Abstract
Background
Heart failure is a growing epidemic and a typical aspect of heart failure pathophysiology is altered calcium transients. Normal cardiac calcium transients are initiated by Cav1.2 channels at cardiac T-tubules. BIN1 is a membrane scaffolding protein that causes Cav1.2 to traffic to T-tubules in healthy hearts. The mechanisms of Cav1.2 trafficking in heart failure are not known.
Objective
To study BIN1 expression and its effect on Cav1.2 trafficking in failing hearts.
Methods
Intact myocardium and freshly isolated cardiomyocytes from non-failing and end-stage failing human hearts were used to study BIN1 expression and Cav1.2 localization. To confirm Cav1.2 surface expression dependence on BIN1, patch clamp recordings were performed of Cav1.2 current in cell lines with and without trafficking competent BIN1. Also, in adult mouse cardiomyocytes, surface Cav1.2 and calcium transients were studied after shRNA mediated knockdown of BIN1. For a functional readout in intact heart, calcium transients and cardiac contractility were analyzed in a zebrafish model with morpholino mediated knockdown of BIN1.
Results
BIN1 expression is significantly decreased in failing cardiomyocytes at both mRNA (30% down) and protein (36% down) levels. Peripheral Cav1.2 is reduced 42% by imaging and biochemical T-tubule fraction of Cav1.2 is reduced 68%. Total calcium current is reduced 41% in a cell line expressing non-trafficking BIN1 mutant. In mouse cardiomyocytes, BIN1 knockdown decreases surface Cav1.2 and impairs calcium transients. In zebrafish hearts, BIN1 knockdown causes a 75% reduction in calcium transients and severe ventricular contractile dysfunction.
Conclusions
The data indicate that BIN1 is significantly reduced in human heart failure, and this reduction impairs Cav1.2 trafficking, calcium transients, and contractility.
Keywords: calcium, L-type calcium channel, trafficking, cardiomyopathy, heart failure, ion channels, calcium transient
INTRODUCTION
Heart failure (HF) is the clinical consequence of multiple forms of heart disease and the fastest growing cardiovascular disorder in the world. In the United States, adults over the age of 40 have a 20% lifetime risk of developing HF [1]. The most common type of non-ischemic HF is dilated cardiomyopathy (DCM), which remains the most common preoperative diagnosis in heart transplant recipients. The failing hearts of DCM patients undergo a complicated progressive remodeling process that leads to terminal disease. The cell biology of failing cardiomyocytes remains poorly understood.
Membrane associated scaffolding proteins have been reported to have important roles in normal cardiomyocyte organization and function. Known mutations within these proteins are associated with many severe cardiovascular diseases. For example, mutations in desmosome proteins lead to life-threatening arrhythmogenic right ventricular cardiomyopathies [2]. In addition, mutations in ankyrin B, the protein that targets Na/K ATPase and Na/Ca exchanger 1 (NCX1) to T-tubules, cause long QT syndrome [3]; whereas mutations in Nav1.5 that interfere with its binding to the intercalated disc scaffolding protein ankyrin G cause Brugada Syndrome [4].
The BAR domain superfamily is a class of scaffolding proteins important in membrane curvature formation and organization [5]. Mutations and alternate splicing of Bridging Integrator 1 (BIN1, Amphiphysin 2), a BAR protein [5], result in inheritable skeletal muscular diseases of central nuclear myopathy [6] and myotonic dystrophies [7]. We recently found that, in cardiomyocytes, BIN1 facilitates microtubules based delivery of Cav1.2 channels directly to T-tubules [8] for normal calcium transient development. The normal cardiac calcium transient begins with calcium entry through L-type calcium channels (with pore-forming α subunit Cav1.2), inducing a large release of calcium from the intracellular sarcoplasmic reticulum via ryanodine receptors. In ventricular cardiomyocytes, close association of Cav1.2 channels with ryanodine receptors is necessary for efficient calcium release and this association is achieved by Cav1.2 enrichment within the T-tubule invaginations of the plasma membrane [9], a process controlled by the integrity of BIN1 at cardiac T-tubules [8]. The role of BIN1 in failing cardiomyocytes, however, remains un-identified. Given the fact that a major aspect of heart failure pathophysiology is perturbed calcium regulation, understanding BIN1 biology may reveal novel aspects of mechanisms underlying heart failure progression.
