Abstract
Study Objective
American Indians experience high rates of cervical cancer, which is preventable by vaccination against human papillomavirus (HPV). We sought information on funding, barriers, education, and policies regarding HPV vaccination from clinics that serve tribal members in the Pacific Northwest.
Design
We surveyed staff either by telephone or by using a mailed survey at 31 tribal and Indian Health Service clinics serving the 29 federally recognized American Indian tribes in Washington State. The survey included 11 items on policies and use of the HPV vaccine.
Main Outcome Measures
Primary outcomes were funding options for HPV vaccine administration, barriers to vaccine delivery, potential gaps in education, and determination of tribal or clinic-specific policies.
Results
Thirty-one clinics provided information; 28 administered the vaccine. Vaccination was supported by federal, private, and tribal sources. Barriers were reported by 89% of clinics, most commonly patients’ lack of knowledge, distrust of the medical system, and lack of funding. Patient and provider information was widely available. Thirteen clinics had either tribal or internal clinic vaccination policies, or both.
Conclusion
HPV vaccine is available, but complex policies on administration could result in tribal differences in vaccination rates.
Keywords: HPV, vaccination, American Indians
INTRODUCTION
The American Cancer Society estimated that 4,070 U.S. women died from cervical cancer in 2009.1 Almost all cervical cancers are causally related to infections by human papillomavirus (HPV),2–6 which is present in 99.7% of cases.7 Notably, 2 high-risk types – HPV-16 and HPV-18 – are responsible for approximately 70% of all cervical cancers in the U.S.8 Several studies have shown that, for most U.S. regions, cervical cancer mortality rates among American Indian/Alaska Native (AI/AN) women are consistently higher than those for all other races.9–15 The reasons behind this disparity are unknown, but one study found that AI women in the Northern Plains had a high prevalence of HPV infection, especially with high-risk types, less than half of which were HPV-16 or HPV-18.16 Although Papanicolau testing and early treatment of pre-cancerous lesions have greatly reduced cervical cancer rates in the U.S. since the 1950s, an effective vaccine against HPV is an important component of prevention efforts.17,18 The availability of such a vaccine is especially relevant to AI/AN communities, in part because of a lower prevalence of screening, which can affect stage at diagnosis and clinical outcomes.19,20
In 2006, the Food and Drug Administration approved Merck’s Gardasil, a vaccine designed to protect against HPV types 6, 11, 16, and 18.21 In 2009, the agency approved GlaxoSmithKline’s Cervarix, a vaccine that also protects against HPV-16 and -18.18 Each vaccine requires a series of 3 injections spaced over 6 months to confer full protection. Shortly after the licensing of Gardasil, the Advisory Committee on Immunization Practices of the Centers for Disease Control (CDC) unanimously recommended that all females aged 9–12 years receive the 3-dose series, and that all females aged 13–26 years receive catch-up immunizations.22 Gardasil was also added to the federally-funded Vaccines for Children program, which provides immunizations to children through 18 years of age who are Medicaid eligible, AI/AN, uninsured, or underinsured.22,23
In April 2007, the Washington State Legislature provided funding to include Gardasil in its statewide childhood vaccination program.24 Later that year, the legislature passed a bill mandating that parents of sixth-grade girls receive information about HPV and where to receive the vaccine,25 without requiring vaccination as a condition of school attendance. However, in response to a budget crisis, the State Department of Health stopped buying the HPV vaccine in July 2009. This change did not affect the eligibility of AI/ANs covered by the Vaccines for Children program, and the Indian Health Service (IHS) continues to recommend adherence to CDC guidelines. The IHS also provides each state with data on its service-eligible female patients, aged 9–18 years, to use for state funding requests. It can independently negotiate with the CDC for funds in states unable or unwilling to vaccinate females of certain ages.26
Washington State is home to 29 federally recognized AI tribes. Because these tribes are sovereign nations that can make decisions independent of state government, they may support diverse policies on HPV vaccination. However, a review of Internet resources and medical and legal literature returned no information on potential tribal policies. Because little is known about usage and funding of the HPV vaccine among tribes, and because their practices, intentions, and policies remain undocumented, we surveyed clinics serving all 29 tribes in Washington State. Our aims were to describe 1) funding sources for the HPV vaccine; 2) patterns of vaccine use, as well as related barriers and concerns; 3) educational needs regarding the vaccine; and 4) clinic or tribal vaccination policies.
