhibitor, excluding a non-specific effect.
In preliminary data we have used mitochondria-targeted antioxidants to confirm the hypothesis. Vitamin E covalently bonded to triphenylphosphonium ion is taken up into the mitochondrial matrix 100-200 fold more than in the cytosol. This is due to the marked membrane potential gradient created by hydrogen ion extrusion. Vitamin E (1 mM) had no effect on FMD, but a similar quantity of mito-Vitamin E eliminated both the increase in mitoSOX formation and FMD in these vessels. Another intramitochondrial antioxidant, mito-PBA which is selective for peroxynitrite and H2O2 showed similar results [71]. These data confirm a role for endothelial mitochondrial generation of hydrogen peroxide, the transferrable factor mediating vasodilation, in FMD of HCA in subjects with CAD.
Mechanochemical signaling of FMD in the human heart
It is interesting to consider how a mechanical stress applied to the surface of an endothelial cell would stimulate the release of H2O2 from the mitochondria. Multiple mechanochemical signaling options have been described [13], however we have evidence supporting the role of two in the human coronary circulation. Endothelial cells contain an extensive actin filamentous and microtubular subarchitecture that provides structural stability and anchorage to the underlying basement membrane, and also serves to transduce mechanical signals [1]. For example actin filaments connect the endothelial surface with focal adhesion complexes on the basal segment of the cell that anchor the cell to the basement membrane through integrins. Mechanical perturbation of these cytoskeletal fibers (e.g. through shear stress), tug on focal adhesion kinases at the cell’s base leading to their activation, which in turn phosphorylates and stimulates eNOS, with NO release, resulting in dilation [61]. This is a key component of the mechanism of FMD in rat gracilis arterioles [60].
We examined whether a similar mechanical stress could also activate mitochondrial release of hydrogen peroxide. HCA treated with nocodazole (to disrupt microtubules) or cytochalasin D (to block actin filament elongation) prevented the shear-induced increase in fluorescence from DCF (peroxides) and 2-hydroxy ethidium (superoxide formation) in HCA [37]. Both inhibitors also blocked mitochondrial superoxide elevations during shear and reduced FMD, with an additive effect using both inhibitors together [37]. Neither inhibitor affected the dilation to bradykinin or papaverine. These data show that intact cytoskeletal elements play a key role in shear-induced mitochondrial ROS generation and dilation in HCA.
Other mechanosensitive elements are present in endothelial cells. The glycocalyx[23;23] [29] is a known mechanotransducer in endothelial signaling. Intact caveolae are required in some vessels for FMD [69]. In addition, a variety of ion channels have been postulated to sense shear in endothelium. Among these, the transient receptor potential vannilloid channel 4 subtype cation channel (TRPV4) has been implicated in multiple vascular beds [40;42;44]. TRPV4 conducts calcium as well as sodium into the cell. They are constitutively expressed and located in caveolae [53]. We examined the role of TRPV4 in FMD of coronary arterioles. In mouse coronary vessels, inhibition of TRPV4 with ruthenium red abrogated FMD [44]. Furthermore TRPV4 KO mice showed a mild increase in blood pressure and absence of coronary FMD. These data show that TRPV4 are critical for FMD. In preliminary studies we examined the role for TRPV4 in signaling FMD in HCA. The TRPV4 opener, 4αPDD_- phorbol 12,13-didecanoate (4αPDD).
stimulated mitochondrial ROS formation and dose-related endothelium-dependent dilation [3]. Inhibition of TRPV4 (ruthenium red) blocked dilation to 4aPDD and to shear in HCA but had no effect on the dilation to papaverine. Since ruthenium red is not specific, we also used siRNA to knock down TRPV4 channel expression in HCA [3]. After siRNA treatment, vasodilation to papaverine was maintained but FMD was significantly reduced, suggesting a role for TRPV4 in mediating FMD in HCA. It is of interest that EETs are known to stimulate TRPV4 opening [67], providing a possible mechanism by which EET is involved in the FMD signaling pathway. This will require further study.
Vascular Smooth Muscle relaxation to endothelial derived hydrogen peroxide
The intraendothelial signal transduction pathway for FMD in HCA is described above. As a final event, H2O2 is released from the endothelium to elicit smooth muscle relaxation. There are a number of pathways by which H2O2 can elicit dilation. Most involve the NO-cGMP-PKG-BKCa pathway leading to membrane hyperpolarization (thus denoted as an EDHF dilator mechanism) and reduced calcium entry, resulting in relaxation. Much of the biological activity involving H2O2 results from thiol oxidation, leading to RSSG or RSSR (disulfide) bond formation [17;68]. However this fundamental redox effect can also lead to diametrically opposite vascular responses via modification of proteins in the same pathway. For example H2O2 can activate eNOS (through phosphorylation of S1179) as a result of oxidation and inhibition of specific phosphatases [64]. This results in BKCa opening. However H2O2 can also inhibit opening of the human Slo1 channel (calcium activated potassium channel homolog of KCa alpha subunit) expressed in HEK cells (BKCa alpha subunit) [62] by oxidizing cysteine residues in the calcium-sensing bowl region of the channel’s pore-forming unit located in the cytosolic domain.
