Abstract
Proton MR spectroscopic imaging of the human brain at ultra high field (≥7T) is challenging due to increased RF power deposition, increased magnetic field B0 inhomogeneity and increased RF magnetic field inhomogeneity. And, especially for multi-slice sequences, these effects directly inhibit the potential gains of higher magnetic field and can even cause a reduction in data quality. However, recent developments in dynamic B0 magnetic field shimming and dynamic multi-transmit RF control allow for new acquisition strategies. Therefore, in this work slice-by-slice B0 and B1 shimming was developed to optimize both B0 magnetic field homogeneity and nutation angle over a large portion of the brain. Together with a low-power water and lipid suppression sequence and pulse-acquire spectroscopic imaging, a multi-slice MRSI sequence is shown to be feasible at 7T. This now allows for multi-slice metabolic imaging of the human brain with high sensitivity and high chemical shift resolution at ultra high field.
Introduction
Proton MR spectroscopic imaging (MRSI) at ultra high field strength, in principle, allows for acquisition of metabolic information with a high sensitivity and accuracy due to an increased intrinsic signal to noise ratio (SNR) and an increase in chemical shift dispersion. However, the increases in chemical shift dispersion also requires higher bandwidth (BW) radio frequency (RF) pulses in suppression and localization schemes. At the same time RF power deposition increases, putting a limit on the amount and strength of the RF pulses that can be used. In addition, the homogeneity in both static magnetic field (B0) and transmit magnetic field (B1+) decreases with higher field strength, since both the magnetic susceptibility artifacts and the RF wave interference effects become more pronounced, causing severe spatial variation in spectral linewidth and the attained nutation angle. As a consequence, traditional localization and suppression sequences for (multi-slice) MRSI are not directly transferable to human ultra high field systems such as 7T since they would lead to severe SNR loss, large chemical shift displacement artifacts, incomplete water and lipid suppression and compromised spectral linewidth. However, recent developments in B0 shimming and RF transmit systems allow for the development of new acquisition strategies. First, the B0 homogeneity in multi-slice imaging and spectroscopy sequences has been shown to benefit from the slice-specific, dynamic application of up to third order spherical harmonic shim fields, as to insure optimal shimming for every individual slice, a technique known as dynamic shim updating (DSU) (1–2). Secondly, for MR (spectroscopic) imaging, multi-element transmit arrays show an important advantage over volume coils or surface coils since they allow homogenization of the transmit B1+ field in the brain over large regions (3), or to define custom shaped B1+ fields for purposes of outer volume suppression (4–5), or inner volume selection (6). This is mostly performed by a person-to-person (numerical) optimization of the transmit amplitudes and phases of multiple transmit elements, so called RF shimming (or B1+ shimming). With these novel developments, homogenization of both the B0 and B1+ field of the human brain becomes possible, thereby possibly opening the way for multi-slice spectroscopic imaging of large areas at 7T.
Several approaches for single-slice MRSI in the human brain have been shown at ultra high field (5,7–11), however, none of these methods appears to be readily extendable to a multi-slice acquisition scheme in order to achieve coverage over a larger area of the human brain. All proposed sequences are limited with respect to RF power deposition, therefore restricting the minimum achievable repetition time (TR) and therefore the number of slices that can be acquired. Especially high BW adiabatic inversion or refocusing pulses have not been considered for multi-slice MRSI yet, due to the high duty cycle on RF power and the associated high RF power deposition (5,10). The method of Xu et al (12) does achieve 3D coverage by performing 3D phase-encoding in a PRESS-selected box. Also Scheenen et al (13)have shown a 3D acquisition, albeit with a surface coil. While these methods provides high-quality data, it precludes the use of dynamic B0 shimming to improve the magnetic field homogeneity.
With the FID-acquisition technique (7–8), signal is acquired immediately after slice-selective excitation and phase encoding, thereby eliminating the slice-selective refocusing pulse needed for spin echo formation. Therefore, this approach lowers the RF power deposition substantially. At the same time, such a pulse-acquire technique reduces B1+ sensitivity, minimizes chemical shift displacement artifacts and maximizes sensitivity, since very short echo times can be realized (e.g. in the order of a few milliseconds). The published FID-acquisition methods are not readily modified and extended for multi-slice acquisitions due to the high RF power deposition associated with the water and lipid suppression schemes.
In this work a low power multi-slice MRSI sequence is presented that allows for a short effective TR and therefore enables the acquisition of multi-slice MRSI. A B1+ and T1 insensitive water and lipid suppression has been developed which is based on a limited number of low RF power pulses. The optimization of the RF powers and interpulse delays resulted in excellent water and lipid suppression over the whole volume. Slice-based B0 and B1+ updating was used to improve the homogeneity of both the static magnetic field and the transmit B1+ field. MRSI from five slices in the human brain at 7T is presented, acquired in 18 minutes with a voxel size of 8×8×8mm3.