MATERIALS AND METHODS
Study Design
We collected data from tribal and IHS clinics serving the 29 federally recognized tribes in Washington State. Data were collected from July 2009 through March 2010 by means of a survey administered by telephone or completed on paper and returned to the study team. Our primary outcomes were funding options for HPV vaccine administration, barriers to vaccine delivery, potential gaps in education, and determination of tribal or clinic-specific policies.
Study Setting and Eligibility
Clinics were identified by using the Northwest Portland Area Indian Health Board’s online database, supplemented by a database of tribally-affiliated clinics in Washington State created by University of Washington staff.27 We identified 32 eligible clinics: 30 were operated either by a tribe or by the IHS, and 2 were non-profit clinics dedicated to providing care to AIs. Twenty-two Washington tribes are land-based and have at least one clinic on reservation land; 7 tribes do not have reservation lands, but 5 of them operate associated clinics.
Data Collection
To collect data, 5 trained staff used a standardized script and operating procedures to telephone the clinics. On the initial call to each clinic, the interviewers introduced themselves, provided a brief summary of the project, and asked to be directed to the person best able to complete the survey. Telephone calls were repeated until the interviewers reached a qualified person at each clinic.
The interviewers obtained verbal consent and offered respondents the option of completing the survey over the telephone or receiving it by e-mail. When clinic staff chose electronic communication, we sent them an introductory e-mail explaining the purpose of the study and providing contact information, along with the survey and consent form. Respondents returned the completed surveys and consent forms by e-mail or fax. Follow-up e-mails and telephone calls were conducted as needed to complete the surveys.
Survey
A team of researchers familiar with tribal healthcare systems, HPV vaccination, and tribal laws created the survey, which was evaluated by an expert in organizational research and the human subjects policies of the IHS. The survey consisted of 11 yes/no questions. The first question asked if the clinic delivered the HPV vaccine; if respondents answered no, the interview concluded. The remaining questions focused on types of funding used to pay for the vaccine, barriers to vaccine administration observed by clinic staff, educational resources for providers and patients on vaccine eligibility, availability of vaccine to the clinic, and tribal or clinic policies regarding the vaccine. Respondents who affirmed the existence of policies and concerns were asked to describe them. Three questions had an “Other” response category, which allowed respondents to expand on their answers. In addition, we solicited comments about the survey questions and each clinic’s experience with the vaccine.
Data Analysis
