Introduction
Many papers have described the biochemistry of the renin-angiotensin system (RAS). Simply put, renin and ACE produce angiotensin II, which affects the brain (increased thirst), the gut (increased salt absorption), the adrenals (aldosterone production), the kidneys (salt and water retention), the heart (increased cardiac output), and vascular smooth muscle (vasoconstriction). Inhibitors of ACE and the AT1 receptor are now widely used to reduce blood pressure, and to treat hypertension, heart failure and renal disease.1 The effectiveness of these drugs and abundant data from animal models show that the RAS is the body’s central mechanism for regulating blood pressure.
Modern studies of the RAS have substantially broadened the known roles of this system by showing that angiotensin II influences cellular proliferation and the inflammatory response. While this wider interpretation of the RAS encompasses more than control of blood pressure, it still focuses on the effects of angiotensin II in the context of vascular injury. This increased understanding of the RAS inevitably leads to the question of whether, even now, we fully understand all the physiologic roles of the RAS. Here, we argue that the answer is no, that nature uses the components of the RAS, and in particular ACE, for a wide variety of physiologic tasks.
ACE is necessary for renal development and normal male fertility
In understanding the function of the RAS, a powerful tool is the ability to genetically alter mouse genes. Knockout studies of angiotensinogen, renin, ACE or the AT1 receptor have confirmed the important role of these proteins in regulating blood pressure, but they also underline the wider physiologic role for the RAS.2 For example, adult mice lacking ACE show marked abnormalities of renal development characterized by hypertrophy of small arteries, interstitial fibrosis, atrophy of the inner medulla and renal papilla, and dilation of calyces. Renal pathology is due to the lack of angiotensin II, as identical pathology is present in mice deficient for either angiotensinogen or renin, and similar renal pathology is seen in rats treated at an early age with an ACE inhibitor. While the pathogenesis of the hypertrophy of the small renal arteries is not known, Ichikawa has demonstrated that the renal papillary blunting is due to a functional hydronephrosis; in the absence of angiotensin II generation, there is abnormal development of the smooth muscle layer along the renal pelvis, lack of normal ureteric peristalsis and elevation of intrapelvic urinary pressure.3 Thus, ACE and angiotensin II contribute to normal development of urinary track, apart from effects on blood pressure.
While angiotensin II is critical for renal development, this peptide is not the cause of the reproductive defect observed in male ACE knockout (KO) mice. ACE KO mice make phenotypically normal sperm, engage in intercourse but are nearly infertile.4,5 While the exact mechanism is not known, studies have shown that it is the lack of testis ACE enzymatic activity, and not the lack of ACE protein per se, that is responsible for the phenotype. As male angiotensinogen knockout mice are not reported as infertile, the infertile of male ACE KO mice must be mediated by effects on other peptides besides angiotensin II. This emphasizes that the RAS has effects on many other important peptides besides angiotensin I.6 While renin is enzymatically specific, ACE is much more promiscuous in its substrate specificity. ACE cleaves angiotensin I, but it also cleaves bradykinin, substance P, luteinizing hormone-releasing hormone, acetyl SerAspLysPro (AcSDKP) and β amyloid1–42 among other peptides.7,8 ACE has carboxy dipeptidase activity. It can also cleave tripeptides and even be endopeptolytic. While a single polypeptide chain, ACE contains two independent catalytic domains, often termed the N- and C-terminal domains. In vitro, the two catalytic sites show differences in binding and sensitivity to pharmacologic inhibitors.9,10 Even more important, the two catalytic sites differ in their affinity and effectiveness in cleaving individual peptide substrates. An example is the peptide AcSDKP, which is cleaved almost exclusively by the N-terminal catalytic domain.11 In contrast, the C-terminal domain is the major catalytic site responsible for the conversion of angiotensin I to angiotensin II.12
