Abstract
Oral gavage dosing can induce stress and potentially confound experimental measurements, particularly when blood pressure and heart rate are endpoints of interest. Thus, we developed a pill formulation that mice would voluntarily consume and tested the hypothesis that pill dosing would be significantly less stressful than oral gavage. C57Bl/6 male mice were singly housed and on four consecutive days were exposed to an individual walking into the room (week 1, control), a pill being placed into the cage (week 2), and a dose of water via oral gavage (week 3). Blood pressure and heart rate were recorded by radiotelemetry continuously for 5 hr after treatment, and feces collected 6–10 hr after treatment for analysis of corticosterone metabolites. Both pill and gavage dosing significantly increased mean arterial pressure (MAP) during the first hour, compared to control. However, the increase in MAP was significantly greater after gavage and remained elevated up to 5 hr, while MAP returned to normal within 2 hr after a pill. Neither pill nor gavage dosing significantly increased heart rate during the first hour, compared to control; however, pill dosing significantly reduced heart rate while gavage significantly increased heart rate 2–5 hr post dosing. MAP and heart rate did not differ 24 hr after dosing. Lastly, only gavage dosing significantly increased fecal corticosterone metabolites, indicating a systemic stress response via activation of the hypothalamic-pituitary-adrenal axis. These data demonstrated that this pill dosing method of mice is significantly less stressful than oral gavage.
Keywords: oral gavage, stress, blood pressure, alternative, corticosterone
Introduction
The advent of radiotelemetry in laboratory rodents has allowed for direct and continuous measurement of blood pressure, heart rate, and activity in conscious freely moving animals (Kramer et al., 2000; Mills et al., 2000; Whitesall et al., 2004). Further, it is an ideal method for measurements of baroreceptor function, avoiding the stress-related increases in blood pressure observed when using an indirect method such as tail cuff occlusion (Pickering et al., 2005). In addition, this method is not limited to measuring blood pressure changes in physiological or pre-clinical pharmacological studies, but is also used in various toxicological studies of environmental xenobiotics (Aragon et al., 2008; Kopf et al., 2008). Accumulating evidence suggests that environmental pollutants contribute to the overall cardiovascular disease burden and thus changes in blood pressure, heart rate, and heart rate variability are becoming increasingly important endpoints when assessing cardiovascular toxicity of environmental pollutants (O’Toole et al., 2008).
Regardless of the type of study being conducted, any source of external stress on the rodents, such as routine handling, can significantly increase heart rate and blood pressure (Irvine et al., 1997; Brown et al., 2000; Kramer et al., 2000; Balcombe et al., 2004), potentially confounding the experimental measurements. One important source of stress is the manner in which rodents are commonly exposed to drugs or xenobiotics. Oral gavage is the principal method used to achieve consistent and accurate dosing of rodents in both pharmacological and toxicological studies. However, research has shown that dosing of rats and mice via oral gavage can induce significant stress, including increased blood pressure, heart rate, and plasma corticosterone levels (Brown et al., 2000; Bonnichsen et al., 2005). This is of significant concern, since the standard route of oral delivery used in most preclinical and toxicological studies could by itself confound the results due to dosing-induced stress. Thus, we developed an oral dosing formulation to incorporate therapeutic drugs as well as xenobiotics into a pill that mice would voluntarily consume when it was placed into their cage. We then tested the hypothesis that this pill dosing method would be significantly less stressful than oral gavage.
Methods
Pill Formulation
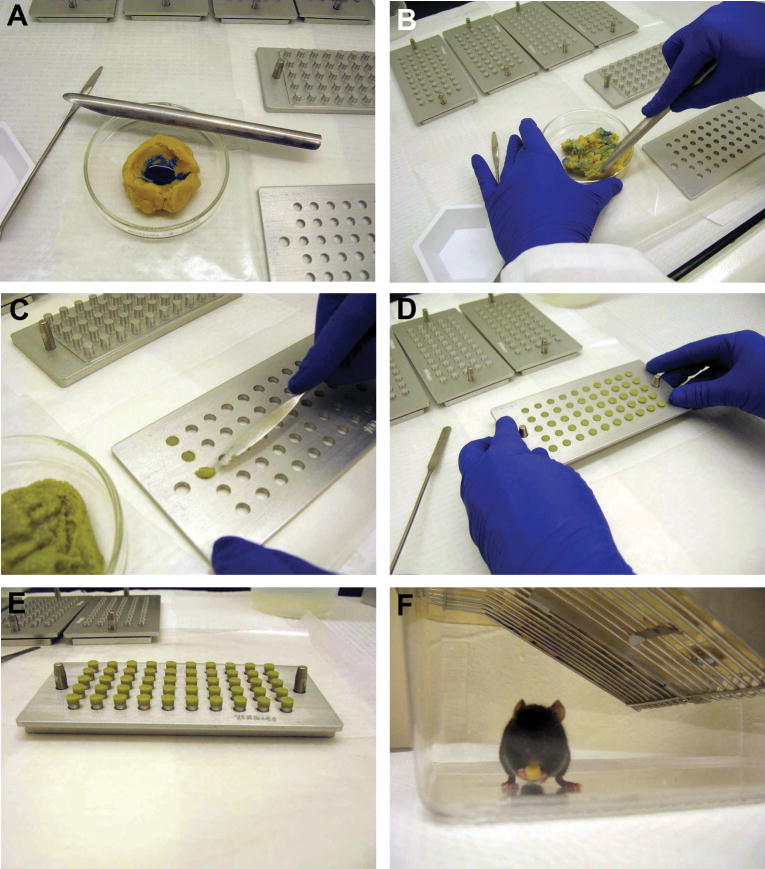
