Abstract
Integrins play myriad and vital roles in development and disease. They connect a cell with its surroundings and transmit chemical and mechanical signals across the plasma membrane to the cell’s interior. Dissecting their roles in cell behavior is complicated by their overlapping ligand specificity and shared downstream signaling components. In principle, synthetic peptides can be used to modify surfaces to mimic extracellular matrix proteins by supporting integrin-mediated adhesion, but most short peptide sequences lack selectivity for one integrin over others. In contrast, synthetic integrin antagonists can be highly selective. We hypothesized that this selectivity could be exploited if antagonists, when immobilized, could support cellular adhesion and activate signaling by engaging specific cell-surface integrins. To investigate this possibility, we designed a bifunctional (RGD)-based peptidomimetic for surface presentation. Our conjugate combines a high affinity integrin ligand with a biotin moiety; the former engages the αvβ3 integrin and the latter allows for presentation on streptavidin-coated surfaces. Surfaces decorated with this ligand promote both cellular adhesion and integrin activation. Moreover, the selectivity of these surfaces for the αvβ3 integrin can be exploited to capture a subset of cells from a mixed population. We anticipate that surfaces displaying highly selective small molecule ligands can reveal the contributions of specific integrin heterodimers to cell adhesion and signaling.
Keywords: Integrin, Small Molecules, Biomaterials, Human Pluripotent Stem Cells, Peptidomimetic
Virtually every cell in the human body, with the exception of erythrocytes, produces a member of the integrin family of receptors (1). This family of cell-surface adhesion molecules provides a pivotal connection between a cell and its surroundings. Integrins are noncovalent heterodimeric transmembrane proteins composed of one a and one β subunit (2). In humans, there are 18 a subunits and 8 β subunits that form 24 unique αβ combinations (1). Both of the subunits contribute to the recognition of insoluble extracellular matrix (ECM) proteins (3). Integrin—ECM interactions are stabilized through integrin clustering (4, 5). Cytoplasmic proteins such as talin, vinculin, and paxillin concentrate at these adhesion complexes to regulate kinases, including focal adhesion kinase (FAK) and Src (4). Through these kinases, integrins mediate a variety of biological processes including anchorage, motility, and cell shape. Integrin engagement also controls cell survival, proliferation, and differentiation (4). Different integrin heterodimers activate many of the same downstream signaling kinases (4); however, differences among integrin intracellular domains and associated transmembrane proteins can result in integrin-specific signaling events (4, 6). Discriminating these integrin-specific signaling events is difficult because of integrins’ overlapping ligand specificities (7, 8). Monoclonal antibodies for specific integrins have proved valuable tools for dissecting integrin function, but these agents typically act by blocking the binding and signaling of specific integrins. Materials that can engage specific integrins and thereby recruit them for adhesion and signaling would complement antibody approaches. We envisioned combining modern surface fabrication methods in conjunction with highly selective integrin ligands to probe and exploit integrin function.
Our strategy requires access to integrin ligands that target specific heterodimers selectively. The tripeptide, arginine-glycine-aspartic acid (RGD) (9) was identified as the minimal recognition sequence for a group of integrins (10). This sequence is found within several ECM proteins including fibronectin, vitronectin, fibrinogen, von Willebrand factor, thrombospondin, laminin, entactin, tenascin, osteopontin and bone sialoprotein, an insight that led to its identification as an integrin binding motif (8, 11). Subsequently, synthetic peptides derived from ECM proteins or phage display screens have been appended to many diverse materials to support cell adhesion (11, 12). In most applications explored to date, linear RGD-containing peptides are employed. For dissecting integrin function, however, RGD-containing peptides have two drawbacks. First, the affinity of these integrins for unstructured linear RGD is weak; the GRGDSP hexapeptide derived from fibronectin is 1000 times less effective at supporting cell adhesion than the full-length protein (13). The second is that the tripeptide RGD is recognized by one-third of all integrins (αIIbβ3, αvβ3, αvβ5, αvβ6, αvβ8, αvβ1, α5β1, and α8β1) (11). The ability of these integrins to bind RGD is not indicative of shared function. Genetic knockout studies suggest that the functions of many integrins are distinct (14). For instance, both αvβ3 and α5β1 integrins recognize RGD, but the activity of intracellular kinases in response to integrin-mediated adhesion depends on whether the cells express αvβ3 or α5β1 integrin (15). These studies offer incentive to devise new strategies to probe integrin function.
Synthetic small molecules have been found that engage select integrins (16). One factor that affects integrin specificity for RGD is the conformation of the ligand (17–19). Preorganization of key functional groups can yield ligands that bind their target with high affinity and greater specificity (20). Accordingly, small molecules targeting several integrins including αvβ3, α4β1, αIIbβ3, and α5β1 have been generated (21–24). These small molecules can act as potent integrin antagonists and tumor targeting agents (21, 25). We have previously employed one such RGD-based peptidomimetic for selective targeting of tumor cells expressing αvβ3 integrin (26, 27). Although designed to antagonize integrin functions, we postulated that these small molecules should support cell adhesion and signaling when appended to a surface. In this way, surfaces could be tailored to recruit specific integrin functions.
A limited number of specific integrin-targeting small molecules have been tested for their ability to support cell adhesion when immobilized on a surface (28–31). It is unknown, however, if the resulting synthetic surfaces could mediate adhesion through a specific integrin. Moreover, no such surface or material has been shown to activate integrin-mediated signaling (28–31). If such surfaces activate integrin-mediated signaling, they could selectively promote signal transduction pathways downstream of a specific integrin. Moreover, surfaces presenting highly selective small molecules could be useful for capturing desired cell types from a heterogeneous population. Thus, we set out to investigate the properties of surfaces modified with a selective integrin ligand, as they could yield insights that complement those obtained from traditional loss-of-function studies.