ACE and hematopoiesis
It has been known for many years that, in humans, ACE inhibitors induce a small reduction of hematocrit levels. This is reflected in ACE KO mice which exhibit a normocytic anemia due to reduced red cell mass, a phenotype independent of renal function. Short term administration of angiotensin II to ACE KO mice increases hematocrit to near normal levels.13 Moreover, a variety of studies have suggested that angiotensin II acts as a regulator of erythropoiesis through its actions on erythroid precursors in the bone marrow and as an EPO secretagogue.14
AcSDKP is a 4 amino acid peptide released from the precursor thymosin β4. When normal volunteers were administered ACE inhibitors (which block both ACE domains), plasma and urine levels of AcSDKP rose 5-fold, showing that ACE is the major enzyme responsible for the degradation of this peptide.15 Initial investigations of AcSDKP indicated that this peptide inhibited the recruitment of primitive hematopoietic progenitors into active proliferation.16,17 Thus, by degrading AcSDKP, ACE may help recruit stem cells into S-phase. AcSDKP has been reported to have several other effects, including promoting angiogenesis.18,19
Other ACE peptides such as angiotensin II and substance P also appear to have effects on hematopoietic cell development. This became clear when the analysis of ACE KO mice showed that this enzyme plays a critical role in the development of myeloid cells.20,21 For example, ACE KO bone marrow shows a shift toward more myeloid precursors, such as myeloblasts and myelocytes. The expansion of myeloid cells was also associated with increased extramedullary hematopoiesis and splenomegaly. Not only did the larger spleens contain more cells, but there is expansion of immature myeloid cells (CD11b+Gr1dim/− cells).
To study the role of ACE in myelopoiesis, ACE activity was eliminated with pharmacologic ACE inhibitors in an in vitro myeloid colony-forming assay.20 When wild type (WT) bone marrow culture was stimulated with GM-CSF, M-CSF or G-CSF, the inhibition of ACE consistently led to a significant increase in colony number. Further analysis strongly suggested that this was due to high levels of substance P in the absence of ACE. Evaluation of bone marrow in the ACE KO mice demonstrated elevated levels of substance P. This peptide is normally destroyed by ACE; in the absence of ACE activity, substance P induced bone marrow stromal cells to secrete growth factors that contributed to increased colony formation.20
While substance P appears to influence myeloid precursor number, additional experiments suggest that cellular differentiation is effected by both substance P and angiotensin II. For example, the up-regulation of the early myeloid maturation marker FcγR II/III is dependent upon the presence of angiotensin II, while the up-regulation of the granulocyte marker Gr1 and the macrophage marker F4/80 appears dependent on both angiotensin II and substance P.
Angiotensin II formation is also necessary for the functional maturation of macrophages; macrophages derived from ACE KO mice showed reduced levels of secreted pro-inflammatory cytokines, surface MHC class II protein, and surface density of the co-stimulatory factors CD80 and CD86.20 Angiotensin II supplementation fully or partly rescued these defects. Finally, when ACE KO mice were treated by acute intra-peritoneal injection of methicillin resistant S. aureus, these mice showed deficient bacterial clearance, resulting in greater blood dissemination than in WT mice. Thus, ACE appears necessary for normal myeloid cell differentiation and function.