H2O2 stimulates guanylyl cyclase directly leading to formation of cGMP in pulmonary arteries [5], leading to PKG activation. Alternatively, H2O2 can oxidize cysteine 42 on PKG-1α monomers to create a disulfide bond, resulting in dimerization and activation of the kinase [4]. In HCA we tested whether hydrogen peroxide-induced opening of BKCa channels involves activation of the cGMP-PKG pathway.
In preliminary studies, dilation of HCA to H2O2 was not altered by L-NAME, an inhibitor of NOS, or by ODQ, a selective inhibitor of guanylyl cyclase [70]. The dilation was inhibited by 8-Br-cGMP, a specific inhibitor of PKG-1α. Treatment of cultured human coronary endothelial cells with H2O2 increased dimerization of PKG-1α, an effect that could be blocked with dithiothreitol [70]. These data indicate that PKG-1α is activated directly by H2O2 through dimerization, and that this activation is responsible for dilation in HCA.
A summary of the pathways involved in FMD of the human coronary microcirculation are depicted in the schematic shown in Figure 3.
Concluding remarks
Shear stress is a ubiquitous mechanism of vasodilation that is arguably the most important endothelium-dependent mechanism of flow regulation. There are a variety of mediators responsible for this response across species and vascular beds, but shear-induced mitochondrial production of H2O2 appears to be a unique mechanism in the coronary microcirculation of people with conduit coronary atherosclerosis. In addition to providing insights into regulating perfusion in the diseased heart, these observations may have broader vascular impact. To the extent that microcirculatory abnormalities predict large vessel dysfunction and atherosclerosis [20], the specific mediators of dilation (NO, prostaglandins, EET, hydrogen peroxide) may have very different effects on prognosis and propensity for atherosclerosis. NO and the EDHF, EET, are antithrombotic, prevent smooth muscle proliferation, and suppress endothelial activation. However H2O2 can stimulate smooth muscle proliferation, endothelial cell adhesion molecule expression, and thrombosis, thus understanding the transition from one dilator agonist to another could be an important prognostic marker and therapeutic or preventive target. Future studies should examine these transitions in dilator mechanism from health to disease, and examine whether the endothelial products released during shear (or agonist stimulation) can be modulated to minimize vascular damage from key atherosclerosis risk factors.
Highlights.
Coronary blood flow is regulated by arterioles between 50-200 microns in diameter
Shear-induced dilation amplifies metabolic dilation by reducing upstream vascular resistance
Shear-induced endothelial hydrogen peroxide originates from mitochondria
This shear-released hydrogen peroxide dilates coronary arterioles from humans with CAD
Transient receptor potential vanilloid 4 channels are required for the dilation to shear
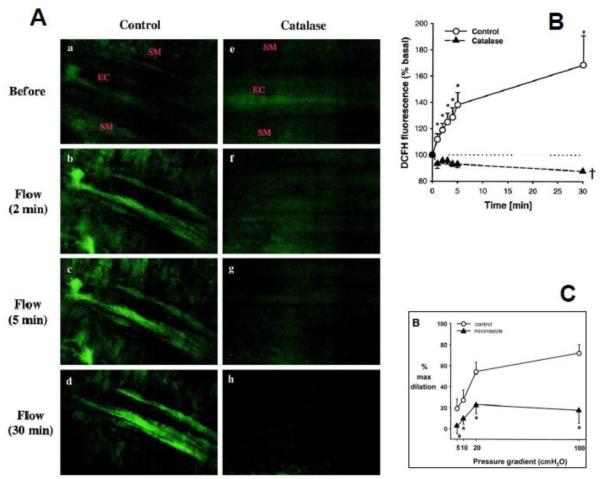
Figure 1. Hydrogen peroxide and epoxyeicosatrienoic acids are necessary for flow-mediated dilation of human coronary arterioles from patients with coronary disease.