Despite the importance of Cav1.2 channels in initiating the calcium transient, their regulation in HF is unclear. Reports of Cav1.2 expression in DCM vary between lower levels [10, 11] to no change [12] in whole-cell Cav1.2 current density, with little difference in transcript and protein expression levels [12, 13]. Given that trafficking of ion channels to the surface membrane is a dynamic process [14] with the surface half life of channels measured on the order of hours [15], it is possible that channel trafficking and surface availability are altered in heart disease without affecting the total cellular content [16]. Previous studies also document that in both human and animal models of acquired heart failure, microtubule dynamics may be altered [16]. Thus it is possible that the pathologic cardiomyocyte remodeling that occurs with acquired human cardiomyopathy involves changes to the cytoskeleton and membrane scaffolding, thus altering the intracellular movement of Cav1.2 channels.
In this study we found a significant reduction in BIN1 mRNA and protein in failing human cardiomyocytes. Furthermore, although the total cellular content of Cav1.2 was not changed, we determined that the channel was less abundant at T-tubules. In adult mouse cardiomyocytes, shRNA knockdown of BIN1 decreased surface Cav1.2 channels, while exogenous BIN1 lacking the Cav1.2 trafficking domain reduced calcium currents in a reductionist cell line. Moreover, loss of BIN1 in both mouse cardiomyocytes and zebrafish hearts resulted in severely altered cardiac calcium transients.
METHODS
Human Tissue Collection and Cardiomyocyte Isolation
With the approval of the University of California, San Francisco (UCSF) Committee for Human Research, we obtained tissue from hearts removed at the time of transplant at UCSF, or from organ donors whose hearts were not transplanted. Full informed consent was obtained from all UCSF transplant recipients prior to surgery. The California Transplant Donor Network (CTDN) provided the unused donor hearts and obtained informed consent for their use from the next of kin.
After immediate perfusion with cold cardioplegia, full-thickness samples from the left ventricular free wall were snap frozen into liquid nitrogen for later protein and mRNA analysis or biochemical analysis, embedded in OCT medium and frozen in liquid nitrogen-chilled isopentane for immunohistochemistry, or subjected to collagenase II digestion for cardiomyocyte isolation experiments. Please see supplemental material for detailed isolation methods as well as imaging and biochemical T-tubule fractionation [17] methods.
Adult Mouse Cardiomyocytes Preparation
Adult mouse cardiomyocytes were isolated and cultured according to a standard protocol [8]. For the knockdown experiments, 40 µM cytochalasin D was added to the culture medium for Ttubule preservation [18]. See supplemental materials for detailed methods.
Patch Clamp Electrophysiology
HEK 293 cells were transfected with Cav1.2 alpha1C-YFP and beta2a-CFP (in a 1:1 ratio). The cells were cotransfected with C-terminus mCherry tagged full length BIN1 or BIN1 truncation (BIN1-BAR*), which loses interaction with Cav1.2 but retains membrane invaginations [8]. Only the cells expressing the three fluorophores were selected for whole cell configuration study of Ltype calcium current. See supplemental materials for detailed methods.
Optical Heartbeat Analysis of Zebrafish Embryonic Hearts
0.5–1 ng of an ATG MO against bin1 (Gene-Tools), 5’ – TGACTCCTTTCCCAACCTCTGCCAT – 3’ was injected into one-cell-stage embryos. In vivo embryonic hearts were imaged at 50 and 70 hours post fertilization (hpf) after 0.1% tricaine immobilization. 100–140 frames per second movies were obtained with a Hamamatsu EMCCD 9300 camera and Simple PCI capture software. Fractional area change measurements from these movies were calculated as (diastolic area – systolic area / diastolic area) x100 [19]. Calcium optical mapping of zebrafish heart was performed as previously described [16].