ACE and MHC class I processing
There are several other areas in which research has indicated a role for the renin-angiotensin system during inflammatory injury. For example, Medhora has summarized evidence showing that ACE inhibitors and, to a lesser extent, AT1 receptor antagonists are some of the most successful drugs yet found to mitigate radiation-induced pneumonitis and fibrosis.22 While many of these studies were performed in animal models, a clinical trial of the ACE inhibitor captopril in patients receiving total body irradiation showed promising results in reducing mortality after hematopoietic stem cell transplantation.23 While some of the effect of an ACE inhibitor may be due to reduced angiotensin II production, there is also evidence that AcSDKP, which is elevated by ACE inhibition, may play an important role in reducing lung and other organ injury.24,25,26 Further, angiotensin 1–7, which is elevated by ACE inhibitors, has also been associated with anti-fibrotic, anti-angiogenic and anti-proliferative effects.27
Several publications have emphasized the role of ACE and angiotensin II in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a model mimicking multiple sclerosis. For example, Platten reported that in EAE an ACE inhibitor or an AT1 receptor antagonist suppressed autoimmune TH1 and TH17 T cells, and also increased FoxP3 positive T regulatory cells.28 This study found that the administration of the ACE inhibitor lisinopril actually reversed paralysis when administered to mice after the establishment of EAE. Further work by this group identified angiotensin II induced expression of TGF-β as being an important pathological mechanism in EAE.29 Additional studies documenting a role of the RAS in autoimmune demyelinating diseases are summarized by Lühder.30
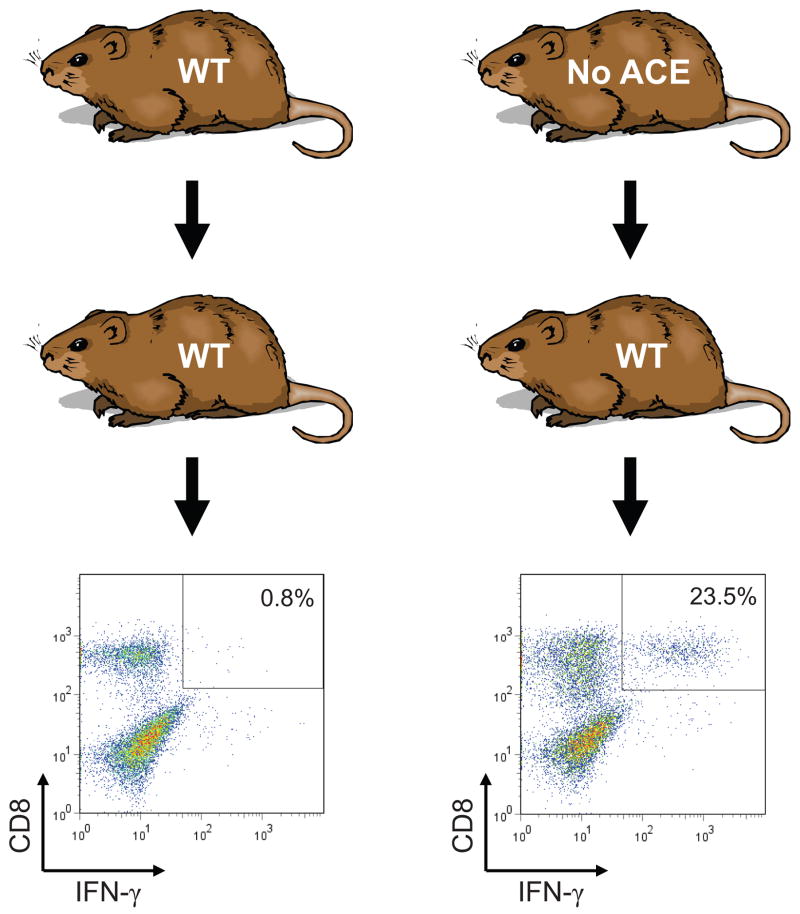
Fundamentally, ACE is a peptidase, and the suggestion that ACE plays a role in the immune response inevitably leads to the question of peptide presentation by major histocompatibility (MHC) class I antigens. All nucleated tissues use MHC class I proteins to present peptide antigens on the cell surface. Under normal circumstances, cytoplasmic proteins are degraded by the proteasome into peptides which are transported into the endoplasmic reticulum where they are trimmed, loaded onto MHC class I molecules, and then transported to the cell surface. Circulating CD8+ T cells are tolerant to the natural repertoire of displayed MHC class I peptides. However, in the presence of viral infection, viral derived peptides also become loaded onto MHC class I molecules, leading to CD8+ T cell recognition, activation and ultimately cell destruction. Substantial work had studied the trimming of peptides within the endoplasmic reticulum and implicated an aminopeptidase called ERAP as critically important. In 1992, Eisenlohr showed that, when overexpressed in cells, ACE was able to trim the carboxy termini of MHC class I peptides.31 However, little follow-up work was performed and, until recently, it was not thought that carboxypeptidases played any natural role in MHC class I peptide trimming. Ultimately, it was the development of ACE KO and ACE over-expressing mice that led us to re-examine ACE in MHC class I peptide processing.32 The critical experiment implicating ACE in this process was cross immunization analysis (Fig. 1). If a mouse is immunized with cells