The foundation for the pill formulation was a bacon-flavored, transgenic dough diet (BioServ, Frenchtown, NJ USA) that had the consistency and color of standard cookie dough. To formulate a batch of 500 (~125 mg) pills, 62.5 g of transgenic dough was placed into a petri dish. Bromophenol blue (7.5 μl of 2% w/v in water, Fisher Scientific, Pittsburgh, PA, USA) was folded into the dough until the blue color became visually uniform (Fig. 1A,B). In experimental studies, the drug or xenobiotic would be added to the dough simultaneously and the use of the dye ensures that the chemical is evenly distributed throughout the dough. Our previous studies have shown that incorporation of bromophenol blue into the pills does not affect blood pressure, heart rate, or activity of mice (unpublished data). The die cavities of a pill mold base plate (Gallipot, St. Paul, MN USA) were then filled with the dough mixture and the pills were ejected out of the die by placing the base plate on top of the pill mold punch pins (Fig. 1C,D). The pill mold with the pills resting on the top of the punch pins was placed in a labeled, ventilated container, and stored in the chemical fume hood for 18–24 hr to allow the pills to dry (Fig. 1E). The pills were then transferred into a glass vial and stored at 4°C. Unused pills were discarded after 6 mo, based on the reported shelf life of the transgenic dough diet when stored at 4°C. After a pill was placed into a cage, the mouse quickly investigated it and typically consumed it within 30 min (Fig. 1F). The cage in the photograph is shown without standard bedding and enrichment items for clarity purposes only.
Figure 1.
Pill preparation and consumption. (A) A solution containing bromophenol blue is added to the transgenic dough diet and (B) the dough is folded repeatedly until the blue color appears uniform. (C) The die cavities of the pill mold are filled with the dough mixture; (D) pills are ejected from the die cavities by placing the pill mold on top of the pill mold punch pins, and (E) pills are allowed to dry for 18–24 hrs while sitting on top of the pill mold punch pins. (F) After placing a pill into the cage, a mouse would readily handle and consume it. The bedding and enrichment items were removed from the cage solely for the purpose of taking the picture. For all experimental studies the bedding was never removed from the cage.
Blood Pressure, Heart Rate, and Activity Analysis
Three month old C57BL/6 male mice were surgically implanted with blood pressure/activity telemeters (PA-C10, Data Sciences International, St. Paul, MN USA) as previously described (Kopf et al., 2008). Briefly, mice were anesthetized with isoflurane, telemeter catheter inserted into the left carotid artery, and body of the transmitter placed subcutaneously. Blood pressure, heart rate, and activity were recorded beginning seven days after surgery. These values were captured directly to computer software for 10 s every 15 min for 24 hours/day.
Animal Treatments
A total of nine mice were used in the study; n=5 with telemeters and n=4 without telemeters and the study was conducted over three weeks. In week 1 (control), a person walked into the mouse room and passed the mouse cages at 0700 on four consecutive days. In week 2 (pill), a person walked into the mouse room and placed a pill into each cage at 0700 on four consecutive days. In week 3 (gavage), a person walked into the mouse room and administered a 3 ml/kg dose of water by oral gavage to each of the mice at 0700 on four consecutive days. Blood pressure, heart rate, and activity were recorded by radiotelemetry continuously for 5 hr after treatment. Then, each mouse from each treatment group was placed into a cage with a wire mesh bottom to collect feces. Feces were collected for 4 hr, 6–10 hr after treatment (i.e. 1300–1700 hr) and stored at −20°C until analyzed for corticosterone metabolites.
Fecal Corticosterone Metabolite Analysis
Samples were homogenized and 0.05 g feces were extracted with 1 ml of 80% methanol. Subsequently, the samples were analyzed with a 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay, previously established and successfully validated to measure corticosterone metabolites in mice (Touma et al., 2003; Touma et al., 2004).
Statistical Analysis
The treatment responses in telemetry measurements and in fecal corticosterone metabolites were measured on four consecutive days for each mouse. These values were averaged for each mouse. These weekly averages were then averaged for all mice (n=5 telemetry data, n=9 fecal corticosterone metabolites) and expressed as mean ± SE. The data from each treatment were then compared by one way, repeated measures analysis of variance, using Holm-Sidak posthoc comparisons and p < 0.05 was considered significant.