Because we obtained completed surveys from almost all clinics that we identified, we did not use statistical inference techniques such as confidence intervals and p values. We therefore report only descriptive statistics and pertinent qualitative comments to illustrate our findings.
RESULTS
We obtained information on 31 of the 32 clinics, as 1 clinic declined to complete the survey. Three clinics did not deliver the vaccine.
Funding
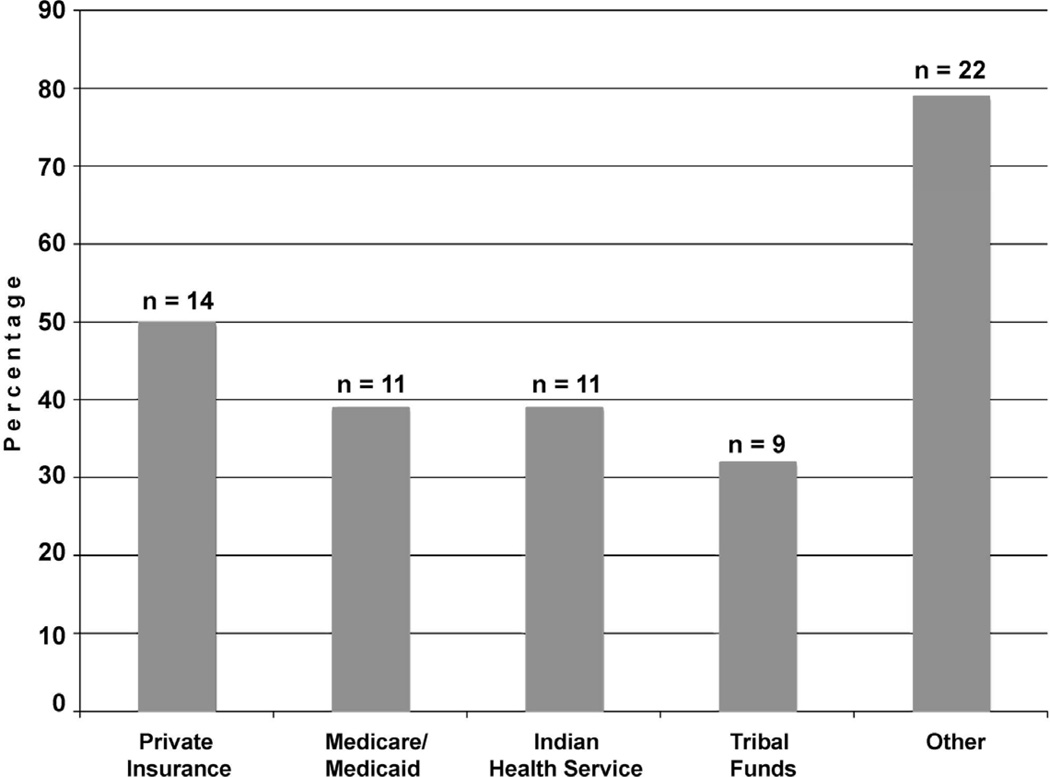
Among the 28 clinics that administered the HPV vaccine, 11 (39%) used only 1 source of funding, while 8 (29%) used as many as 4 different sources (Figure 1). The use of private insurance was quite common, with half of all clinics reporting this source, whereas Medicare/Medicaid or IHS support was reported by 11 (39%), and tribal funding by only 9 (32%). However, the single most widely endorsed source of funding was the Vaccines for Children program, reported by 20 (71%) clinics in the “Other” response category. In addition, one clinic noted that it received funding from patient assistance grants from a pharmaceutical company. Many respondents commented that state or federal sources paid for vaccination of females aged 11–18 years, but for those aged 19–26 years, either the tribe or the patients were responsible. This situation could become problematic if a patient was unable to receive all 3 of the required injections before turning 19. One clinic did not offer the vaccine to women aged 19–26 years because of a lack of internal funding, ambiguity regarding the reimbursement policies of patients’ insurance providers, and patients’ inability to pay for the vaccine out of pocket.
Figure 1.
Funding sources used by clinics
Note: 20 out of 22 responses in the Other category referred to the Vaccines for Children program.
Barriers and Concerns
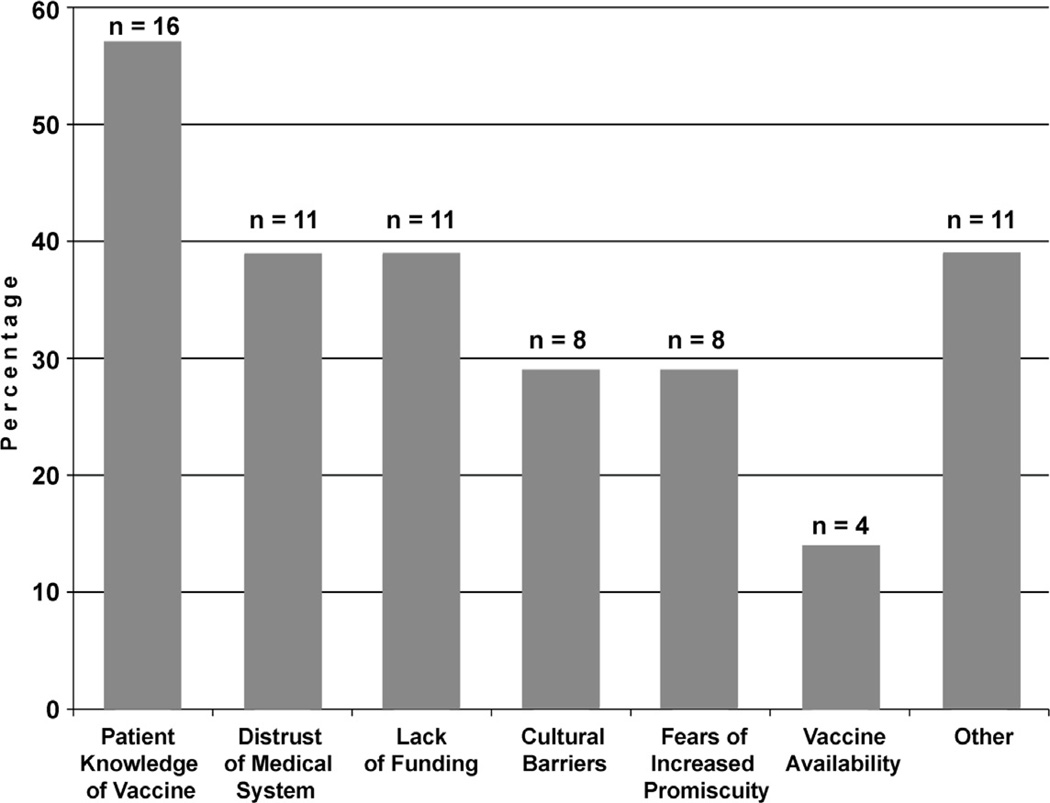
Overall, 25 clinics (89%) reported experiencing at least 1 barrier to administering the vaccine, with a large minority (22%) reporting 5 or more barriers (Figure 2). The most commonly cited barrier was patients’ lack of knowledge of the vaccine (57%), followed by patients’ distrust of the medical system (39%) and overall lack of funding (39%). Among the 11 clinics reporting specific barriers that were not identified by the survey (i.e., responses in the “Other” category), 5 cited patient anxiety about pain or side effects of the injections, 3 cited the number of injections required, 3 cited difficulty recruiting eligible patients (either transportation barriers or lack of interest among eligible females), and 2 cited the age limit. Other clinic-specific barriers included sex restrictions, paperwork, and clinic workload.
Figure 2.
Barriers to HPV vaccination reported by clinics
Educational Materials and Needs
Twenty-five clinics (89%) had information for healthcare providers on HPV vaccine eligibility and 13 (46%) conducted an educational session about the vaccine for providers. Two clinics (7%) were unable to answer questions about vaccine education for providers.