RESULTS
Design and Synthesis of Bifunctional Conjugates
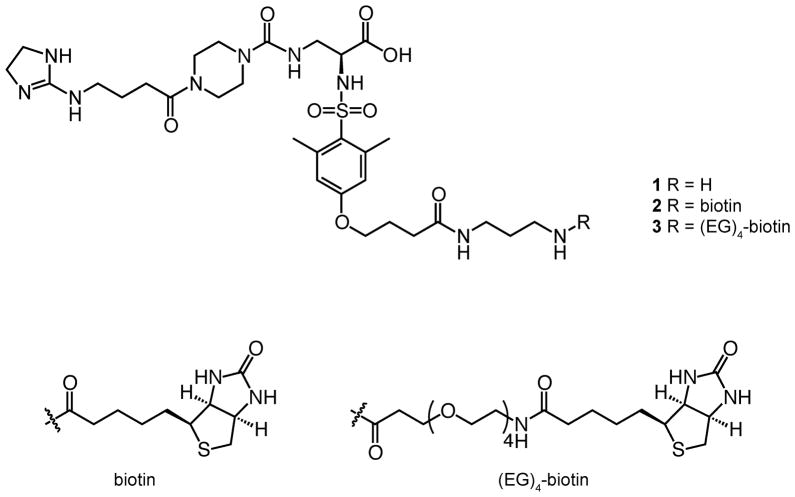
To test the utility of highly specific, synthetic integrin antagonists in adhesion, we devised a modular method for ligand immobilization. The ligand we tested is a synthetic antagonist targeting αvβ3 integrin. This compound is a variation of an inhibitor identified by Degrado and coworkers (32). We previously developed a route to append a linker terminating in an amino group (compound 1) (27). The amine can be coupled to additional functional units to afford bifunctional compounds (27). We envisioned that a biotinylated version of the αvβ3 antagonist would yield a bifunctional compound that could be presented on readily available polystyrene plates coated with streptavidin (33). Streptavidin-coated surfaces can display adhesion ligands at a density of approximately 5.2 pmol cm−2 (34), which is within the range of other methods for immobilizing adhesion ligands to polystyrene (35). This strategy has also been used to generate defined, peptide-substituted surfaces for propagating human pluripotent stem cells (36). We therefore synthesized a bifunctional molecule with two distinct motifs: the αvβ3 integrin targeting non-peptidic small molecule (32) and a biotin moiety.
A key parameter in implementing this immobilization strategy is the length of the linker separating the integrin-binding and biotin groups. Inspection of the streptavidin-biotin complex suggests the binding pocket is buried (33). To explore the spacer requirements, we generated bifunctional ligands that differ in the length of the spacer separating the integrin-binding and biotin groups (Figure 1). Both compounds were derived from amine 1 (27). This synthetic building block (27) was exposed to a biotin derivative bearing a succinimidyl ester (NHS-biotin) to yield conjugate 2 (Figure 1). Similarly, we prepared bifunctional compound 3 with a longer spacer between the two binding moieties (Figure 1) (37).
Figure 1.
Chemical structures of the bifunctional compounds used for surface production. The parent compound 1 is an RGD-based small molecule, which possesses a linker bearing an amine group for conjugation to other moieties. Amine 1 can be functionalized to append biotin (2) or a biotin with an oligoethylene glycol ((EG)4) linker (3).
Cell Adhesion
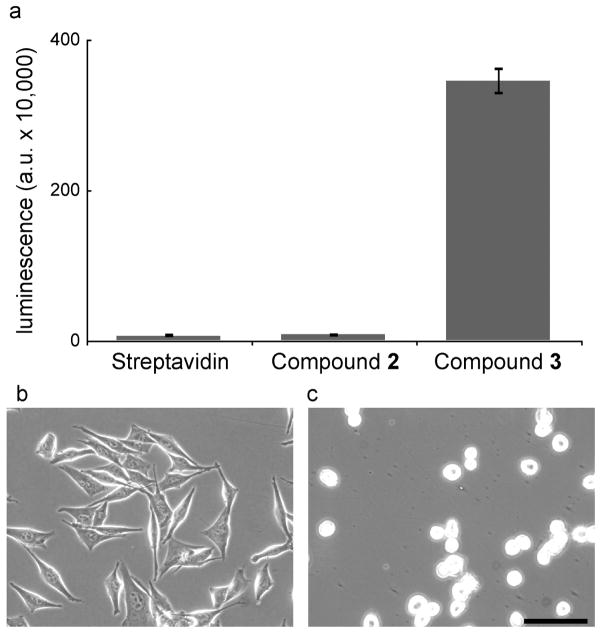
We evaluated streptavidin-coated surfaces presenting the biotinylated small molecules in a cell adhesion assay. We employed M21 cells, a melanoma line with high levels of αvβ3 integrin (26). These cells did not adhere to streptavidin-coated surfaces (Figure 2, panel a), nor did they bind to surfaces functionalized with compound 2, which bears the shorter linker (Figure 2, panel a). We postulated that the accessibility of the integrin binding group was the problem. Consistent with this idea, surfaces generated with compound 3, which has a longer connector separating the biotin and the integrin-binding group, support cell adhesion (Figure 2, panel a). Thus, linker length is a critical factor for ligand accessibility on streptavidin-coated surfaces, and the linker in compound 3 possesses the requisite properties.
Figure 2.