Statistical Analysis
Prism 5 software (GraphPad) was used for all statistical analysis. For comparison between two groups, paired or unpaired two-tail student’s t-test was performed. For comparison among three and more treatment groups, one-way ANOVA followed by Dunnet post test was performed.
RESULTS
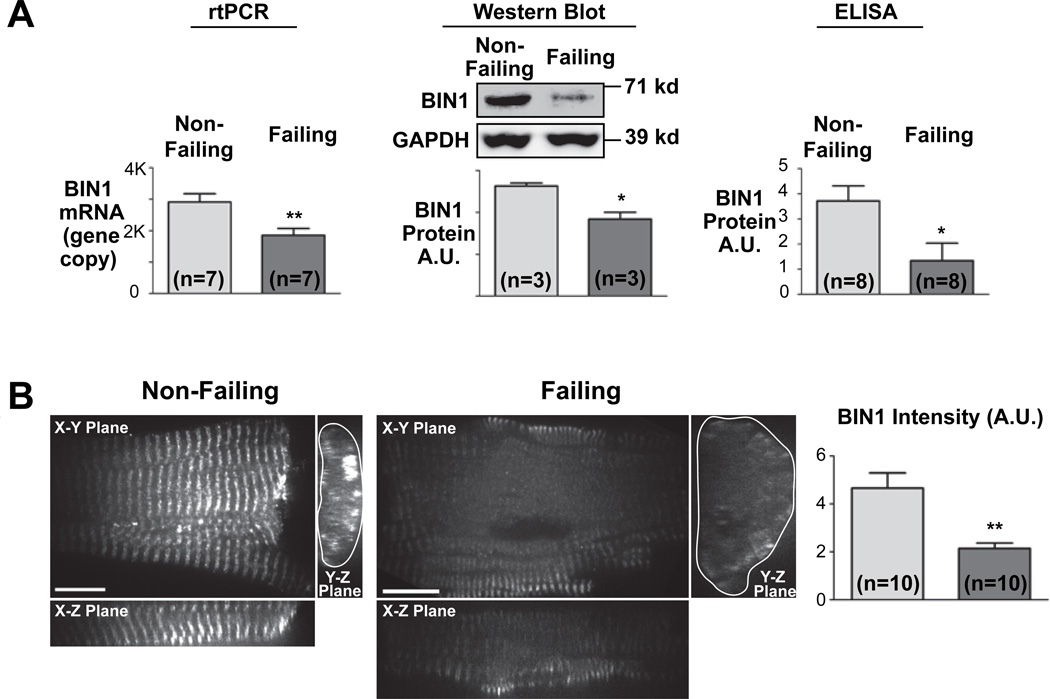
To examine BIN1 expression in failing human hearts, we obtained freshly explanted hearts from transplant recipients with end-stage non-ischemic DCM and from organ donors whose hearts were not used for transplantation. After immediate perfusion with cardioplegia, we both isolated ventricular cardiomyocytes and froze ventricular tissue for cryosectioning. In the lysates prepared from the frozen myocardium, BIN1 expression was determined. Quantitative RT-PCR indicated that BIN1 mRNA level is reduced 30% in failing human hearts (n=7, P<0.01) and Western blotting indicated a 36% reduction of BIN1 protein (n=3, P<0.05), and a sensitive ELISA test detected a 64% reduction of BIN1 protein in heart lysates (n=8, P<0.01) (Figure 1A). To further study the expression of BIN1 at a cellular level, freshly isolated human cardiomyocytes were fixed, immunolabeled for BIN1, and imaged with spinning disc confocal microscopy. We observed that failing cardiomyocytes have a larger cell volume with less organized periodicity of BIN1 than controls (Figure 1B, right panel). Immunofluorescence identified a 54% reduction of BIN1 fluorescent intensity in failing cardiomyocyte T-tubules (Figure 1B, n=10, P<0.001).
Figure 1. Failing cardiomyocytes express lower BIN1.