from an identical animal, it will not activate CD8+ T cells. However, when WT mice (having WT ACE) were immunized with cells from an ACE KO mouse, there was significant expansion and activation of CD8+ T cell. This phenomenon was also observed when ACE KO mice were immunized with cells from a WT mouse. These experiments were carefully controlled; all mice were backbred to a common genetic background (C57BL/6), and the donor and recipient mice were the same sex. Further strengthening the argument were experiments in which a WT mouse was immunized with cells from an ACE KO mouse, but then challenged with cells from either a WT mouse or a WT mouse treated for several days with the ACE inhibitor ramipril. When the immunized WT mouse was challenged with WT cells, there was virtually no T cell reaction. However, when the immunized WT mouse was challenged with cells from a syngeneic WT mouse treated with ramipril, there was a significant CD8+ T cell response. In other words, treating a mouse with an ACE inhibitor changed the repertoire of displayed MHC class I peptides. Finally, when a cross immunization study was performed using ACE KO cells pre-treated with a blocking antibody specific for the MHC class I protein H-2Db (one of the two MHC class I proteins present in strain C57BL/6 mice) the ACE KO cells were significantly less efficient as immunogens. Such experiments argue that it is differences in MHC class I peptide repertoire, and not differences in minor histocompatability antigens, that are responsible for the immunogenicity of ACE KO vs. WT mice. These and other experiments provide very strong evidence that, even under normal physiologic conditions, ACE affects MHC class I peptide repertoire in WT mice. Thus, ACE affects one of the central means by which the immune system detects viral infection and tissue transplantation. It also raises the question as to whether conditions such as hypertension may subtly change the repertoire of expressed MHC class I peptides, perhaps leading to some form of immune activation and cytokine release affecting blood pressure.
Fig. 1.
Cross-immunization of WT and ACE KO mice. WT mice were immunized with peritoneal macrophages from either ACE WT or ACE KO mice. After 10 days, the recipient mice were sacrificed and splenocytes were expanded in vitro by restimulating with macrophages equivalent to those used for immunization. After 7 days, lymphocytes were again restimulated with macrophages equivalent to those used for immunization, but for 5 hrs. FACS analysis was then used to stain for CD8 and IFN-γ. The percentage of CD8+ cells that are IFNγhigh is indicated.
ACE over expression and resistance to tumors
Additional evidence for a significant role for ACE in the immune response resulted from analysis of mice called ACE 10/10. In this model, targeted homologous recombination was used to place ACE gene expression under the control of the c-fms promoter.33 c-fms is expressed by myelomonocytic lineage cells where it encodes the receptor for macrophage colony-stimulating factor.34,35 Thus, ACE 10/10 mice markedly over express ACE in monocytes, macrophages and other myelomonocytic lineage cells and lack ACE expression by endothelial cells, which do not recognize the c-fms promoter. Because of the high levels of ACE in monocytic cells, care must be taken in extrapolating from the ACE 10/10 model to the normal physiologic role of ACE.