Surfaces presenting the bifunctional small molecule 3 support cell adhesion. a) M21 cells bind to the indicated surfaces as measured by a luminescence assay. Error bars represent the standard deviation (n=3 technical replicates). b–c) Micrographs of M21 cells cultured on surfaces presenting 3 (b) or a biotinylated heparin-binding peptide (FHRRIKA) (c). Scale bar, 100 μm.
Cell spreading can be an important determinant of cell survival (4, 38); therefore, we investigated the extent of cell spreading on surfaces modified with compound 3. The resulting surfaces mediate both cell attachment and spreading (Figure 2, panel b). To compare the influence of integrin engagement with other modes of adhesion, we plated cells on a streptavidin-coated surface modified with the heparin-binding peptide FHRRIKA derived from bone sialoprotein (39). This peptide supports cell attachment by binding to cell surface glycosaminoglycans (GAGs); therefore, cell adhesion is independent of integrin engagement. Although cells attached to this surface, they did not spread (Figure 2, panel c). These data reveal that our RGD-based small molecule supports robust cell adhesion. We next examined the key issue of ligand specificity.
Evaluating the Specificity of the Integrin-Ligand Presenting Surfaces
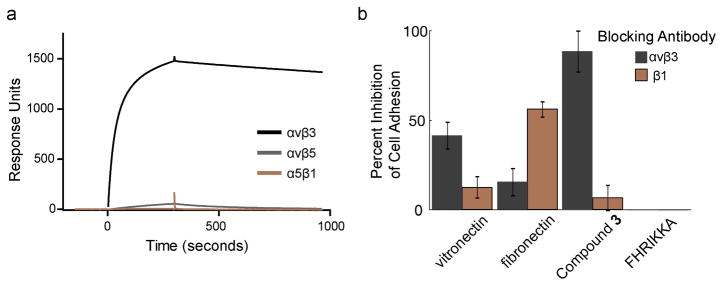
The peptidomimetic group in our bifunctional ligand was designed to target the αvβ3 integrin (32). To assess the specificity of the conjugate, we used surface plasmon resonance (SPR) experiments. The bifunctional ligand 3 was immobilized on streptavidin-functionalized sensor chips, and the resulting surface was exposed to several recombinant, soluble integrin heterodimers. We compared the binding of αvβ3 with that of the closely related αvβ5 integrin and another RGD-recognizing integrin, α5β1. Of these integrins, only αvβ3 integrin interacted with the surface; no binding of αvβ5 and α5β1 integrins was detected (Figure 3, panel a). These data highlight the excellent αvβ3 integrin selectivity of the small molecule ligand.
Figure 3.
Adhesion to the surface functionalized with small molecule 3 is mediated by the αvβ3 integrin. a) Binding of recombinant αvβ3, αvβ5, or α5β1 integrin to 3 was assessed using surface plasmon resonance spectroscopy. b) Percent inhibition of M21 cell binding to the indicated surfaces in the presence of antibodies that block either the αvβ3 or the β1 integrins as measured by a luminescence assay (100 minus the average ratio of the mean luminescence of cell lysates plated in the presence of a blocking antibody versus those without antibody). Error bars represent the standard deviation (n=3 biological replicates).
To determine whether cell binding to the surface was mediated by the αvβ3 integrin, we used an antibody blocking experiment. Antibodies against either αvβ3 integrin or β1 integrin were tested for their abilities to inhibit all adhesion of the M21 melanoma cell line. We postulated that the activity of the β1 would be especially diagnostic because it forms heterodimers with a variety of a subunits, many of which recognize the RGD motif (2). For comparison, cell adhesion to surfaces coated with fibronectin and vitronectin also was assessed, because these proteins interact with a spectrum of integrins (8, 40). As expected, adhesion to the protein-coated surfaces was inhibited to some extent by both antibodies. Adhesion to vitronectin was inhibited to a greater extent by the αvβ3 blocking antibody, and adhesion to fibronectin was inhibited to a greater extent by the β1 blocking antibody (Figure 3, panel b). In contrast, adhesion to surfaces presenting the small molecule ligand was unaffected by the β1 blocking antibody, but was almost completely ablated by the αvβ3 blocking antibody (Figure 3, panel b). To control for any integrin-independent effects, we plated cells treated with the antibodies onto surfaces presenting a heparin-binding peptide. The blocking antibodies did not inhibit cell interactions with the glycosaminoglycan-binding surfaces (Figure 3, panel b). Together, the data indicate that adhesion to surfaces presenting the small molecule relies upon αvβ3 integrin engagement.
Comparison of Integrin Ligands
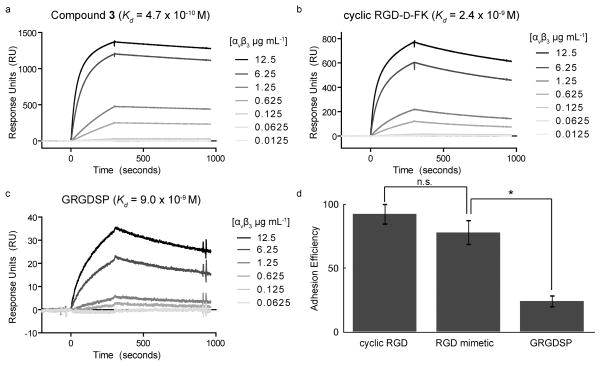
Our next objective was to determine whether differences in integrin-binding affinity were manifested in surfaces decorated with different integrin ligands. Specifically, we generated surfaces presenting the small molecule ligand, a short linear RGD-containing peptide (9), or a cyclic RGD derivative that is selective for αvβ3 and αvβ5 integrins (41). From the sensorgrams obtained from the αvβ3 integrin interacting with these surfaces, dissociation constants for the ligands were determined (Figure 4, panels a-c). Of the three ligands, our small molecule peptidomimetic had the highest affinity for αvβ3 (Kd = 4.7 × 10−10 M). Thus, the small molecule, when presented on a solid substrate maintains its high affinity for the αvβ3 integrin.