(A) Quantitative RT-PCR analysis (left), semi-quantitative western blotting and quantitative ELISA indicate significant reduction of BIN1 expression in failing human hearts. (B) Confocal images (100x) of isolated human cardiomyocytes stained with mouse anti-BIN1 antibody indicate decreased expression of BIN1. (*, P<0.05; **, P<0.01)
Because BIN1 has a critical role in targeting Cav1.2 to cardiac T-tubules, we hypothesized that a reduction of BIN1 in failing cardiomyocytes may be associated with altered intracellular localization of Cav1.2 channels. Using OCT-embedded frozen myocardium, immunofluorescence of Cav1.2 reveals that the channels are enriched at T-tubules, and this enrichment is substantially decreased in failing cardiomyocytes (Supplemental Figure 1). To better understand the subcellular distribution of Cav1.2, immunofluorescence of Cav1.2 in isolated human cardiomyocytes was imaged at Z-depth increments of 0.1 μm using spinning disc confocal microscopy (Figure 2), with only rod-shaped cells with clear striation pattern were selected for data analysis (Figure 2A). Next, collapsed projections of reconstructed slices of cardiomyocytes (represented as red frames in schematic) were then used for quantification of peripheral Cav1.2 (Figure 2B, left panel). Unlike murine cardiomyocytes [20], human cardiomyocytes contain a large population of small 1–2 micron deep T-tubules in addition to the observed sparse elongated T-tubules which run into the center of cardiomyocytes (Supplemental Figure 2, average T-tubule depth is 1.2 ±0.1 microns, n=9 cells). Therefore, quantification of Cav1.2 from the cortical 2 micron regions of these cardiomyocytes represents surface Cav1.2 from both sarcolemma and the majority of primary T-tubules. As seen in the projection of a non-failing cardiomyocyte (Figure 2B, top), Cav1.2 is enriched in these cortical regions, whereas in diseased cardiomyocytes this distribution pattern is less evident (Figure 2B, bottom). Cav1.2 quantification (approximately 30 cardiomyocytes isolated from five separate hearts for each condition were used) indicates that the cortical proportion of Cav1.2 (within 2 μm from cell edges containing the majority of T-tubule Cav1.2) is significantly lower in failing cardiomyocytes (Figure 2B, bottom right). To correct for cell volume difference between the two populations, mean fluorescent intensity per pixel at cortical portion were further analyzed and compared between non-failing and failing cardiomyocytes. As indicated in Supplemental Figure 3, mean surface Cav1.2 fluorescence intensity, normalized to general sarcoplasmic intensity, is significantly lower in failing cardiomyocytes. The data indicate less Cav1.2 per surface area in failing cardiomyocytes.
Figure 2. Intracellular Cav1.2 distribution in failing human cardiomyocytes.
(A) Two dimensional frame view of confocal images (100x) of Cav1.2 immunofluorescence in human non-failing (left panel) and failing cardiomyocytes (right panel). (B) Volume view of intracellular Cav1.2 distribution reconstructed from a stack of 100x confocal image frames acquired at a z-step of 0.1 μm. The Z-axis cross section volume views of subsection cardiomyocytes were generated from the original z-stack and shown in the left panel (top, representative non-failing cardiomyocyte; bottom, representative failing cardiomyocyte). The top right cardiomyocyte schematic illustrates the imaging frames (green), the viewing sections (red frames), as well as the analysis of the peripheral Cav1.2 (blue area). Cortical Cav1.2 at the cell periphery within 2 μm of cell edges (blue area indicated in the schematic) were quantified and normalized to total cellular Cav1.2 and are presented in the bottom right panel. Peripheral Cav1.2 fluorescent signal is significantly reduced in failing cardiomyocytes. (***, P<0.001)
Despite the reduction in surface Cav1.2, we confirmed that total Cav1.2 protein by Western blotting (Figure 3A) and mRNA by RT-PCR (Supplemental Figure 4) are not different between failing and non-failing hearts, consistent with previous reports [12, 13]. To further explore the T-tubule content of Cav1.2 channels in failing myocardium, a conventional biochemical membrane fractionation assay was employed, based on a sucrose gradient with ultracentrifugation, to isolate T-tubule membranes [17]. As seen in Figure 3B, Cav1.2 is enriched in the T-tubule fraction from non-failing myocardium but is barely present in T-tubules from failing myocardium. A positive control for T-tubule membrane channel whose trafficking is not BIN1 dependent [8] is the NCX1 [21]. We found NCX1 to be similarly enriched in the T-tubule fractions of non-failing and failing hearts (Figure 3B). From this technique we cannot measure the relative amount of T-tubule membrane in failing versus non-failing heart, but rather the trafficking of Cav1.2 relative to the trafficking of NCX1. We find that the ratio of T-tubule Cav1.2 to T-tubule NCX1 is significantly reduced in failing myocardium (Figure 3B), indicating that BIN1 dependent trafficking in heart failure is reduced.