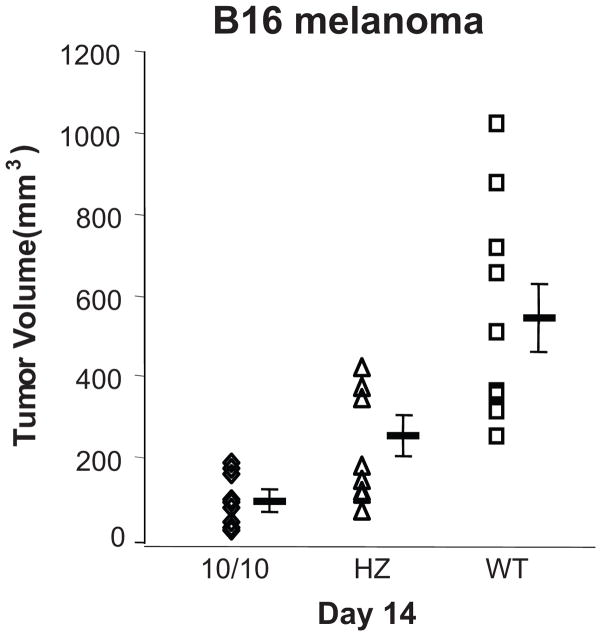
The basal physiology of the ACE 10/10 mice is very similar to that of WT mice. ACE 10/10 animals have normal blood pressures, renal function and appearance of both bone marrow and peripheral blood. However, when immunologically challenged, ACE 10/10 mice have a marked enhancement of their innate and adaptive immune responses. This first became apparent when we studied the growth of the B16 melanoma in ACE 10/10 mice.33 B16 is an aggressive mouse neoplasm which is commonly used to evaluate tumor immunology. Tumor growth in ACE 10/10 mice was evaluated by implanting melanoma cells intradermally and then measuring tumor volume 14 days later. Tumor in WT mice averaged 540 mm3, while heterozygous and ACE 10/10 mice averaged only 252 and 90 mm3, respectively (Fig. 2). This difference in tumor size was observed whether the ACE 10/10 mice were on a pure C57BL/6 background, a mix of C57BL/6 and 129 or partially outbred to CD1(Swiss) mice. In all experiments, the ACE 10/10 phenotype was associated with significantly smaller tumor growth compared to genetically matched mice having WT ACE expression.
Fig. 2.
Tumor growth in ACE 10/10 mice. Wild type (WT), heterozygous (HZ) and ACE 10/10 mice were injected intradermally with 1 × 106 B16 melanoma cells. The mice were sacrificed 14 days later and tumor volume was determined. Each point is an individual mouse. Group means are also shown. Tumor growth in ACE 10/10 mice was greatly suppressed as compared to WT mice.
While ACE 10/10 mice have several differences from WT animals, we believe it is the presence of ACE over expression by myelomonocytic cells that is central to ACE 10/10 behavior. For example, we studied tumor growth in ACE 10/10 and WT mice in which both groups were treated with the ACE inhibitor captopril.33 With ACE inhibition, tumor growth was similar between the two groups. Thus, catalytically active ACE in myelomonocytic cells is important for the resistance of ACE 10/10 mice to melanoma.
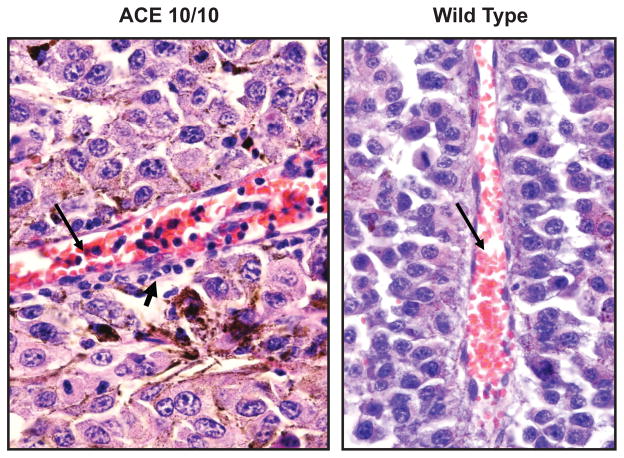
Histologic examination of the tumors provided insight into how ACE 10/10 mice suppress tumor growth. Large numbers of intravascular and tumor associated monocytes and macrophages were found in the small tumors present in ACE 10/10 mice (Fig. 3). In fact, occasional vessels were almost engorged by a monocytic response. As expected, these cells showed strong expression of ACE. Other inflammatory cells, including T cells, were also more abundant in the tumors of the ACE 10/10 mice as compared to the larger tumors in WT mice. Further, the in vitro analysis of cytokine production by macrophages from ACE 10/10 mice consistently showed an enhanced pro-inflammatory phenotype, with increased expression of the pro-inflammatory cytokines TNFα, IL-12, and nitric oxide, and decreased expression of the anti-inflammatory cytokine IL-10, as compared to cells from WT mice.