Figure 4.
Cell binding to different surfaces that present specific integrin ligands. a–c) SPR sensorgrams showing the binding of recombinant αvβ3 integrin to streptavidin-functionalized flow cells presenting b) the small molecule ligand 3 c) cyclic RGD-D-FK or d) the linear peptide GRGDSP. A streptavidin-functionalized flow cell saturated with biotin was used as a control. d) Percentage of cells binding to indicated surfaces relative to cells binding to vitronectin-coated surfaces as measured by a luminescence assay (average ratio of the mean luminescence of cell lysates for cells bound to vitronectin-coated surfaces after 18 h versus the mean luminescence of cell lysates for cells bound to the indicated surfaces). Error bars represent the standard deviation (n=3 biological replicates). Statistical analysis was performed using a two-tailed Student t test. Statistically significant P values < 0.01 are indicated with an asterisk. The abbreviation n.s. denotes a value that is not statistically significant.
The peptide-integrin ligand might be less selective than a cell binding experiment, as cells can exploit multivalent interactions. We therefore used a cell adhesion assay to compare surfaces displaying the small molecule to those presenting other integrin ligands. M21 cells were allowed to attach overnight, unbound cells were removed by washing, and the remaining bound cells were lysed. The concentration of ATP in the lysate was used as a measure of viable cell number and therefore number of cells bound. The results indicate that surfaces bearing the cyclic RGD derivative were almost as effective as the extracellular matrix protein vitronectin at supporting adhesion (Figure 4, panel d). As expected, the percentage of cells bound to the selective small molecule-substituted surfaces was slightly less than that observed with cyclic RGD-modified substrates (Figure 4, panel d). The surfaces presenting the promiscuous and low affinity ligand RGD peptide were the least effective at supporting cell adhesion (Figure 4, panel d). In general, surfaces with high affinity ligands were the most adhesive. Substrates capable of interacting with multiple cell surface receptors (vitronectin, cyclic RGD derivative, linear RGD), however, can compensate for their lower affinity. The discrepancy among the synthetic ligands’ binding affinities for recombinant αvβ3 integrin (Figure 4, panels a-c) and their effectiveness as cell adhesion ligands (Figure 4, panel d) suggests a role for both integrin-ligand affinity and selectivity.
Integrin Activation
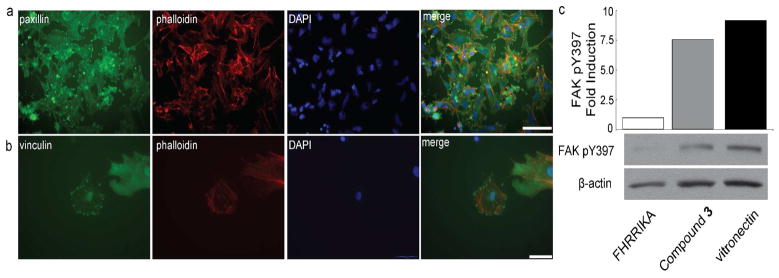
The previous results indicate that substrates displaying the small molecule ligand mediate cell adhesion; however, they do not address whether those tailored surfaces activate integrin signaling. A hallmark of integrin signaling is the formation of focal adhesions and actin stress fibers (4). Focal adhesions can be detected by staining for the adaptor proteins paxillin and vinculin, which bind the cytoplasmic tail of integrin β subunits upon integrin activation (4). Cells cultured overnight on surfaces presenting compound 3 exhibited punctate staining of paxillin and vinculin as well as the formation of defined actin stress fibers (Figure 5, panels a and b). Thus, the small molecule-presenting surfaces promote focal adhesion formation.
Figure 5.
Surfaces displaying the small molecule 3, which binds the αvβ3 integrin, activate signaling. a–b) M21 cells cultured on a surface presenting 3 were stained for paxillin (a, green) or for vinculin (b, green) and counterstained with phalloidin (red) and DAPI (blue). Scale bars, 100 μm (a) 50 μm (b). c) Histogram depicting change in focal adhesion kinase (FAK) phosphorylation (pY397) for cells cultured on the indicated surfaces; values were normalized to β-actin levels. Immunoblot analysis for cells cultured on indicated surfaces using a phospho-specific antibody against FAK pY397 and an antibody against β-actin.
Focal adhesion formation results from the activation of integrin-responsive kinases (4). Integrin-mediated adhesion to the ECM triggers autophosphorylation of focal adhesion kinase (FAK) in the activation loop residue tyrosine 397 (42). We therefore measured the levels of phosphorylated FAK in cells cultured upon the functionalized surfaces. To benchmark FAK pY397 levels, we seeded cells onto vitronectin-coated surfaces. We also measured FAK pY397 levels for cells grown on surfaces either modified with 3 or presenting a heparin-binding peptide. The latter serves as a useful control, because adhesion through GAGs should not activate integrin signaling. After three hours, bound cells were lysed; the resulting samples were subjected to immunoblotting to detect FAK pY397. Cells cultured on vitronectin contained high levels of FAK pY397 relative to those cultured on the surfaces modified with heparin-binding peptide (Figure 5, panel c). Interestingly, the level of FAK phosphorylation in cells grown on the small molecule-coated surfaces was similar to that obtained for cells cultured on vitronectin (Figure 5, panel c). Together, these data demonstrate that surfaces presenting a small molecule integrin ligand can indeed promote the formation of focal adhesions and stimulate kinase activity downstream of integrin signaling.