Figure 3. Reduced T-tubule abundance of Cav1.2 in failing human myocardium.
(A) Western blot of protein lysates prepared from human myocardium indicates no significant change of Cav1.2 protein expression level in failing myocardium. (B) A sucrose gradient was generated for isolation of T-tubule membranes from the microsomes prepared from human myocardium. As indicated, fractions recovered from 32%/35% interface are enriched with T-tubule localized proteins like Cav1.2 and NCX1. In the same T-tubule fraction prepared from failing myocardium, Cav1.2 expression is significantly reduced whereas NCX1 expression is preserved. T-tubule Cav1.2 protein level is normalized to NCX1 and presented in the right panel. (n=3 hearts, P<0.05)
Given that Cav1.2 is less abundant in T-tubules from failing cardiomyocytes (Figures 2 and 3) and that BIN1 is reduced at T-tubules in these cells (Figure 1), we examined whether altered Cav1.2 localization during heart failure could be a function of decreased BIN1. Lentiviral-transduced shRNA was used to knock down BIN1 in isolated adult mouse cardiomyocytes, which retain T-tubules during extended culture when supplemented with cytochalasin D [18] (Supplemental Figure 5). Partial BIN1 knockdown results in a significant diminishment of surface Cav1.2 (Figure 4A). In the absence of cytoskeleton stabilizers such as cytochalasin D, adult mouse cardiomyocytes lose BIN1 and surface Cav1.2 expression [18] during extended culture in vitro. Replenishment of BIN1 by lentiviral mediated expression of exogenous BIN1 (BIN1-V5), partially rescued surface expression of Cav1.2 (Figure 4B). To further explore whether functional calcium channels are localized to cell surface by BIN1, electrophysiology studies were carried out in a cell line. Exogenous GFP tagged Cav1.2 was co-transfected with either an mCherry tagged truncated form of BIN1 (BIN1-BAR*) which causes membrane invagination but does not have the BIN1 domains necessary for Cav1.2 targeting [8], or full length mCherry tagged BIN1 as a control. As seen in Figure 4C (left), nonfunctional mutant BIN1 results in 41% (P < 0.001) reduction in peak surface calcium current. There was no difference in the half maximal activation voltage (V1/2) indicating that channel trafficking (and not kinetics) is altered by changing BIN1 expression (Figure 4C, right panel). These electrophysiology studies, together with the biochemical biotinylation data, indicate that the level of BIN1 expression regulates surface availability of functional Cav1.2 channels. The reduction of BIN1 in human failing cardiomyocytes can therefore contribute to the decreased cell surface Cav1.2 proteins (Figure 2 and 4A) and to the reduced whole cell L–type calcium current [10, 11].
Figure 4. BIN1 level controls surface availability of functional Cav1.2 channels.