All clinics surveyed provided patient information on the vaccine, often obtained from Vaccine Information Statements or other materials issued by the CDC, or from pharmaceutical companies. All clinics had information for patients on vaccine eligibility (Table 1); 27 (96%) had information about federal and state recommendations; 23 (82%) had information about federal and state policies; and 17 (61%) had information on funding options. Some misconceptions about the vaccine were spontaneously reported in anecdotal form. One site reported that they had to educate providers who initially (and mistakenly) required girls and young women to undergo Pap smears and pregnancy tests before being vaccinated. At another site, only a subset of female patients was vaccinated because providers held the erroneous belief that the vaccine was approved only for women younger than 24 years.
Table 1.
Availability of information on HPV immunization in reservation-based clinics
| Type of Information | Information Available (%) n = 28 |
Unaware of Information Availability (%) n = 28 |
|---|---|---|
| Eligibility | 28 (100) | 0 (0) |
| Funding options | 17 (61) | 0 (0) |
| Current federal and state recommendations | 27 (96) | 0 (0) |
| Current federal and state policies | 23 (82) | 3 (11) |
| Other | 0 (0) | 0 (0) |
Policies
Only 6 clinics (21%) reported that their associated tribes had policies regarding the HPV vaccine (Table 2). Examples of tribal policies included 1) establishing an age range for vaccination (variously 14–26 years, 11–26 years, or 0–19 years); and 2) following guidelines from the Advisory Committee on Immunization Practices.23 In contrast, 12 (43%) clinics reported variable internal clinic policies regarding vaccine administration. In 11 clinics the policy followed guidelines from the Advisory Committee on Immunization Practices, the Vaccines for Children Program, or Washington State. One clinic administered the vaccine unless the patient’s family opted out. Another clinic was developing a standing order protocol to enable nurses to administer the vaccine without a physician’s order at the time of administration. Other policies included offering the vaccine to all females aged 9–26 years and supporting programs that targeted girls aged 11–12, teenagers, and women aged 19–26 years. One clinic planned to give the vaccine to males, but was waiting for CDC recommendations.
Table 2.
Clinic and tribal policies regarding HPV immunization
| Policy Type | Number of Clinics (%) n = 28 |
|---|---|
| Clinic-only | 7 (25) |
| Tribal-only | 1 (4) |
| Both clinic and tribal | 5 (18) |
| No knowledge of either type | 2 (7) |
| No policy | 13 (46) |
DISCUSSION
We found that almost all reservation-affiliated clinics that serve AI/AN women in Washington State deliver the HPV vaccine. We also found substantial variation among clinics in funding for vaccine delivery. The Vaccines for Children program was used by 71% of clinics, and for at least 36%, it was the only source of funding. Unfortunately, as some clinics noted, primary or exclusive use of this source can create problems, because a woman who does not receive all 3 injections before turning 19 must use another funding source to complete the series.