Manipulating Mixed Populations of Cells
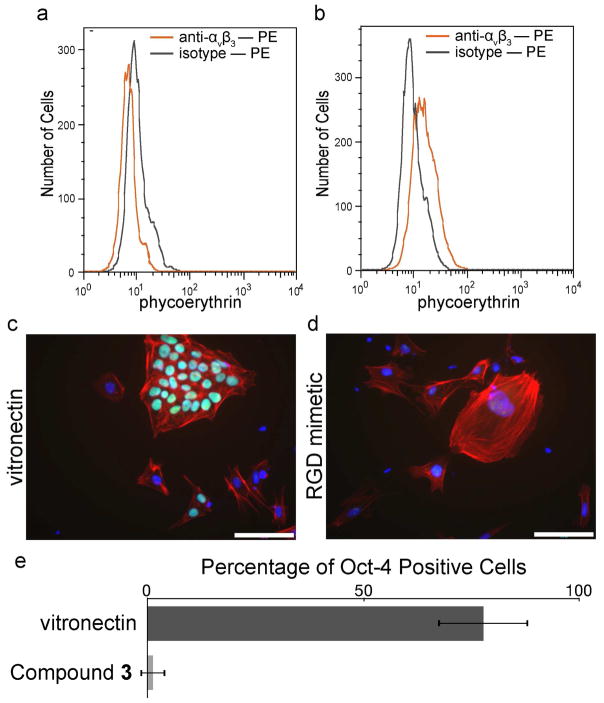
The selectivity of the small molecule-decorated surfaces was tested by exposing them to a mixed population of cell types. To generate such a population, we differentiated human embryonic stem (hES) cells. Embryonic stem cells are unique in their ability to self-renew indefinitely and differentiate into many different cell types (43). To initiate differentiation, hES cells were placed in suspension culture and allowed to form spheroid structures termed embryoid bodies (EBs); these cellular aggregates differentiate in a manner similar to the early embryo (44). In their pluripotent state, hES cells do not express αvβ3 integrin (Figure 6, panel a), but a subset of differentiated cells derived from EBs does (Figure 6, panel a). The differences in cell surface receptors led us to postulate that a synthetic surface could retain cells with specific integrin expression profiles.
Figure 6.
A surface decorated with the αvβ3 integrin-binding small molecule 3 supports the adhesion of differentiated cells but not undifferentiated human embryonic stem (hES) cells. a–b) Flow cytometry analysis of αvβ3 integrin levels in the cell population. a) Human ES cells (WA07) were analyzed by flow cytometry for αvβ3 integrin. b) Differentiated cells derived from embryoid bodies formed from the WA07 hES cell line were analyzed by flow cytometry for αvβ3 integrin. An increase in αvβ3 integrin is seen for the differentiated cell population. c–d) A mixed population of undifferentiated and differentiated cells was seeded onto vitronectin-coated surfaces and immunostained for Oct-4 (green) and counterstained with phalloidin (red) and DAPI (blue). Scale bars, 100 μm. e) High-content imaging was used to determine the number of Oct-4 positive cells from the mixed population bound to the indicated surfaces.
We seeded a population of cells containing undifferentiated hES cells and EB-derived cells onto surfaces presenting either our αvβ3 ligand or vitronectin. After 48 hours, cells were fixed and stained for the marker of pluripotency Oct-4. The presence or absence of the transcription factor Oct-4 marks undifferentiated and differentiated cells, respectively (43). Cells also were counterstained with phalloidin and DAPI; the former indentifies actin filaments, and the latter serves to visualize the location of cell nuclei. As expected, vitronectin-coated surfaces support the adhesion of both undifferentiated and differentiated cell types (Figure 6c). In contrast, surfaces presenting the αvβ3 specific ligand only bind differentiated cells (Figure 6d). High-content imaging of cells cultured on the surfaces decorated with the small molecule further demonstrates the selectivity of the tailored surfaces (Figure 6e). These data emphasize the utility of surfaces that engage specific cell surface receptors. They also indicate derivatives of hES cells can be manipulated based on substratum selectivity.
DISCUSSION
Biocompatible materials can mimic features identified as important from studies of cell—matrix interactions, cellular signaling mechanisms, and developmental pathways (45). An additional advantage of using synthetic materials over those that occur naturally is that the former can be tailored to a specific application. We reveal here a high affinity, selective small molecule can be used to functionalize surfaces to engage only those cells that produce the target αvβ3 integrin. Traditionally, cell adhesive surfaces have been decorated with small synthetic fragments derived from ECM proteins (46). Although effective at supporting cell adhesion, these ligands typically lack selectivity for specific cell surface receptors. In contrast to linear peptides, small molecules can exhibit higher affinity, selectivity, stability, and are resistant to enzymatic degradation (12). Thus, small molecules offer advantages over traditional methods for generating defined, synthetic surfaces and for probing integrin-mediated biological processes.
Our small molecule-modified surfaces not only mediate cell adhesion but they also activate integrin-mediating signaling. This activation is significant because crosstalk between specific integrins and growth factor receptors provides important contextual signals during development and regulates cell migration and differentiation (47). Integrin αvβ3, for example, cooperates with growth factors to promote angiogenesis (6). Synthetic strategies are being devised to direct growth factor signaling using insoluble substrata. Recently, we demonstrated that synthetic peptides displayed on a surface could nucleate the transforming growth factor β receptor signaling complex, and sensitize bound cells to the soluble growth factor (48, 49). These surfaces can potentiate specific growth factor signaling to deliver signals with spatial control (49). The use of insoluble cues to promote synergistic signals from integrins and growth factors to direct cell behavior and perhaps even cell fate decisions.