(A) Surface biotinylation indicate that reduced BIN1 expression following lentiviral mediated shRNA knockdown (left) causes less surface expression of Cav1.2 in adult mouse cardiomyocytes. (B) Exogenous BIN1 rescues Cav1.2 trafficking. In three-day cultured cardiomyocytes (compared to freshly dissociated) BIN1 expression is decreased and Cav1.2 is internalized. Introduction of V5-tagged exogenous BIN1 (indicated as anti-V5 staining) by lentiviral construct normalizes BIN1 level and rescues Cav1.2 surface expression. (C) Non Cav1.2 targeting BIN1 mutant (BIN1-BAR*) decreases full length BIN1 induced surface Cav1.2 current in HEK 293 cells (n=9). The half maximal activation voltage (V1/2) remains the same. (*, P<0.05; **, P<0.01; ***, P<0.001)
To further examine whether loss of BIN1 affects calcium regulation in adult mouse cardiomyocytes, calcium transients were followed using the fluorescent calcium sensor Fluo-4AM. BIN1 knockdown resulted in slower development of calcium transients (Figure 5), which is an expected consequence of decreased calcium entry [8]. As indicated in the representative supplemental movies 1 (control cardiomyocytes) and 2 (BIN1 knockdown cardiomyocytes), only uniform calcium waves were used for analysis of calcium transient kinetics. Next, we were interested in studying the effect of BIN1 knockdown on whole heart calcium transients however, in mice, BIN1 knockout is perinatal lethal [22]. As zebrafish do not require a functional circulatory system during early development, we used zebrafish embryos to evaluate whether in vivo loss of BIN1 could affect calcium transients. Within 72 hours post fertilization (hpf), zebrafish hearts display organized cardiac Z-discs with associated Cav1.2 localization [23], and thus can be used to study the in vivo effects of BIN1 function. Utilizing a cardiac-specific fluorescent calcium indicator zebrafish transgenic line Tg(cmlc2:gCaMP)s878 that allows for in vivo optical mapping analysis in intact animals [24], we evaluated the effect of BIN1 on calcium transients in hearts subjected to bin1 morpholino knockdown. Imaging of calcium transients in these intact hearts indicates a diminished calcium transient (Figure 6). BIN1 knockdown in zebrafish also resulted in significantly impaired ventricular contractility (fractional area change was reduced from 28.6±1.8% to 5.5±1.7%, n=9, P<0.001) (Supplemental Figure 6, Supplemental Movie 3, 4), a functional consequence of altered Cav1.2 localization and calcium transients. Changes in gross morphology were also apparent with BIN1 knockdown resulting in zebrafish embryos with a more curved phenotype (Supplemental Figure 7A), likely due to interference with T-tubule development in skeletal muscle [25]. In addition, BIN1 knockdown significantly decreases heart rate (Supplemental Figure 7B) which may result from abnormal calcium channel trafficking in pacemaker cells. Morpholino zebrafish hearts occasionally developed unusual beating patterns consistent with non-nodal arrhythmias, however bradycardia was the only consistent finding in each zebrafish morpholino studied.
Figure 5. BIN1 knockdown delays calcium transient in adult mouse cardiomyocytes.
BIN1 knockdown by shRNA (indicated in Figure 4A) delays calcium transient development in adult mouse cardiomyocytes. Average time to half maximal signal (T1/2max) is presented in the bar graph (bottom). (**, P<0.01)
Figure 6. BIN1 knockdown diminishes cardiac calcium transient in zebrafish.
Calcium transients are decreased after bin1 morpholino injection in zebrafish. Top panel includes representative cardiac calcium transients in whole zebrafish hearts 70 hpf with control morpholino (top) or bin1 morpholino (bottom). Baseline fluorescent (F0) and maximal fluorescent (Fmax) are indicated. The amplitude of calcium transients (ΔF/F0) are averaged and presented in the bottom panel. (***, P<0.001)
DISCUSSION
Our data indicate that the membrane scaffolding protein BIN1 is significantly reduced in failing human cardiomyocytes. As a result, surface membrane localization of Cav1.2 is reduced, impairing the calcium transient and contributing to excitation-contraction uncoupling in human heart failure (schematic in Figure 7). Ion channels have half lives that are typically reported to occur in the order of hours [15], and therefore their function is highly dependent on cardiomyocyte structure and trafficking pathways. In this study we have found that the BIN1-mediated Cav1.2 trafficking apparatus appears to be relevant to the biology of acquired HF.
Figure 7. Reduced BIN1 impairs Cav1.2 trafficking and calcium transient regulation.
Model of Cav1.2 trafficking and calcium transient regulation in non-failing and failing cardiomyocytes.