Methods
MR measurements were performed on a 7 Tesla actively shielded, 68 cm inner diameter MR magnet interfaced to a Direct Drive spectrometer with 8 transmit and receive channels (Agilent, Santa Clara, CA). The asymmetric gradient system (Agilent, Santa Clara, CA) included a full set of third order shim coils equipped with preemphasis and B0 compensation on all shim terms, which was essential for successful DSU (2). All data were acquired using an eight element cylindrical transceiver array (10) driven by an eight channel (8×1 kW) RF amplifier (CPC, Hauppauge, NY). In this study, five human subjects were scanned with the MRSI sequence on five axial slices through the brain. All subjects were studied in accordance with Yale Institutional Review Board guidelines for research on human subjects.
MRSI sequence
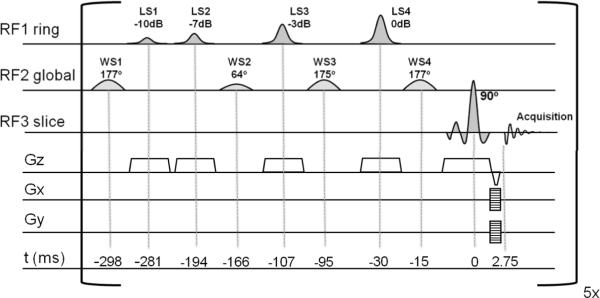
Multi-slice MRSI was performed on five axial slices with an (interleaved) slice-selective pulse-acquire MRSI sequence (figure 1). The Shinnar Le Roux optimized (14) slice-selective 90 degrees excitation pulse (BW=2.3kHz) was followed by phase encoding gradients and a refocusing gradient, resulting in a (gradient) echo time (TE) of 2.75 ms and a TR of 2500 ms (e.g. effective TR=500ms). A chemical shift displacement artifact was only present in the direction of the slice selective excitation (1 mm/ppm). MRSI data was acquired with 25×25 circular sampling of k-space over a 200×200 mm2 field-of-view, resulting in a total acquisition time of 18 min. With a slice thickness of 8 mm, the nominal and actual voxel sizes were 512 μL and 875 μL respectively. Signal acquisition was performed for 170 ms (512 complex points over a 3 kHz spectral BW). Water and lipid suppression modules were inserted before every slice-selective excitation pulse, where the duration of the suppression module was optimized in order not to exceed the 320 ms available between the end of the signal acquisition and the next slice-selective excitation pulse.
Figure 1.
The multi-slice MRSI sequence consists four RF- and slice-selective lipid suppression pulses (LS1-4), four global water suppression pulses (WS1-4) and a slice-selective 90 degree excitation pulse, combined with 2-dimensional phase encoding for signal localization. This sequence was repeated every 500 ms, resulting in a TR of 2.5 s for the five-slice MRSI sequence. B0 and RF shim settings were updated before every RF pulse. Pulse-timing is indicated in milliseconds, relative to the center of the excitation pulse. As the RF1 ring mode provides nonuniform nutation, the values are expressed in dB attenuation of the maximum RF power.
An additional, fast MRSI scan to acquire the water signal was performed, at identical resolution, but with a shorter readout (128 points), a reduced excitation angle to prevent T1 saturation and without water and lipid suppression (TR=260ms, scan time=10s) to estimate the required correction coefficients for coherent summation of the signals from the different receive channels (15). No receiver or intensity correction was performed on the spectra.
Water suppression
Global water suppression in the steady-state (effective TR=500 ms) was performed with an optimized WET scheme (16) that consisted of four frequency selective Gaussian excitation pulses (figure 1). Using the Bloch equations, and by taking the effective TR of the water suppression scheme into account, the nutation angles and delays between the four pulses were numerically optimized to maximize the suppression efficiency. This resulted in the situation where the steady-state magnetization of the water signal was effectively nulled at the time of the excitation (figure 2a). To compensate for residual B0 variations in the brain, the numerical optimization comprised the frequency range of +/− 20 Hz around the Larmor frequency. With this approach, the average suppression factor in the steady-state was 980 fold (figure 2b) within the range of 60% and 140% of the nominal B1+ and for typical T1 values (17) of water in CSF, gray and white matter of the human brain at 7T.
Figure 2.