Precision control over cell binding and cell signaling would advance the fields of regenerative medicine and stem cell biology. Controlling the differentiation of human pluripotent cells to specific cell types remains a challenge. Typically, complex purification protocols employing fluorescence or magnetic-activated cell sorting are used to enrich for desired cells types or to separate differentiated cells from pluripotent cells. Such separations are required as pluripotent cells have the potential to form teratomas (50, 51). Our results indicate that surfaces selective for a specific cell-surface protein provide an alternative, rapid, and convenient method for capturing cells from a mixed population. Tailored surfaces could, therefore, augment or perhaps even replace current purification protocols. Unlike traditional methods for cell sorting (52), our strategy relies not only on the presence of a specific cell-surface marker, but its functionality as well. Moreover, surfaces presenting combinations of small molecules targeting specific adhesion receptors and growth factor receptors can provide a powerful method to sequester specific cell types and even guide their subsequent differentiation.
To accomplish these strategies, we must expand our ability to target specific cell-surface receptors using synthetic molecules. Small molecule binders selective for integrins other than αvβ3 have been reported. These small molecules target RGD-recognizing integrins (αIIbβ3, αvβ5, α5β1, αvβ6) (21, 53, 54) as well as ligands for RGD-independent integrins (α2β1, α4β1, α4β7, αLβ2) (21, 22). Although the integrin ligands reported to date were designed to be antagonists, the general, modular strategy outlined here could readily be applied to identify small molecules that promote cell adhesion. Tailored surfaces presenting these highly selective ligands could thus be used to unravel the contributions of specific integrins to cellular adhesion and signaling. Moreover, we anticipate that selective integrin-binding surfaces could also promote specific cell decisions.
METHODS
Synthesis of Bifunctional Conjugates
The synthesis of the key building block 1 has been published (27). Procedures for the synthesis of Compounds 2 and 3 are detailed in the Supporting Information. Other bifunctional molecules used in this study included biotin-Ahx-GFHRRIKA-NH2 (Biomatik), biotin-(OEG)4-GRGDSP (Anaspec), and cyclo RGD-D-FK-(OEG)2-(OEG)2-biotin (Peptides International).
Cell Culture
Human M21 melanoma cells were maintained in RPMI medium containing fetal bovine serum (10%), pen-strep antibiotics (100 U), and glutamax (2 mM)(Gibco). Human embryonic stem cells (WA07) were maintained as described previously (36). Cells were induced to differentiate as embryoid bodies in medium consisting of Iscove’s modified Dulbecco’s medium, 15% fetal bovine serum, 1% non-essential amino acids (Gibco). Cells were maintained at 37 °C in 5% carbon dioxide. For adhesion experiments, cells were seeded in serum-free mTeSR medium (Stem Cell Technologies).
To display the adhesion ligands on polystyrene, non-tissue culture treated plates (BD Falcon) were coated with 10 μg mL−1 streptavidin (Prospec) in Hanks’ balanced salt solution (HBSS) (Gibco). Wells were washed twice with HBSS and then coated with a 5 μM solution of biotinylated ligand diluted in HBSS.
Adhesion Assays
M21 cells were detached from cell culture flasks using 1 mM EDTA in PBS for 5–10 minutes. Cells were resuspended in a serum free medium at a concentration 6000 cells mL−1. Cell suspensions were seeded on to vitronectin-coated surfaces (5 μg mL−1, R&D Systems), fibronectin-coated surfaces (2.5 μg mL−1, R&D Systems), or streptavidin-coated surfaces (10 μg mL−1, Prospec) functionalized with a biotinylated small moleucle (5 μM). Blocking antibodies used in this study included the αvβ3 (clone LM609, 5 μg mL−1, Millipore) and β1 (clone 6s6, 10 μg mL−1, Millipore) blocking antibodies. After 3 h, surfaces were washed 2 times with PBS and the cells were lysed with radioimmunoprecipitation assay buffer containing 10% CellTiter-Glo (Promega), which is a homogenous and sensitive method to determine the number of viable cells in culture based on the presence of ATP. The luminescence was measured using an Infinite M1000 plate reader (Tecan).
Microscopy and Immunostaining
Images were collected with a Hamamatsu digital camera mounted onto an Olympus IX81 microscope. Primary antibodies used in this study included antibodies against paxillin (1:250, BD), vinculin (1:250, Sigma), and Oct-4 (1:250, R&D Systems). Cells were fixed with PBS containing 4% formaldehyde for 20 minutes and then permeabilized and blocked with PBS containing 0.1% Triton X-100 and 2% bovine serum albumin (BSA). Antibodies were incubated in blocking buffer overnight at 4 °C. Secondary staining was performed with Alexa Fluor 488-conjugated antibodies (1:1000, Invitrogen), which were diluted in blocking buffer and exposed to cells for 1 h at rt. Cells were counterstained with Alexa Fluor 594-conjugated phalloidin and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen). Image overlays were generated using ImageJ software. High-content imaging was performed using a BD Pathway 855 with laser autofocus. Image analysis was performed using BD AttoVision 1.6.2. Nuclear regions of interest were determined using DAPI stained nuclei and constrained using Oct-4 stained nuclei. Data analysis was performed using BD Image Data Explorer in Microsoft Excel.