Impaired Forward Trafficking of Ion Channels in Heart Failure
Cardiomyocyte structure is highly organized with important subdomains in the plasma membrane that are enriched with specific types of ion channels. For instance, connexin based gap junctions are enriched at the intercalated disc regions to provide rapid cell-to-cell conduction along the lengths of cardiac fibers, and L-type calcium channels are enriched in T-tubule membrane invaginations to be in close proximity to ryanodine receptors on the sarcoplasmic reticulum. Understanding that the forward trafficking of ion channels to their respective subdomains is altered during disease provides insight into disease mechanisms and may suggest new therapeutic approaches.
We have proposed a model in which ion channels exit the Golgi Apparatus in vesicles which are loaded onto microtubules and delivered to specific cellular regions that are determined by a combination of membrane anchoring proteins, microtubule plus-end tracking proteins, and the ion channel itself [26]. In the case of Cx43 based gap junctions, the membrane anchor is the adherens junction structure [14]. Similarly, BIN1 is the membrane anchor for Cav1.2 [8]. Other channels such as Na/K ATPase, NCX1 and Nav1.5 may use Ankyrin-B/G as their target of microtubule delivery [27, 28].
In end-stage ischemic cardiomyopathy adherens junctions (the membrane anchor for Cx43 delivery [14]) are preserved [16]. However, in this study we find that BIN1 (the membrane anchor for Cav1.2 delivery[8]) is reduced in failing human hearts (Figure 1) and BIN1 replenishment rescues Cav1.2 delivery to the plasma membrane (Figure 4). However, at least in our isolated cardiomyocyte system, the rescue is incomplete. It is possible that changes of microtubules and their associated plus-end-tracking proteins also contribute to impaired Cav1.2 forward trafficking [16].
Since BIN1 may induce tubulogenesis in skeletal muscle [25], limited BIN1 in failing myocardium could induce loss of T-tubules [29] and a consequent reduction of surface Cav1.2 channels. While this possibility cannot be excluded, the decrease in Cav1.2 with preserved NCX1 content in failing cardiac T-tubules (Figure 3B) indicates that Cav1.2 trafficking to the remaining T-tubules is impaired in failing myocardium. Furthermore, it is important to note that it remains unknown whether BIN1 has a role in cardiac tubulogenesis, which we speculate is a complicated process involving multiple T-tubule membrane junction proteins such as junctophilin [30]. A potential role for BIN1 in cardiac T-tubule formation, in addition to its ability to facilitate Cav1.2 channel trafficking, would increase the significance of our finding that BIN1 is reduced in failing myocardium. Much like adherens junctions, BIN1 can theoretically have a role in determining cardiomyocyte structure as well as channel trafficking.
The Cardiac Calcium Transients during Heart Failure
The cardiac calcium transient is an extraordinarily dynamic and complex intracellular process. Calcium overload is a general feature of failing cardiomyocytes, and known contributors include leaky ryanodine receptors [31], failure of SERCA [32], and pathological buffering by Ca2+/calmodulin-dependent kinase II [33]. In comparison, there are less data available regarding Cav1.2 channels (pore forming alpha subunit of L-type calcium channels), probably in part due to the lack of changes in protein and mRNA expression in diseased human cardiomyopathy (Figure 3 and reference[12, 13]).
The finding that Cav1.2, in end stage DCM hearts, has similar mRNA and protein levels yet is less abundant at T-tubules helps to explain findings of less surface current density in failing cardiomyocytes [10, 11]. Meanwhile, BIN1, the T-tubule membrane anchor for Cav1.2 [8], is also significantly reduced in DCM (Figure 1). Causality between reduced BIN1 and reduced surface Cav1.2 (and by extension calcium current) is supported by the in vitro knockdown and biochemical experiments (Figure 4A), partially in vitro rescue with exogenous BIN1 (Figure 4B), and the cell line electrophysiological studies (Figure 4C), as well as our previous finding that Cav1.2 is targeted to BIN1 more than general T-tubule-like invaginations (Figure 6 in Reference [8]). It is possible that reduced BIN1 can cause a compensatory change in Cav1.2 kinetics in adult cardiomyocytes despite not affecting channel kinetics in the cell line, or ironically increase Cav1.2 single channel conductance. However, a link between reduced surface Cav1.2 and diminished calcium transient is supported by calcium imaging experiments in adult mouse cardiomyocytes (Figure 5) as well as intact zebrafish hearts, in which BIN1 knockdown is sufficient to impair the overall calcium transient (Figure 6).