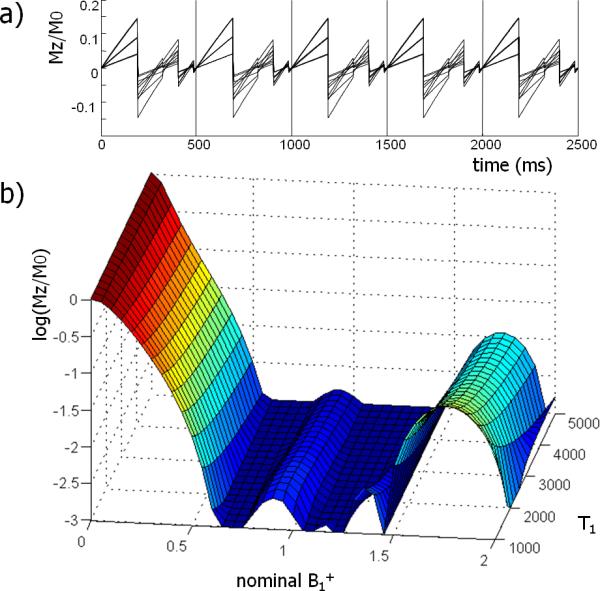
The steady-state water suppression was developed by minimization of the longitudinal magnetization after four suppression pulses in the steady-state. The time evolution of the longitudinal magnetization (Mz) is shown (a) for different T1 (GM, WM, CSF) and B1+ (70%, 100%, 130%) values. Using the steady-state behavior, the magnetization is effectively nulled at the time of the slice-selective excitation. In (b) the suppression factor is shown for a range of T1 and B1+ values at the time of the slice-selective excitation. The suppression is highly effective for a range of B1+ values and nearly insensitive to T1 values of water in the human brain. For B1+ between 60–140% and T1 between 1–5 seconds, the average suppression factor was 980.
Lipid suppression
Lipid suppression pulses were interleaved with the water suppression scheme (figure 1) and was performed for a ring shaped RF shim setting on the skull (see below). Care was taken to assure that the four slice-selective excitation pulses did not to perturb the magnetization on neighboring slices nor saturate the brain metabolites. However, since the B1+ transmit field in the skull close to the transmit coils was highly inhomogeneous, adequate lipid suppression demanded a method that was insensitive to a large range of B1+ values. For this purpose, the nutation angles and delays of a four pulse WET sequence (16) were numerically optimized through Bloch simulations to provide a 30-fold suppression over a factor of three times the nominal B1+ value and T1 values for lipids between 400 and 600 ms. Slice-selective hyperbolic secant pulses (18) were used since they provide a B1+ insensitive slice profile, thereby minimizing the impact on the magnetization in adjacent slices. A uniform suppression of all lipid resonances was achieved by matching the BW of the lipid suppression pulses to the BW of the slice-selective excitation pulses.
The suppression efficiency of the water and lipid suppression was measured by calculating the ratio of two images with similar scanning parameters as the MRSI sequence. One image was acquired with the suppression pulses on, and one image with the suppression pulses off. A suppression map was then calculated as the ratio of these two images.
B0 shimming
Magnetic field B0 homogenization was based on gradient echo B0 field mapping from 15 slices with four echo times, that covered the same volume as the multi-slice MRSI sequence (TR=450ms, FOV=200×200mm2, slice thickness=3.3mm, matrix=50×50, scan time=90 seconds). In order to accommodate the shimming requirements for both non-slice-selective and slice-selective pulse sequence elements, a global B0 shim over the whole brain volume as well as slice-specific B0 shims were calculated. To compensate through-plane magnetic field inhomogeneity, the slice-specific shims were calculated over three adjacent slices of the B0 mapping dataset that covered the spatial extent of the MRSI slice. The global B0 shim setting was employed during the water and lipid suppression. Before the slice-selective excitation and acquisition, the shims were switched to the slice-specific B0 shim setting. Eddy currents and off resonance effects during the fast updating of high order shims were prevented by preemphasis and B0 compensation, which was incorporated for each of the second and third order shim coils (2). No current limits were employed in the fitting of the shim fields, at maximum 20% of the dynamic range of the shim amplifiers (20A) was required for first-through-third order shimming. The effectiveness of the slice-based DSU was assessed from the B0 maps for first through third order DSU, and compared to the results with static B0 shimming (measured up to third order and simulated up to fourth order). In addition, water MRSI measurements with DSU and with static shimming were performed to assess the impact of third order DSU on the water linewidth. All MRSI metabolite measurements were performed with third order slice-based DSU. The spectral linewidth was measured by manually estimating the full width at half max of the creatine CH3 resonance at 3.0 ppm.