Western Blotting
To determine the levels of FAK phosphorylation, M21 cells were transferred to the relevant surfaces in serum-free medium. After 3 h, cells were lysed at a final concentration of 1% Triton-X 100, 1 mM EDTA, 2 mM Na3VO4, 2 mM Na2MoO4, 2 mM NaF and 1:100 HALT protease inhibitor mixture (Pierce). Samples were resuspended in SDS-PAGE sample buffer and run on 4–20% gradient polyacrylamide Tris-HCl Ready gel (Bio Rad). For immunoblot analysis, samples were transferred to a 0.45-μm PVDF membrane (Millipore) in transfer buffer (14.4 g glycine, 3.03 g Tris base, and 10% methanol to 1 L, pH 8.3). Membranes were blocked for 1 h at rt using blocking buffer (TBS containing 0.1% Tween-20 (TBS-T) and 5% nonfat dry milk) before incubating with primary antibody overnight at 4 °C. The primary antibodies used in this study included antibodies against β-actin (Cell Signaling, 1:5000) and phospho-FAK Y 397 (BD, 1:2000) and were diluted in 5% BSA in TBS-T. Blots were rinsed for 3 times for 10 min with TBS-T before incubating with horseradish peroxidase (HRP)-conjugated secondary (Jackson Laboratories) antibodies diluted in blocking buffer for 1 h at rt. Blots were then rinsed 3 times for 10 min with TBS-T and then developed with chemiluminescent substrate (ECL; Pierce) and X-ray film (Thermo). Blots were analyzed using the ImageJ.
Flow Cytometry
To determine the levels of αvβ3 integrin, cells were detached with 1 mM EDTA in PBS and resuspended at 4 × 10−5 mL−1 in integrin binding buffer (IBB) (25 mM Tris HCl, 150 mM NaCl, 1.5% BSA, 5 mM glucose, 1.5 mM MgCl2, 1.5 mM MnCl2). Surface marker staining using an αvβ3 integrin phycoerythrin (PE) conjugated antibody (BD) was performed on ice in integrin binding buffer for 30 minutes with. After antibody exposure, the cells were washed once with IBB and then analyzed using a FACSCalibur flow cytometer and FlowJo software (Tree Star, Inc.).
Surface Plasmon Resonance Spectroscopy
SPR analysis was performed using a BIAcore 2000 instrument (GE Healthcare) to determine the binding affinities of immobilized biotinylated integrin ligands. Streptavidin-coated SA sensorchips (GE Healthcare) were used according to the manufacturer’s instructions and washed with two 60 second pulses of a solution containing 1 M NaCl with 50 mM NaOH prior to peptide immobilization. Biotinylated peptides (10 μM) were immobilized at a flow rate of 10 μL min−1 in sample running buffer (HBS-P supplemented with 1 mM MgCl2, GE Healthcare) using two 60 second pulses to ensure maximum immobilization. A reference channel was functionalized using biotin (Sigma-Aldrich). Sensorgrams were collected using soluble integrins (R&D Systems) injected in running buffer at a flow rate of 10 μL min−1 with surface regeneration performed using two 60 second pulses of a solution containing 1 M NaCl with 50 mM NaOH. Sensorgram analysis yielded dissociation constants (Kd) by kinetic analysis (simultaneous ka/kd) using BIAevaluation Software (GE Healthcare).
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (N.I.H.) (R01 GM049975) and the Department of Defense (DoD) (W81XWH-08-1-0648). We thank the W. M. Keck Foundation for supporting the Center for Chemical Genomics and the University of Wisconsin Paul P. Carbone Comprehensive Cancer Center Flow Cytometry Facility (5P30 CA014520-3S). SPR data were obtained at the University of Wisconsin—Madison Biophysics Instrumentation Facility (BIF). The NMR facilities at UW-Madison are funded by the NSF (CHE-9208463) and NIH (RR08389-01). The Shimadzu LCMS-2010A was purchased in 2000 with funds from a Keck grant, a Shimadzu grant, and the Chemistry Department. We thank D. R. McCaslin, L. Li, and M. R. Levengood for helpful conversations. R.T.C.S. was supported by the N.I.H. Chemistry-Biology Interface Training Program (T32 GM008505). P.J.W. was supported by the N.I.H. Molecular Biology Training Grant (grant no. T32 GM007215).