Past and present policies of the states and the IHS are complex, but they indicate that funding should be available to cover vaccination for many young Native women. Nevertheless, we found that clinics experienced numerous barriers to vaccination, including logistical issues, patients’ lack of knowledge about the vaccine, and cultural perceptions of the medical system and vaccines in general. Despite controversy regarding the HPV vaccine’s potential to increase adolescent promiscuity, only 29% cited parental fears about increased promiscuity as a barrier. Perhaps most troubling is the fact that over 57% of clinics reported patient knowledge of the HPV vaccine as a major barrier, even though patient information on vaccination was available at all clinics. This finding suggests that educational efforts are either not reaching many patients in these clinics or not adequately addressing patients’ concerns. Their doubts most often focused on the efficacy, necessity, and safety of the vaccine, consistent with concerns voiced by the general population regarding the vaccine’s high cost and potential side effects.18,28
Tribal and clinic policies varied widely across sites. Because each tribe and clinic is empowered to define its own policies, inequities in coverage could arise as a function of tribal affiliation or clinic location. Such variation is likely to be most evident in females aged 19 years and older and in males served by clinics without HPV immunization policies, because the Vaccines for Children program does not support vaccination of these groups. The rate of incomplete vaccination could also increase among tribes that lack explicit policies regarding females who reach the Vaccines for Children age threshold without completing the 3-dose series and without individual means of payment.
The present study has several noteworthy limitations. First, we used telephone inquiries to identify the most appropriate and knowledgeable person to interview. However, our interviewees fulfilled diverse professional roles, and their knowledge of the HPV vaccine varied, likely resulting in some inaccurate responses. Second, we did not inquire about vaccination practices for males, primarily because the study was designed before the vaccine was approved for use in this population.29 Third, the amount of qualitative information volunteered varied by site, thus biasing our presentation of solicited and unsolicited comments in favor of interviewees who gave more expansive answers.
In summary, among clinics serving AI/ANs in Washington State, all but 3 of the 31 clinics participating in this study administered the HPV vaccine. Nevertheless, clinics varied with regard to who gets vaccinated, how the vaccine is funded, and what level of patient and provider education is available. These variations likely result in regional- and tribal-dependent differences in rates of HPV vaccination. A more stable, accessible, and well-understood funding source, as well as more detailed knowledge regarding support by the Vaccines for Children program, could help more Native girls and women get vaccinated.
Our results underscore the need for educational materials and venues intended for providers as well as patients. Providers need to know more about eligibility criteria for vaccination and availability of federal funding sources, especially for patients who do not qualify for Vaccines for Children. Patients need appropriate education to address the concerns and misconceptions about the HPV vaccine that we document here. To inform these educational efforts, we recommend qualitative research using focus groups and key informant interviews among providers, among AI/AN parents of teenage children, and among AI/AN teens themselves. Such work is likely to uncover rich data on knowledge, attitudes, and beliefs relevant to HPV vaccination.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the clinical and administrative staff of the health clinics serving American Indians in Washington State for their time and cooperation. We also thank William Freeman, MD, MPH; Craig Sawchuk, PhD; and Eric Strachan, PhD, for helping to guide this project through the review process. We are grateful to Janice Sabin, PhD, MSW; Kate Murray, MPH; Corrinal Tordillos; and Ann Weinberg, MA, for their help with data collection, and to Jack Goldberg, PhD, and Joan Russo, PhD, for their help with data analysis.