B1+ shimming
Using an eight channel transceiver array, it was possible to optimize the excitation pattern for every RF pulse. Optimization of the B1+ transmit field was performed by numerical optimization of the transmit amplitudes and phases, after measurement of the complex transmit B1+ fields. This was performed by combining phase information from gradient echo images with B1+ amplitude field maps obtained by Bloch-Siegert shift B1+ mapping (19). The transmit phase information was measured by sequentially turning on each transmit channel in a multi-slice gradient echo acquisition (TE/TR=3.5/100 ms, FOV=200×200mm2, 5 slices, slice thickness=8mm, matrix=50×50, total scan time=40s). From the transmit B1+ phase information, an RF shim setting was derived that created a constructive interference between the eight coils in the center of the brain. This RF phase-shim was used for the excitation pulse in the B1+ mapping sequence to generate a high overall nutation angle, and therefore high measurement sensitivity. B1+ amplitude mapping was performed with a five slice gradient echo sequence, employing the Bloch-Siegert B1+ mapping technique (TE/TR=13.5/650 ms, FOV=200×200mm2, slice thickness=8mm, matrix=50×50,scan time=65 s per transmit channel). The off-resonant Fermi calibration pulse (8 ms duration, placed at ±1.5 kHz) was turned on for each of the eight transmit channels in consecutive measurements. Pixels with low SNR that could corrupt the phase measurement, and therefore the B1+ estimation, were removed from the dataset. An extrapolation of the B1+ map through these points was performed by 2D-polynomial fitting.
With both the phase and amplitude information of the B1+ field in the five slices, RF shim settings were calculated by a numerical least squares minimization of the variation of the B1+ field. Several RF shim settings were generated to ensure an optimal B1+ field for every RF pulse; a slice-specific uniform excitation, a ring shaped excitation pattern on the skull area for lipid suppression on every slice and a global uniform excitation for water suppression in all five slice. During the MRSI sequence the RF system was switched to the appropriate RF shim for every RF pulse.
The RF power deposition was monitored at the input power of all the individual transmit elements. RF power was mostly required for water suppression (46%) and lipid suppression pulses (43%). The localization (with slice-selective excitation) only required a small fraction of the total RF power (11%). The global RF power deposition of 2.3 ± 0.3 W/kg (mean ± SD, n = 5) was well within FDA guidelines. Local SAR values were estimated at 5 W/kg from numerical simulations (5) of the homogeneous and ring mode SAR distributions of the coil.
Water residuals were subtracted from the spectra by HLSVD filtering (20). Although no correction for acquisition delay is required to perform further spectral analysis and metabolite fitting (7), a correction for the missing first points was performed for display purposes. To this end, an HSVD fit of the time domain signal was performed, and the first 8 missing data points were extrapolated before Fourier transform.
Results
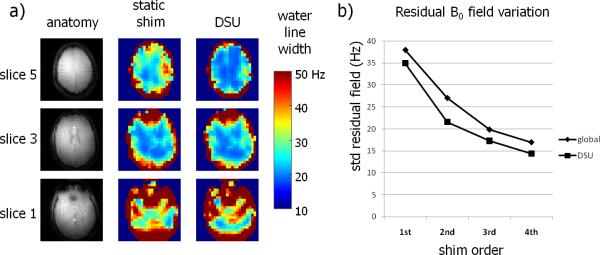
Optimization of the uniformity of the static B0 magnetic field on a slice-by-slice basis showed an improved homogeneity compared to a global optimization strategy. Figure 3a shows the effect of slice-specific DSU on the water linewidth in one volunteer, as determined by the fast MRSI measurement without water suppression. The slice-based B0 optimization led to improved B0 magnetic field homogeneity compared to a global optimization, especially in the upper and lower slices. However, close to the nasal cavity, large residual B0 inhomogeneities remained which could not be compensated with the third order spherical harmonic fields. The outcome of slice-specific DSU was analyzed in the five subjects by means of the average residual B0 field inhomogeneity (figure 3b). For the slice positions and coverage used in this study, slice-based DSU improved the B0 magnetic field homogeneity compared to a global optimization by approximately one order of spherical harmonic fields (e.g. third order DSU is comparable with full fourth order static shimming).
Figure 3.
B0 homogeneity for slice-based DSU compared to global B0 shimming. The impact of DSU on the measured water linewidth with third order shimming is shown (a). Limited gain is seen in the center slice, however, the upper and lower slices show clear linewidth reductions. Severe inhomogeneities remain in the lower slice, above the nasal and ear cavities, which are not easily compensated by spherical harmonic shimming. Improvement in the B0 homogeneity with DSU was simulated (b) for different shim orders (first through fourth order shimming).
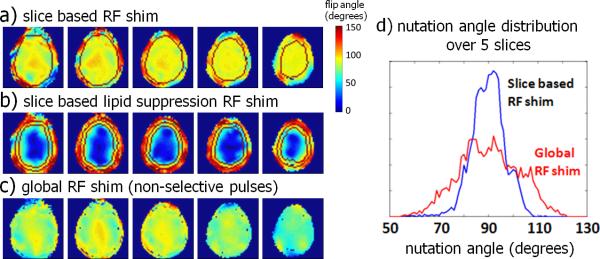
Pulse specific RF shimming was performed by calculating three sets of RF shim settings. Firstly, slice-specific RF shims (figure 4a) were calculated for slice-selective excitation by finding the optimal combination of transmit phases and amplitudes which maximized the nutation angle homogeneity over the brain. Secondly, slice-specific lipid suppression RF shims (figure 4b) were calculated by maximizing the excitation on the skull while simultaneously minimizing the excitation over the brain, resulting in a excitation of the skull only (figure 4b). Thirdly, a global excitation RF shim for non-slice-selective pulse sequence elements was calculated (figure 4c), by minimizing the variation of the field in the brain volume over all slices. Figure 4d displays the nutation angle distribution over the five slices and shows how the slice-by-slice optimization of the B1+ magnetic field leads to a more homogeneous distribution of the excitation nutation angles over the whole volume, compared to a single RF shim for optimization of the B1+ field on the center slice.