Representative images of DCFH fluorescence (A) in an human coronary arteriole before (a and e) and after exposure to shear stress (b through d and f through h) in the absence or presence of catalase (1000 U/mL). a through d, Shear stress induces an increase in fluorescence intensity in the endothelial cell layer (EC) but not in the VSMC layer (SM). e through h, Increase in fluorescence to shear stress was not observed in vessels treated with catalase. Summary data (B) shows that shear stress induced endothelial H2O2 production occurred in a time-dependent manner (*P<0.05 vs baseline, n=5). Treatment with catalase completely abolished H2O2 production (P<0.05 vs control, n=4). Flow-induced dilation is also markedly inhibited by miconazole (C) or the selective epoxygenase inhibitor 17-ODYA. Reprinted from [45] and [46]. Reproduced with permission from the American Heart Association.
Footnotes
Disclosures: none declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bevan JA, Kaley G, Rubanyi GM. Flow-dependent regulation of vascular function. Oxford University Press; New York: 1995. [Google Scholar]
- 2.Bell MR, Lerman LO, Rumberger JA. Validation of minimally invasive measurement of myocardial perfusion using electron beam computed tomography and application in human volunteers. Heart. 1999;81:628–635. doi: 10.1136/hrt.81.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubolz AH, Zhang DX, Larsen BT, Gutterman DD. TRPV4 mediates flow - induced dilation in human coronary arterioles. Faseb Journal. 2007;21:A1234. [Google Scholar]
- 4.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 5.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 6.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med. 2009;50:1076–1087. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 8.Canty JM, Jr., Schwartz JS. Nitric oxide mediates flow-dependent epicardial coronary vasodilation to changes in pulse frequency but not mean flow in conscious dogs. Circ. 1994;89:375–384. doi: 10.1161/01.cir.89.1.375. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res. 1991;69:561–570. doi: 10.1161/01.res.69.3.561. [DOI] [PubMed] [Google Scholar]
- 11.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251:H779–H788. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 12.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circ. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 13.Davies PF. Flow-mediated signal transduction in endothelial cells. In: Bevan JA, Kaley G, Rubanyi GM, editors. Flow-dependent regulation of vascular function. Oxford University Press; New York: 1995. pp. 46–61. [Google Scholar]
- 14.Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circ. 1999;100:1951–1957. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- 15.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 16.Feigl EO. Control of myocardial oxygen tension by sympathetic coronary vasoconstriction in the dog. Circ Res. 1975;37:88–95. doi: 10.1161/01.res.37.1.88. [DOI] [PubMed] [Google Scholar]
- 17.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, Bornstein SR, Duerrschmidt N, Wagner AH, Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res Cardiol. 2011;106:551–561. doi: 10.1007/s00395-011-0170-3. [DOI] [PubMed] [Google Scholar]
- 19.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48–55. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 20.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circ. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 21.Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9:135–143. doi: 10.1067/mnc.2002.120680. [DOI] [PubMed] [Google Scholar]
- 22.Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol. 2010;105:1–5. doi: 10.1007/s00395-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Deussen A. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res. 1983;53:8–15. doi: 10.1161/01.res.53.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- 25.Hundley WG, Lange RA, Clarke GD, Meshack BM, Payne J, Landau C, McColl R, Sayad DE, Willett DL, Willard JE, Hillis LD, Peshock RM. Assessment of coronary arterial flow and flow reserve in humans with magnetic resonance imaging. Circ. 1996;93:1502–1508. doi: 10.1161/01.cir.93.8.1502. [DOI] [PubMed] [Google Scholar]
- 26.Kaul S. Instrumentation for contrast echocardiography: technology and techniques. Am J Cardiol. 2002;90:8J–14J. doi: 10.1016/s0002-9149(02)02859-x. [DOI] [PubMed] [Google Scholar]
- 27.Knaapen P, Camici PG, Marques KM, Nijveldt R, Bax JJ, Westerhof N, G÷tte MJW, Jerosch-Herold M, Schelbert HR, Lammertsma AA. Coronary microvascular resistance: methods for its quantification in humans. Basic Res Cardiol. 2009;104:485–498. doi: 10.1007/s00395-009-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990;67:529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai R, Lu X, Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2009;47:600–607. doi: 10.1016/j.freeradbiomed.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991;261:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 31.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol. 1988;255:H1558–H1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- 32.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and - independent vascular responses in the coronary microcirculation. Circ. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- 33.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen BT, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Bubolz AH, Zhang DX. H2O2 Is the Transferrable Factor Mediating Flow-Induced Dilation in Human Coronary Arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Li H, Bubolz AH, Zhang DX. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput. 2008;46:469–473. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 39.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Jr., Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. NEJM. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010;30:851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 41.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circ. 1990;82:1–7. doi: 10.1161/01.cir.82.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Marrelli SP, O’neil RG, Brown RC, Bryan RM., Jr. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 43.Meimoun P, Tribouilloy C. Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: a magic tool for the real world. Eur J Echocardiogr. 2008;9:449–457. doi: 10.1093/ejechocard/jen004. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza SA, Fang J, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 46.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr., Saito T, Miura M. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circ. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffari MS, Baban B, Liu JY, Abebe W, Sullivan JC, El-Marakby A. Mitochondrial complex I and NAD(P)H oxidase are major sources of exacerbated oxidative stress in pressure-overloaded ischemic-reperfused hearts. Basic Res Cardiol. 2011;106:287–297. doi: 10.1007/s00395-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 48.Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res. 1996;32:668–678. [PubMed] [Google Scholar]
- 49.Mulvagh SL, DeMaria AN, Feinstein SB, Burns PN, Kaul S, Miller JG, Monaghan M, Porter TR, Shaw LJ, Villanueva FS. Contrast echocardiography: current and future applications. J Am Soc Echocardiogr. 2000;13:331–342. doi: 10.1067/mje.2000.105462. [DOI] [PubMed] [Google Scholar]
- 50.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagel E, Bornstedt A, Hug J, Schnackenburg B, Wellnhofer E, Fleck E. Noninvasive determination of coronary blood flow velocity with magnetic resonance imaging: comparison of breath-hold and navigator techniques with intravascular ultrasound. Magn Reson Med. 1999;41:544–549. doi: 10.1002/(sici)1522-2594(199903)41:3<544::aid-mrm17>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 52.Najibi S, Cowan CL, Palacino JJ, Cohen RA. Enhanced role of potassium channels in relaxations to acetylcholine in hypercholesterolemic rabbit carotid artery. Am J Physiol. 1994;266:H2061–H2067. doi: 10.1152/ajpheart.1994.266.5.H2061. [DOI] [PubMed] [Google Scholar]
- 53.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 54.Onoue H, Tsutsui M, Smith L, Stelter A, O’Brien T, Katusic ZS. Expression and function of recombinant endothelial nitric oxide synthase gene in canine basilar artery after experimental subarachnoid hemorrhage. Stroke. 1998;29:1959–1965. doi: 10.1161/01.str.29.9.1959. [DOI] [PubMed] [Google Scholar]
- 55.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- 56.Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–123. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Randall MD, Kendall DA. Anandamide and endothelium-derived hyperpolarizing factor act via a common vasorelaxant mechanism in rat mesentery. Eur J Pharmacol. 1998;346:51–53. doi: 10.1016/s0014-2999(98)00003-x. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Recalde A, Galeote G, Moreno R, Dobarro D, Gomez-Rubin MC, Calvo L, Sobrino N, Martin-Reyes R, Jimenez-Valero S, Sobrino JA, Lopez-Sendon JL. Long-term prognostic value of endothelial dysfunction in patients with chest pain and angiographically normal coronary arteries. Rev Port Cardiol. 2009;28:785–791. [PubMed] [Google Scholar]
- 59.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun D, Huang A, Sharma S, Koller A, Kaley G. Endothelial microtubule disruption blocks flow-dependent dilation of arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2087–H2093. doi: 10.1152/ajpheart.2001.280.5.H2087. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Ishida T, Traub O, Corson MA, Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res. 1997;34:212–219. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- 62.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 63.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circ. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 64.Thomas S, Kotamraju S, Zielonka J, Harder DR, Kalyanaraman B. Hydrogen peroxide induces nitric oxide and proteosome activity in endothelial cells: a bell-shaped signaling response. Free Radic Biol Med. 2007;42:1049–1061. doi: 10.1016/j.freeradbiomed.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 66.Vanhoutte PM. COX-1 and vascular disease. Clin Pharmacol Ther. 2009;86:212–215. doi: 10.1038/clpt.2009.108. [DOI] [PubMed] [Google Scholar]
- 67.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 68.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 69.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang DX, Gebremedhin D, Gutterman DD. H2O2 dilates human coronary arterioles by stimulating the large-conductance Ca2+-activated K+ channel activity. FASEB. 2011;25:379. [Google Scholar]
- 71.Zinkevich NS, Gutterman DD. Role of mitochondrial reactive oxygen species on flow- induced dilation in human arterioles with coronary artery disease. FASEB. 2011;25:273. [Google Scholar]
- 72.Zinkevich NS, Wittenburg AL, Gutterman DD. Role of Cyclooxygenase in flow-induced dilation of human coronary arterioles depends upon age. Circulation. 2010;vol:122 [Google Scholar]