Fig. 3.
Tumor inflammation. Photos of blood vessels (long arrows) from melanomas in ACE 10/10 and WT mice. Many more mononuclear inflammatory cells are present in the blood vessels and the tumor (short arrow) of the ACE 10/10 mouse as compared to WT.
If an enhanced inflammatory response is responsible for smaller tumors in ACE 10/10 mice, then bone marrow transplant should endow a recipient WT mouse with enhanced tumor resistance. In fact, precisely this was observed.33 When WT mice were irradiated and then transplanted with either ACE 10/10 or WT bone marrow, and then challenged with melanoma, the WT mice with ACE 10/10 bone marrow had substantially smaller tumors (141 ± 18 mm3 vs 342 ± 33 mm3 for mice with WT bone marrow, p<0.0001). Thus, transfer of ACE 10/10 bone marrow to WT recipients significantly increased resistance to melanoma. This is not due to enhanced local production of angiotensin II, since ACE 10/10 mice on a genetic background in which angiotensin II production was impossible (ACE 10/10:angiotensinogen double knock-out mice), also showed increased resistance to tumor. Rather, abundant evidence indicates that the over expression of ACE by myelomonocytic cells renders a mouse more immunologically capable of resisting tumor growth.33
ACE over expression and bacterial resistance
The thesis that ACE 10/10 mice show an enhanced immune response led us to test the behavior of the ACE 10/10 model when challenged with bacterial infection.36 More than tumor models in mice, infections mimic the biological behavior of human disease. Our approach was to challenge ACE 10/10 with L. monocytogenes (listeria), a mainstay in the study of innate immunity, and with methicillin resistant Staphylococcus aureus (MRSA). Both systems demonstrated a substantially better innate immune response in ACE 10/10 mice as compared to WT. For example, when mice were challenged with an IV injection of listeria and then sacrificed three days after inoculation, there was more than 6-fold the bacteria (colony forming units or CFU) in the spleens of WT mice than in the spleens of ACE 10/10. At 5 days after bacterial infection, CFUs in the spleens of WT mice were 8-fold those of ACE 10/10. This difference between WT and ACE 10/10 mice is all the more remarkable since WT mice are fully immunocompetent and very capable of clearing a listeria infection. A similar result was found when mice were challenged by an intradermal injection of MRSA. Four days after infection, the quantity of bacteria in the skin of WT mice was greater than 50-fold the CFUs found in ACE 10/10 mice. Our study of tumor in the ACE 10/10 mice showed that the enzymatic activity of ACE was critical for an enhanced immune response. Experiments with ACE inhibitors showed a similar result for resistance to bacteria; when treated with ACE inhibitors, ACE 10/10 mice were equivalent to WT in their response to either listeria or MRSA.
Many studies have documented that two major mechanisms used by macrophages to kill bacteria are the generation of reactive oxygen species by NADPH oxidase (Nox2) and the production of nitric oxide (NO) by inducible nitric oxide synthase (iNOS).37,38 When reactive oxygen species were measured, there was no difference between macrophages from WT or ACE 10/10. However, studies of either iNOS induction after LPS or nitrite production in response to listeria consistently showed a marked increase of iNOS and NO by ACE 10/10 macrophages. This increased iNOS production is critical to the enhanced innate bacterial resistance in ACE 10/10 mice, as treating these mice with the iNOS inhibitor 1400W, rendered them equivalent to the WT mice when challenged with either listeria or MRSA.