Figure 4.
RF shimming over five slices; a slice-by-slice optimization was used for the slice-selective excitation pulses (a), a slice-by-slice ring mode was used for lipid suppression (b) and a global optimization was used for the water suppression (c). The histogram of the nutation angle (d) demonstrates the improvement in overall nutation angle homogeneity with a slice-based B1+ optimization.
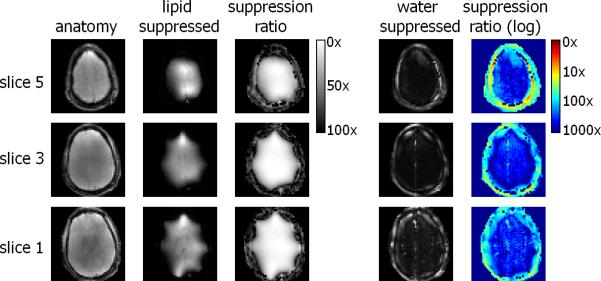
The efficiency of water and lipid suppression was quantified as the ratio of MRI images acquired with and without suppression pulses (figure 5). The optimized four pulse water suppression sequence in the steady-state was very effective, leading to suppression factors >300 fold in the brain. The lipid suppression led to a suppression of approximately 30 fold on the skull without perturbing large areas of brain tissue. However, the outer part of the cortex was still partially excited by the ring mode. Since the lipid suppression was slice-selective, this lead to a uniform signal loss over the spectrum for voxels close to the skull.
Figure 5.
Evaluation of the water and lipid suppression quality in the human brain at 7T. On the left the original anatomical image, the second column shows an anatomical image with the lipid suppression pulses on and the third column shows the division of the original image and an image acquired with the lipid suppression. On the right an image acquired with water and lipid suppression is shown, together with the ratio (suppression factors) on a logarithmic scale. Water signal in the brain is suppressed by >300 fold, signal in the skull is suppressed >30 fold.
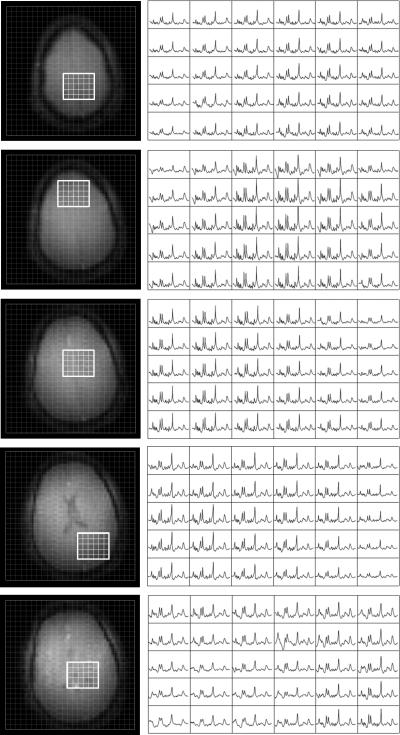
Several selected 5×6 areas from the 20×20 matrix from all five slices in the MRSI dataset are shown in figure 6. High overall SNR and spectral quality are apparent. The stable macromolecular (MM) signal indicates a good lipid suppression over all slices. Intensity variation of the metabolites as seen over the slices is caused by inhomogeneous linewidth (figure 3), by a spatially varying receiver sensitivity, partial volume effects and partly by undesired saturation in the transition zone of the lipid suppression pulses (figure 5). The average (± standard deviation) linewidth of the 3.0 creatine resonance of the MRS voxels in the brain over the five slices was 14.2±5.5 Hz. On the individual slices, the average and standard deviations of the creatine linewidth were (from bottom slice to top slice) 23.6.0±6.2Hz, 16.0±4.0 Hz, 12.8±2.8 Hz, 12.5±3.2 Hz and 10.6±2.0 Hz.
Figure 6.
Five slice MRSI dataset of a healthy volunteer. For every slice 6×5 spectra out of the 20×20 matrix are shown. No linear prediction to improve the baseline or intensity correction or normalization was performed. High SNR and a narrow linewidth are apparent over large parts of the brain. Despite the short TE of 2.75 ms the absence of lipid signals is noticeable.