This work was funded by grant number UO1 CA114642 from the National Cancer Institute, which supports Native People for Cancer Control, a Community Networks Program (D. Buchwald, Principal Investigator), and grant number UL1RR025014 from the NIH National Center for Research Resources, which supports the Institute for Translational Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was conducted through telephone and mail-in surveys of tribal and Indian Health Service clinics that serve the 29 federally recognized American Indian tribes in Washington State.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses: HPV IARC monograph summary. Lancet Oncology. 2005 April;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 3.Dunne EF, Markowitz LE. Genital Human Papillomavirus Infections. Clin Inf Dis. 2006 Sep 1;43:624–629. doi: 10.1086/505982. [DOI] [PubMed] [Google Scholar]
- 4.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995 Aug;33(8):2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 6.Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004 Nov;114(11 Pt 2) Suppl 104:1–23. doi: 10.1097/01.mlg.000148224.83491.0f. [DOI] [PubMed] [Google Scholar]
- 7.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):1–3. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV Infection Among Females in the United States. JAMA. 2007 Feb 28;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 9.Baquet CR. Native Americans' cancer rates in comparison with other peoples of color. Cancer. 1996;78(7 Suppl 1):1538–1544. [PubMed] [Google Scholar]
- 10.Becker TM, Wheeler CM, Key CR, Samet JM. Cervical cancer incidence and mortality in New Mexico's Hispanics, American Indians, and non-Hispanic whites. West J Med. 1992 April;156(4):376–379. [PMC free article] [PubMed] [Google Scholar]
- 11.Bleed DM, Risser DR, Sperry S, Hellhake D, Helgersom SD. Cancer incidence and survival among American Indians registered for Indian Health Service Care in Montana, 1982–1987. J Natl Cancer Inst. 1992 Oct 7;84(19):1500–1505. doi: 10.1093/jnci/84.19.1500. [DOI] [PubMed] [Google Scholar]
- 12.Epsey D, Paisano R, Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska Natives, 1990–2001. Cancer. 2005 Jan;103(5):1045–1053. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- 13.Nutting PA, Freeman WL, Risser DR, et al. Cancer incidence among American Indians and Alaska Natives, 1980 through 1987. Am J Public Health. 1993 Nov;83(11):1583–1588. doi: 10.2105/ajph.83.11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partin MR, Rith-Najarian SJ, Slater JS, Korn JE, Cobb N, Soler JT. Improving cancer incidence estimates for American Indians in Minnesota. Am J Public Health. 1999;89(11):1673–1677. doi: 10.2105/ajph.89.11.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker TM, Epsey DK, Lawson HW. Regional differences in cervical cancer incidence among American Indians and Alaska Natives, 1999–2004. Cancer. 2008. 2008;113:1234–1243. doi: 10.1002/cncr.23736. [DOI] [PubMed] [Google Scholar]
- 16.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. There is a high prevalance of human papillomavirus infection in American Indian women of the Northern Plains. Gynecologic Oncology. 2007 November;107(2):236–241. doi: 10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002 Nov–Dec;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther. 2007 Oct 31;82(6):760–763. doi: 10.1038/sj.clpt.6100397. [DOI] [PubMed] [Google Scholar]
- 19.Steele BC, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives—Findings from the behavioral risk facotr surveillance system, 2000–2006. Cancer. 2008 September 1;113(5):1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 20.Wiggins CL, Epsey DK, Wingo PA, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008 September 1;113(5):1142–1152. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 21.FDA. [Accessed December 29, 2007];FDA Licenses New Vaccine for Prevention of Cervical Cancer and Other Diseases in Females Caused by Human Papillomavirus. 2006 http://www.fda.gov/bbs/topics/NEWS/2006/NEW01385.html.
- 22.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1–24. [PubMed] [Google Scholar]
- 23.CDC. [Accessed December, 2007];ACIP Resolution No. 6/06-2: Vaccine to Prevent Human Papillomavirus (HPV) Infection. 2006 http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/0609mening-mcv-508.pdf.
- 24.Washington State Department of Health. [Accessed December 30, 2007];Immunization Program CHILD Profile Update. 2007 http://www.doh.wa.gov/cfh/childprofile/documents/summer2007.pdf.
- 25.National Conference of State Legislatures. [Accessed December 30, 2007];HPV Vaccine. 2007 http://www.ncsl.org/programs/health/HPVvaccine.htm.
- 26.Groom A, Holve S, Singleton R. Editorial Comments on HPV Vaccine: Newest ACIP Recommendations. Indian Child Health Notes: Indian Health Services. 2007
- 27. [Accessed February 8th, 2010];Washington Member Tribes. 2010 http://www.npaihb.org/member_tribes/washington_member_tribes/, 2010.
- 28.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and yong women: Results of a National Survey. Journal of Adolescent Health. November;45(5):453–462. doi: 10.1016/j.jadohealth.2009.04.021. 29009. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell D. ACIP Supports 'Permissive Use,' But Not Routine Use, of Gardasil in Males. [Accessed January 17, 2010];AAFP News Now. 2009 http://www.aafp.org/online/en/home/publications/news/newsnow/clinical-care-research/20091027acip-hpv-vacc.html.