Great insight into the ACE 10/10 model was obtained from an experiment in which peritoneal macrophages were assessed for their ability to kill listeria in vitro. This showed that, in the absence of immune stimulation, there was no difference between macrophages derived from ACE 10/10 and WT mice. Only after stimulation with interferon-γ (IFN-γ) were cells from ACE 10/10 mice significantly better in killing bacteria than equivalent cells from WT. As previously discussed, the enhanced immune response of ACE 10/10 can be eliminated with an ACE inhibitor. However, this is not an acute effect, meaning that for an assay such as in vitro killing, the addition of an ACE inhibitor during the 8 hour killing assay does not reverse the increased efficacy of the ACE 10/10 macrophages. Rather, ACE 10/10 mice must be treated with an ACE inhibitor for several days in order to revert the cellular phenotype to that of cells from WT mice. These and other data are consistent with a model in which ACE over expression changes the underlying pattern of monocytic differentiation and tilts these cells towards a more pro-inflammatory phenotype. While the biochemical sequence of events leading to the ACE 10/10 phenotype is not fully understood, we postulate that the catalytic actions of ACE on an unidentified peptide substrate are responsible for the phenotypic change.
Perspective
The modern ACE gene resulted from an gene duplication of a primordial ACE having one catalytic site; this duplication is thought to have occurred over 300 million years ago.39 The modern clinical emphasis on the role of ACE in blood pressure control often obscures the implications that this protein maintained two independent catalytic sites throughout millions of years of evolution. ACE is enzymatically much less specific than renin, but it is involved in many more physiologic processes. Understanding the many roles of ACE is important, since the peptide substrates and products of this enzyme have profound physiologic effects.
Acknowledgments
Source of Funding
This work was supported by National Institutes of Health grant T32 DK007770 (WLBB), F32 HL105036 (FSO), R00 DK083455 (RAGV), R00-DK051445 (SF) R01 DK039777 and R01 HL110353 (KEB).
Footnotes
Conflict of Interest: None
References
- 1.Paulis L, Unger T. Novel therapeutic targets for hypertension. Nature Reviews Cardiology. 2010;7:431–441. doi: 10.1038/nrcardio.2010.85. [DOI] [PubMed] [Google Scholar]
- 2.Xiao HD, Fuchs S, Frenzel K, Teng L, Li P, Shen XZ, Adams J, Zhao H, Keshelava GT, Bernstein KE, Cole JM. The use of knockout mouse technology to achieve tissue selective expression of angiotensin converting enzyme. J Mol Cell Cardiol. 2004;36:781–789. doi: 10.1016/j.yjmcc.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Matsusaka T, Miyazaki Y, Ichikawa I. The renin angiotensin system and kidney development. Annu Rev Physiol. 2002;64:551–561. doi: 10.1146/annurev.physiol.64.081501.155721. [DOI] [PubMed] [Google Scholar]
- 4.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O’Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 5.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 6.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O’Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95:2552–2557. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corvol P, Eyries M, Soubrier F. Handbook of proteolytic enzymes. 2. Elsevier Academic Press; Amsterdam, San Diego: 2004. Peptidyl-dipeptidase A/angiotensin I-converting enzyme; pp. 332–346. [Google Scholar]
- 8.Zou K, Maeda T, Watanabe A, Liu J, Liu S, Oba R, Satoh Y, Komano H, Michikawa M. Abeta42-to-Abeta40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J Biol Chem. 2009;28:31914–1920. doi: 10.1074/jbc.M109.011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei L, Clauser E, Alhenc-Gelas F, Corvol P. The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem. 1992;267:13398–13405. [PubMed] [Google Scholar]
- 10.Jaspard E, Wei L, Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 11.Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs S, Xiao HD, Michaud A, Campbell DJ, Hubert C, Capecchi MR, Corvol P, Bernstein KE. The ACE C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008;51:267–274. doi: 10.1161/HYPERTENSIONAHA.107.097865. [DOI] [PubMed] [Google Scholar]