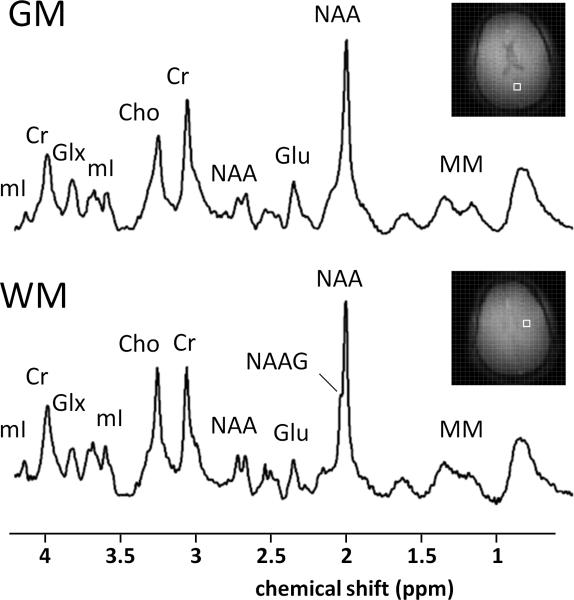
Two representative spectra from the white (WM) and gray matter (GM) (figure 7) show excellent spectral resolution and sensitivity. Clear differences in metabolite content, i.e. decreased glutamate (Glu) and increased N-acetylaspartylglutamate (NAAG) in the parietal white matter compared to occipital gray matter, as well as differences in the ratio of choline (Cho) and creatine (Cr).
Figure 7.
Two example spectra (875 μL, processed with HSVD baseline correction) from frontal white matter (WM) and parietal gray matter (GM) show differences in Glu concentration as well as Cho/Cr ratio and NAAG concentrations between gray and white matter.
Discussion
Proton MR spectroscopic imaging, and especially multi-slice MRSI, of the human brain at ultra high field (≥7T) is challenging due to increased RF power deposition, increased magnetic field B0 inhomogeneity and increased RF magnetic field inhomogeneity. Many of these effects directly inhibit the potential gains of higher magnetic field and can even cause a reduction in data quality due to increased chemical shift displacement artifacts, incomplete water and lipid suppression and a decrease in spectral resolution. In addition, traditional multi-slice spectroscopic imaging cannot be directly translated to ultra-high magnetic fields due to the increased RF power deposition. However, recent developments in dynamic magnetic field shimming and dynamic RF control with multi-element transmit coils allow for novel acquisition strategies.
Several groups have shown promising results with 2D/3DMRSI at ultra high field, where various acquisition strategies as well as suppression techniques for water and lipid signals have been employed. These methods include highly selective lipid suppression with several slice-selective saturation pulses (7), spectrally (9) or spatially selective inversion recovery (5,10), spectral saturation of water and/or lipids (7–8) and spectrally selective refocusing (5,10–11) of the metabolites of interest. Unfortunately, methods that employ such non-selective RF pulses for excitation or refocusing do not allow direct extension to multi-slice acquisition as they perturb the magnetization in adjacent slices. Also, the chemical shift displacement artifacts associated with localization at ultra high field makes the use of traditional refocusing pulses undesired, adiabatic (13)or spatial-spectral pulses (11–12) might overcome this issue, however only with long pulse durations, leading to long echo times. In addition, the significant RF power deposition associated with refocusing and water and lipid suppression for these 2D/3D methods does typically prevent the extension to multiple interleaved slices due to SAR concerns. This is especially true when high-BW adiabatic inversion or refocusing pulses are used for suppression or localization.
Therefore, in this work low-power water and lipid suppression was developed and combined with a pulse-acquire MRSI acquisition to minimize the number of RF pulses, and to allow for a short effective TR as required for multi-slice MRSI. The SNR of the measurement was maximized by using a pulse acquire acquisition with a very short echo time and a multi-element transceiver array. Multi-slice MRSI of the brain was shown to be feasible within a reasonable scan time and with minimal chemical shift displacement artifacts by using dynamic B0 and B1+ optimization. MRSI was performed on five axial slices, where both the B0 and B1+ fields were optimized on a slice-by-slice basis. All B0 and B1+ settings were switched to the appropriate values during the sequence before every RF pulse.
Third order dynamic updating of the B0 shim fields has been shown to improve the magnetic field homogeneity over the five slices compared to a global optimization strategy, to a degree that is comparable to static fourth order shimming. Slice based shim updating was applied in a multi-slice sequence, and can be extended to more slices or be combined with multi-stack 3D acquisitions. It is however limited to sequences where multiple volumes are simultaneously examined. But even if a single volume is excited, dynamic shim updating allows for optimization of the B0 and B1 field for water or lipid suppression pulses, independent of the excitation. After slice based shim updating, in certain regions, the B0 magnetic field homogeneity is still not perfect. Especially above the nasal cavity, considerable B0 magnetic field residuals remained. These distortions could not be compensated with the shallow zero-through-third order spherical harmonic fields, and more sophisticated shim systems are required to also improve field homogeneity in these regions (21–22). However, slice-based DSU maximizes the usefulness of conventional spherical harmonic shim systems.