CONCLUSIONS
In conclusion, the current study identifies that the T-tubule membrane scaffolding protein BIN1 is significantly reduced in failing human cardiomyocytes, which results in reduced membrane trafficking of Cav1.2 and abnormal calcium transient development (schematic in Figure 7). In the larger context, this study highlights the different internal milieu that exists in end-stage failing hearts. Our results point to the need to understand not just ion channel biology but also the accessory proteins such as BIN1 that have a role in disease-related cardiomyocyte function.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr. Paul Simpson for organizing the UCSF human tissue bank. Dr. Hua Wang for power spectrum analysis, Dr. John Fahy for providing us with human housekeeping gene probes, and Dr. Shan-Shan Zhang for critical review of the manuscript.
Funding sources: This work was supported by National Institutes of Health (NCC and RMS), and American Heart Association (TTH, JWS, KO, and RMS).
Glossary of Abbreviations
- HF
Heart failure
- DCM
dilated cardiomyopathy
- BIN1
Bridging integrator 1
- NCX 1
Na/Ca exchanger 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: None
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Bauce B, Nava A, Beffagna G, et al. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2010;7:22–29. doi: 10.1016/j.hrthm.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 3.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 4.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicot AS, Toussaint A, Tosch V, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39:1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 7.Fugier C, Klein AF, Hammer C, et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat Med. 2011;17:720–725. doi: 10.1038/nm.2374. [DOI] [PubMed] [Google Scholar]
- 8.Hong TT, Smyth JW, Gao D, et al. BIN1 Localizes the L-Type Calcium Channel to Cardiac T-Tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bers DM. Ecitation-contraction coupling and cardiac contratile force. 2nd ed. Dordrecht, Nethelands: Kluwer Academic; 2001. [Google Scholar]
- 10.He J, Conklin MW, Foell JD, et al. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 12.Schroder F, Handrock R, Beuckelmann DJ, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 13.Hullin R, Asmus F, Ludwig A, Hersel J, Boekstegers P. Subunit expression of the cardiac L-type calcium channel is differentially regulated in diastolic heart failure of the cardiac allograft. Circulation. 1999;100:155–163. doi: 10.1161/01.cir.100.2.155. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saffitz JE, Laing JG, Yamada KA. Connexin expression and turnover : implications for cardiac excitability. Circ Res. 2000;86:723–728. doi: 10.1161/01.res.86.7.723. [DOI] [PubMed] [Google Scholar]
- 16.Smyth JW, Hong TT, Gao D, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassilopoulos S, Esk C, Hoshino S, et al. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science. 2009;324:1192–1196. doi: 10.1126/science.1171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Fink M, Callol-Massot C, Chu A, et al. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+-Ca2+ exchange activity is localized in the T-tubules of rat ventricular myocytes. Circ Res. 2002;91:315–322. doi: 10.1161/01.res.0000030180.06028.23. [DOI] [PubMed] [Google Scholar]
- 22.Muller AJ, Baker JF, DuHadaway JB, et al. Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol Cell Biol. 2003;23:4295–4306. doi: 10.1128/MCB.23.12.4295-4306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rottbauer W, Baker K, Wo ZG, Mohideen MA, Cantiello HF, Fishman MC. Growth and function of the embryonic heart depend upon the cardiac-specific L-type calcium channel alpha1 subunit. Dev Cell. 2001;1:265–275. doi: 10.1016/s1534-5807(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 24.Chi NC, Shaw RM, Jungblut B, et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee E, Marcucci M, Daniell L, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 26.Smyth JW, Shaw RM. Forward trafficking of ion channels: what the clinician needs to know. Heart Rhythm. 2010;7:1135–1140. doi: 10.1016/j.hrthm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe JS, Palygin O, Bhasin N, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyon AR, MacLeod KT, Zhang Y, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Oort RJ, Garbino A, Wang W, et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehnart SE, Wehrens XH, Reiken S, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.