- 13.Cole J, Ertoy D, Lin H, Sutliff RL, Ezan E, Guyene TT, Capecchi M, Corvol P, Bernstein KE. J Clin Invest. 2000;106:1391–1398. doi: 10.1172/JCI10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56:558–565. doi: 10.1053/j.ajkd.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A, Guigon M. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Seraspenide) on the growth of human CD34+ subpopulations in response to growth factors. Blood. 1993;82:3307–3314. [PubMed] [Google Scholar]
- 17.Waeckel L, Bignon J, Liu JM, Markovits D, Ebrahimian TG, Vilar J, Mees B, Blanc-Brude O, Barateau V, Le Ricousse-Roussanne S, Duriez M, Tobelem G, Wdzieczak-Bakala J, Lévy BI, Silvestre JS. Tetrapeptide AcSDKP induces postischemic neovascularization through monocyte chemoattractant protein-1 signaling. Arterioscler Thromb Vasc Biol. 2006;26:773–779. doi: 10.1161/01.ATV.0000203510.96492.14. [DOI] [PubMed] [Google Scholar]
- 18.Sosne G, Qiu P, Goldstein AL, Wheater M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010;24:2144–2151. doi: 10.1096/fj.09-142307. [DOI] [PubMed] [Google Scholar]
- 19.Myöhänen T, Tenorio-Laranga J, Jokinen B, Vázquez-Sánchez R, Moreno-Baylach M, García-Horsman J, Männistö P. Prolyl oligopeptidase induces angiogenesis both in vitro and in vivo in a novel regulatory manner. Br J Pharmacol. 2011;163:1666–1678. doi: 10.1111/j.1476-5381.2010.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE, Shen XZ. Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 2011;25:1145–1155. doi: 10.1096/fj.10-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen XZ, Bernstein KE. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle. 2011;10:1363–1369. doi: 10.4161/cc.10.9.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: mitigation by angiotensin converting enzyme (ACE) inhibitors. Respirology. 2012;17:66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, Lawton CA, Moulder JE. Mitigation of Late Renal and Pulmonary Injury After Hematopoietic Stem Cell Transplantation. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.05.081. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Xiao HD, Xu J, Ong FS, Kwon M, Roman J, Gal A, Bernstein KE, Fuchs S. Angiotensin-converting enzyme N-terminal inactivation alleviates bleomycin-induced lung injury. Am J Pathol. 2010;177:1113–1121. doi: 10.2353/ajpath.2010.081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800. doi: 10.1161/01.hyp.37.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CX, Rhaleb NE, Yang XP, Liao TD, D’Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrario CM. ACE2: more of Ang-(1–7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lühder F, Lee DH, Gold R, Stegbauer J, Linker RA. Small but powerful: short peptide hormones and their role in autoimmune inflammation. J Neuroimmunol. 2009;217:1–7. doi: 10.1016/j.jneuroim.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Eisenlohr LC, Bacik I, Bennink JR, Bernstein K, Yewdell JW. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992;71:963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 32.Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol. 2011;12:1078–1085. doi: 10.1038/ni.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JW, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Himes SR, Tagoh H, Goonetilleke N, Sasmono T, Oceandy D, Clark R, Bonifer C, Hume DA. A highly conserved c-fms gene intronic element controls macrophage-specific and regulated expression. J Leukoc Biol. 2001;70:812–820. [PubMed] [Google Scholar]
- 35.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 36.Okwan-Duodu D, Datta V, Shen XZ, Goodridge HS, Bernstein EA, Fuchs S, Liu GY, Bernstein KE. Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J Biol Chem. 2010;285:39051–39060. doi: 10.1074/jbc.M110.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 38.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 39.Cornell MJ, Williams TA, Lamango NS, Coates D, Corvol P, Soubrier F, Hoheisel J, Lehrach H, Isaac RE. Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J Biol Chem. 1995;270:13613–13691. doi: 10.1074/jbc.270.23.13613. [DOI] [PubMed] [Google Scholar]