Bloch-Siegert B1+ mapping in combination with short TE gradient echo images was used for RF shimming on a slice-by-slice basis. The high accuracy and large dynamic range of this method allowed for reproducible B1+ mapping and RF shimming. However, as the current B1+ mapping method required up to 10 minutes of acquisition time, image acceleration techniques might be employed to reduce the calibration time of the transmit settings. Slice-based RF shimming improved the overall homogeneity of the nutation angle distribution as compared to a single-slice RF shim optimization. Especially the lower and upper slices of the volume benefited significantly from a slice-based optimization strategy. Further gain is expected when the slice coverage is increased to approach the physical dimensions of the RF transceiver array.
For optimization of the water suppression, a fixed effective TR of 500 ms was used which represents a reasonable compromise between T1 saturation and experiment duration for the typical brain metabolites (TR=5×500ms). A further reduction of the effective TR is possible, but requires a re-optimization of the delays and powers of the water suppression pulses. In addition, less conservative settings for RF power deposition might be applied since the local heat hotspots may be distributed more than was assumed here because of the differences in RF shim settings used for the different slices (23). Careful assessments of these calculations, along with an in vivo validation, as well as more advanced TX array coil design or RF shimming strategies with simultaneous SAR minimization might further increase the number of slices that can be acquired per unit of time.
Lipid suppression was performed with an RF shimmed ring-mode, where the extra-cranial lipids in the slice were saturated with only a single low power RF pulse, relieving much of the RF power deposition which is usually required for lipid suppression. To make the lipid suppression less sensitive to the steep drop off in B1+ close to the transmit coils, four pulses were used with optimized delays and RF powers. However, as with most lipid suppression techniques, there is a tradeoff between adequate lipid suppression and saturation of the metabolites in the outer part of the cortex. Future development of transmit arrays (e.g. more than eight elements) will potentially allow for an even sharper transition zone between extra cranial lipids and brain tissue in the slice. Suppression maps (figure 5) were measured, and can be used retrospectively for data normalization or data rejection.
The acquisition delay, which is inherent to a pulse-acquire approach, is known to cause a first-order phase over the spectra in the frequency domain. The acquisition delay does not pose a problem for spectral quantification, since it can be incorporated into the metabolite basisset as prior knowledge (7). However, for displaying purposes a correction was applied by making an HSVD fit to the time domain signal and consecutive back extrapolation of the missing first data points. The remaining baseline that is seen in the spectra is due to the very short T2 components mainly originating from macromolecules (24) since this short echo time sequence significantly enhances these resonances. For accurate quantification of the metabolite concentrations, a reliable estimation of this macromolecular baseline is essential (25), especially since at ultra high field these macromolecules are very well resolved from one another (26). This macromolecular baseline can be included in the quantification algorithm, as is readily done for short TE single voxel MRS either by simulation or measurement of these macromolecular compounds. Measurements of the macromolecular compounds has been shown to be more accurate than mathematical modeling (27) and these measurements are generally performed by inserting an additional (preferably adiabatic) inversion pulse prior to the excitation in order to null the metabolite signals. However, inserting such a high BW inversion pulse into the multi-slice MRSI sequence would greatly increase the power deposition, rendering it a multi-slice application at short TR impossible. A different strategy for macromolecular estimation must therefore be developed before accurate metabolic fitting can be performed, which is beyond the scope of this paper.
In conclusion, multi-slice MRSI of the human brain has been demonstrated at ultra high field. B0 and B1+ homogeneity was optimized on a slice-by-slice basis with slice-based DSU and pulse specific RF shimming with an eight channel transceiver array. Low-power lipid suppression based on RF shimming and low-power water suppression in the steady-state were employed in a five slice MRSI sequence for metabolic profiling of large areas of the human brain at ultra high field within a reasonable scan time.
Acknowledgements
This research was supported by NIH grants R01 EB000473 and R42 RR031457.