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: Evolutionary trees and model organisms. Biochim Biophys Acta, BBA. 2009;1788:779–789. doi: 10.1016/j.bbamem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong J-P, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αvβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 4.Giancotti FG, Ruoslahti E. Integrin Signaling. Science. 1999;285:1028–1033. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 5.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 7.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals-Distinct functions of α5β1 and αvβ3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–738. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 11.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 12.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 13.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264:1437–1442. [PubMed] [Google Scholar]
- 14.Hynes RO. Targeted Mutations In Cell Ahesion Genes: What Have We Learned From Them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- 15.Danen EHJ, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Update. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Nat Biotechnol. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 18.Pfaff M, Tangemann K, Müller B, Gurrath M, Müller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 19.Bach AC, Espina JR, Jackson SA, Stouten PFW, Duke JL, Mousa SA, DeGrado WF. Type II′ to Type I β-Turn Swap Changes Specificity for Integrins. J Am Chem Soc. 1996;118:293–294. [Google Scholar]
- 20.Cram DJ. The design of molecular hosts, guests, and their complexes. Science. 1988;240:760–767. [PubMed] [Google Scholar]
- 21.Perdih A, Dolenc S. Small Molecule Antagonists of Integrin Receptors. Curr Med Chem. 2010;17:2371–2392. doi: 10.2174/092986710791698558. [DOI] [PubMed] [Google Scholar]
- 22.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 23.Duggan ME, Duong LT, Fisher JE, Hamill TG, Hoffman WF, Huff JR, Ihle NC, Leu CT, Nagy RM, Perkins JJ, Rodan SB, Wesolowski G, Whitman DB, Zartman AE, Rodan GA, Hartman GD. Nonpeptide alpha(v)beta(3) antagonists. 1 Transformation of a potent, integrin-selective alpha(IIb)beta(3) antagonist into a potent alpha(v)beta(3) antagonist. J Med Chem. 2000;43:3736–3745. doi: 10.1021/jm000133v. [DOI] [PubMed] [Google Scholar]
- 24.Miller W, Keenan R, Willette R, Lark M. Identification and in vivo efficacy of small-molecule antagonists of integrin alphavbeta3 (the vitronectin receptor) Drug Discov Today. 2000;5:397–408. doi: 10.1016/s1359-6446(00)01545-2. [DOI] [PubMed] [Google Scholar]
- 25.Cacciari B, Spalluto G. Non peptidic alphavbeta3 antagonists: recent developments. Curr Med Chem. 2005;12:51–70. doi: 10.2174/0929867053363522. [DOI] [PubMed] [Google Scholar]
- 26.Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem Biol. 2007;2:119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]
- 27.Owen RM, Carlson CB, Xu J, Mowery P, Fasella E, Kiessling LL. Bifunctional ligands that target cells displaying the αvβ3 integrin. ChemBioChem. 2007;8:68–82. doi: 10.1002/cbic.200600339. [DOI] [PubMed] [Google Scholar]
- 28.Marchand-Brynaert J, Detrait E, Noiset O, Boxus T, Schneider YJ, Remacle C. Biological evaluation of RGD peptidomimetics, designed for the covalent derivatization of cell culture substrata, as potential promotors of cellular adhesion. Biomaterials. 1999;20:1773–1782. doi: 10.1016/s0142-9612(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 29.Biltresse S, Attolini M, Marchand-Brynaert J. Cell adhesive PET membranes by surface grafting of RGD peptidomimetics. Biomaterials. 2005;26:4576–4587. doi: 10.1016/j.biomaterials.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Rerat V, Dive G, Cordi AA, Tucker GC, Bareille R, Amédée Jl, Bordenave L, Marchand-Brynaert J. αvβ3 Integrin-Targeting Arg-Gly-Asp (RGD) Peptidomimetics Containing Oligoethylene Glycol (OEG) Spacers. J Med Chem. 2009;52:7029–7043. doi: 10.1021/jm901133z. [DOI] [PubMed] [Google Scholar]
- 31.Dahmen C, Auernheimer JR, Meyer A, Enderle A, Goodman SL, Kessler H. Improving Implant Materials by Coating with Nonpeptidic, Highly Specific Integrin Ligands. Angew Chem Int Ed. 2004;43:6649–6652. doi: 10.1002/anie.200460770. [DOI] [PubMed] [Google Scholar]
- 32.Corbett JW, Graciani NR, Mousa SA, DeGrado WF. Solid-phase synthesis of a selective αvβ3 integrin antagonist library. Bioorg Med Chem Lett. 1997;7:1371–1376. [Google Scholar]
- 33.Hendrickson W, Pähler A. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci USA. 1989;86:2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Välimaa L, Pettersson K, Vehniäinen M, Karp M, Lövgren T. A high-capacity streptavidin-coated microtitration plate. Bioconjug Chem. 2003;14:103–111. doi: 10.1021/bc020058y. [DOI] [PubMed] [Google Scholar]
- 35.Harbers GM, Gamble LJ, Irwin EF, Castner DG, Healy KE. Development and characterization of a high-throughput system for assessing cell-surface receptor-ligand engagement. Langmuir. 2005;21:8374–8384. doi: 10.1021/la050396y. [DOI] [PubMed] [Google Scholar]
- 36.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710–713. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- 38.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 39.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 40.Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 41.Hölzemann G, Goodman S, Kessler H. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1:107–114. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Schaller M, Hildebrand J, Shannon J, Fox J, Vines R, Parsons J. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 44.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci USA. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 46.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 47.Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Orner BP, Huang T, Hinck AP, Kiessling LL. Peptide ligands that use a novel binding site to target both TGF-β receptors. Mol Biosyst. 2010;6:2392–2402. doi: 10.1039/c0mb00115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Klim JR, Derda R, Courtney AH, Kiessling LL. Spatial control of cell fate using synthetic surfaces to potentiate TGF-{beta} signaling. Proc Natl Acad Sci USA. 2011;108:11745–11750. doi: 10.1073/pnas.1101454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schriebl K, Lim S, Choo A, Tscheliessnig A, Jungbauer A. Stem cell separation: a bottleneck in stem cell therapy. Biotechnol J. 2010;5:50–61. doi: 10.1002/biot.200900115. [DOI] [PubMed] [Google Scholar]
- 52.Tanke HJ, van der Keur M. Selection of defined cell types by flow-cytometric cell sorting. Trends Biotechnol. 1993;11:55–62. doi: 10.1016/0167-7799(93)90123-Q. [DOI] [PubMed] [Google Scholar]
- 53.Goodman SL, Hölzemann G, Sulyok GAG, Kessler H. Nanomolar small molecule inhibitors for αvβ6, αvβ5, and αvβ3 integrins. J Med Chem. 2002;45:1045–1051. doi: 10.1021/jm0102598. [DOI] [PubMed] [Google Scholar]
- 54.Boxus T, Touillaux R, Dive G, Marchand-Brynaert J. Synthesis and evaluation of RGD peptidomimetics aimed at surface bioderivatization of polymer substrates. Bioorg Med Chem. 1998;6:1577–1595. doi: 10.1016/s0968-0896(98)00083-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.