References
- 1.Koch KM, McIntyre S, Nixon TW, Rothman DL, de Graaf RA. Dynamic shim updating on the human brain. J Magn Reson. 2006;180:286–296. doi: 10.1016/j.jmr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Juchem C, Nixon TW, Diduch P, Rothman DL, Starewicz P, de Graaf RA. Dynamic Shimming of the Human Brain at 7 Tesla. Concepts Magn Reson. 2010;37B:116–128. doi: 10.1002/cmr.b.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao W, Smith MB, Collins CM. Exploring the limits of RF shimming for high-field MRI of the human head. Magn Reson Med. 2006;56:918–922. doi: 10.1002/mrm.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boer VO, Voogt I, van de Bank BL, Kroeze H, van den Berg CAT, van Lier AL, Raaijmakers A, Luijten PR, Klomp DWJ. Ultra high field 1H-MRS: outer volume suppression by local excitation; Proceedings of the ESMRMB; Antalya, Turkey. 2009. p. 74. [Google Scholar]
- 5.Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63:9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katscher U, Börnert P, Leussler C, van den Brink JS. Transmit SENSE. Magn Reson Med. 2003;49:144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 7.Henning A, Fuchs A, Murdoch JB, Boesiger P. Slice-selective FID acquisition, localized by outer volume suppression (FIDLOVS) for 1H-MRSI of the human brain at 7 T with minimal signal loss. NMR Biomed. 2009;22:683–696. doi: 10.1002/nbm.1366. [DOI] [PubMed] [Google Scholar]
- 8.Boer VO, Siero JC, Hoogduin H, van Gorp JS, Luijten PR, Klomp DW. High-field MRS of the human brain at short TE and TR. NMR Biomed. 2011 doi: 10.1002/nbm.1660. In press, DOI 10.1002/nbm.1660. [DOI] [PubMed] [Google Scholar]
- 9.Balchandani P, Spielman D. Fat suppression for 1H MRSI at 7T using spectrally selective adiabatic inversion recovery. Magn Reson Med. 2008;59:980–988. doi: 10.1002/mrm.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avdievich NI, Pan JW, Baehring JM, Spencer DD, Hetherington HP. Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magn Reson Med. 2009;62:17–25. doi: 10.1002/mrm.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balchandani P, Pauly J, Spielman D. Interleaved narrow-band PRESS sequence with adiabatic spatial-spectral refocusing pulses for 1H MRSI at 7T. Magn Reson Med. 2008;59:973–979. doi: 10.1002/mrm.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Cunningham CH, Chen AP, Li Y, Kelley DA, Mukherjee P, Pauly JM, Nelson SJ, Vigneron DB. Phased array 3D MR spectroscopic imaging of the brain at 7 T. Magn Reson Imaging. 2008;26:1201–1206. doi: 10.1016/j.mri.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheenen TW, Heerschap A, Klomp DW. Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. MAGMA. 2008;21:95–101. doi: 10.1007/s10334-007-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm. IEEE Trans Med Imaging. 1991;10:53–65. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 15.Natt O, Bezkorovaynyy V, Michaelis T, Frahm J. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med. 2005;53:3–8. doi: 10.1002/mrm.20337. [DOI] [PubMed] [Google Scholar]
- 16.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 17.Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57:308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- 18.Silver MS, Joseph RI, Hoult DI. Highly selective [pi]/2 and [pi] pulse generation. Journal of Magnetic Resonance. 1984;59:347–351. [Google Scholar]
- 19.Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B1 mapping by Bloch-Siegert shift. Magn Reson Med. 2010;63:1315–1322. doi: 10.1002/mrm.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Boogaart A, van Ormondt D, Pijnappel WWF, de Beer R, Ala-Korpela M. In: Mathematics and Signal Processing III. McWhirter JG, editor. Clarendon Press; Oxford: 1994. [Google Scholar]
- 21.Juchem C, Nixon TW, McIntyre S, Rothman DL, de Graaf RA. Magnetic field homogenization of the human prefrontal cortex with a set of localized electrical coils. Magn Reson Med. 2010;63:171–180. doi: 10.1002/mrm.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juchem C, Nixon TW, McIntyre S, Boer VO, Rothman DL, de Graaf RA. Dynamic Multi-Coil Shimming of the Human Brain at 7 Tesla. Journal of Magnetic Resonance. 2011 doi: 10.1016/j.jmr.2011.07.005. In Press. doi:10.1016/j.jmr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang L, Hue YK, Ibrahim TS. Studies of RF Shimming Techniques with Minimization of RF Power Deposition and Their Associated Temperature Changes. Concepts Magn Reson Part B Magn Reson Eng. 2011;39B:11–25. doi: 10.1002/cmr.b.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 25.Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 26.Otazo R, Mueller B, Ugurbil K, Wald L, Posse S. Signal-to-noise ratio and spectral linewidth improvements between 1.5 and 7 Tesla in proton echo-planar spectroscopic imaging. Magn Reson Med. 2006;56:1200–1210. doi: 10.1002/mrm.21067. [DOI] [PubMed] [Google Scholar]
- 27.Gottschalk M, Lamalle L, Segebarth C. Short-TE localised 1H MRS of the human brain at 3 T: quantification of the metabolite signals using two approaches to account for macromolecular signal contributions. NMR Biomed. 2008;21:507–517. doi: 10.1002/nbm.1219. [DOI] [PubMed] [Google Scholar]