Results
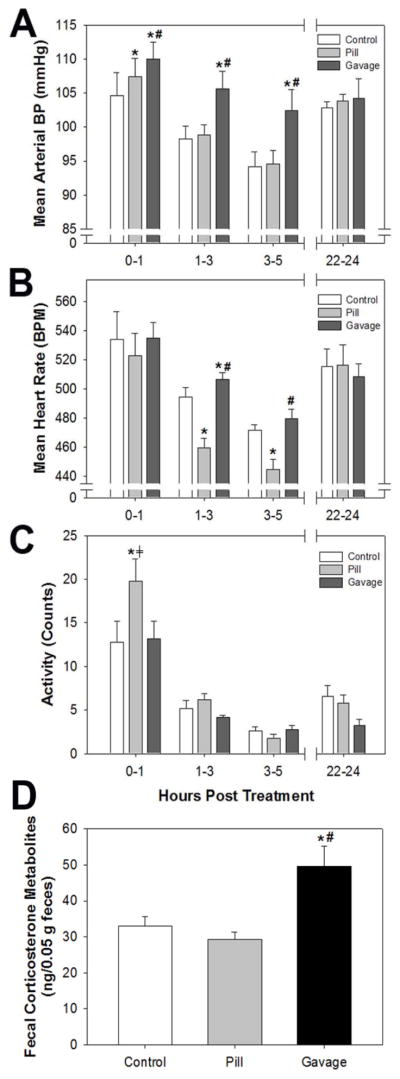
We compared blood pressure, heart rate, and activity for the first 5 hr after pill (week 2) or oral gavage (week 3) dosing to those values recorded after an individual walked in the mouse room (week 1, control). Both pill and gavage dosing significantly increased mean arterial pressure (MAP) in the first hour, compared to control; however, the increase induced by gavage was significantly higher than that induced by a pill (Fig 2A). MAP values of all groups declined over the next 4 hr, reflecting the inactive/sleep period for the mice. While MAP in the pill dosing group returned to control values, MAP of the gavage dosed group remained significantly elevated up to 5 hr. MAP of all three groups was similar 22–24 hr after treatment. Systolic and diastolic blood pressure values mirrored those observed for MAP (not shown).
Figure 2.
Effects of pill or oral gavage dosing on stress responses in the mouse. (A) Mean arterial pressure, (B) Mean heart rate, and (C) Activity measured by radiotelemetry in mice at 0–1, 1–3, 3–5, and 22–24 hr after dosing; and (D) Fecal corticosterone metabolites from feces collected 6–10 hr after treatment. The control values are those recorded when a person walked into the mouse room and past the cages without opening them. Blood pressure data were analyzed by one-way, repeated measures analysis of variance, n=5, and fecal corticosterone metabolites were analyzed by one-way, repeated measures analysis of variance, n=9. *p < 0.05 compared to control, #p < 0.05 compared to pill dosing, ⧧p<0.05 compared to oral gavage.
In contrast to changes in MAP, heart rate was not significantly different among the three groups during the first hour after treatment (Fig 2B). While heart rate declined for all three groups over the next 4 hr, reflecting the inactive/sleep period for the mice, heart rate for the pill dosed group was significantly lower than control and heart rate for the gavage dosed group was significantly higher than control up to 3 hr. As with MAP, heart rate of all three groups was similar 22–24 hr after treatment. Since activity can have a significant influence on MAP and heart rate, we also compared activity levels of mice after pill and gavage dosing to the control. Notably, activity of mice following pill dosing was significantly increased in the first hour, compared to both control and gavage dosing (Fig 2C), but returned to normal in the next 2 hr. Activity declined in all groups with time, reflecting the inactive/sleep period for the mice, and no other differences were apparent at any time points.
Lastly, we measured fecal corticosterone metabolites as a non-invasive index of activation of the hypothalamic-pituitary-adrenal axis, indicative of a systemic stress response and increased corticosterone secretion. We found that only gavage dosing significantly increased fecal corticosterone metabolites, compared to control and pill dosing (Fig 2D).
Discussion
We have developed a pill formulation method for oral dosing of laboratory mice that is significantly less stressful than oral gavage as demonstrated by multiple indices, including blood pressure, heart rate, and activation of the hypothalamic-pituitary-adrenal axis. We found that oral gavage induced a significant increase in MAP and heart rate that remained elevated for 3–5 hours and a significant increase in fecal corticosterone metabolites, reflecting activation of the adrenal stress response. In contrast, pill administration had no effect on fecal corticosterone metabolites, reduced heart rate, and modestly increased MAP only in the first hour after dosing.
Although oral gavage is a well accepted method for drug or toxicant administration, evidence from the literature shows that it can be stressful to the animal and can significantly increase heart rate and blood pressure (Bonnichsen et al., 2005), and plasma corticosterone (Brown et al., 2000). Using radiotelemetry to measure blood pressure and heart rate, Bonnichsen et. al. (2005) found that oral gavage of rats significantly induced increases in blood pressure and heart rate 30 min after dosing, but that the effects did not persist. While we also found that oral gavage of mice significantly increased blood pressure during the first hour, in contrast to the rat study we found that blood pressure in the mice remained significantly elevated for at least 5 hours. Further, we found that heart rate remained significantly elevated for 3 hours. The persistent effect of oral gavage on blood pressure and heart rate in mice may reflect a greater sensitivity of mice than rats to stress induced by this dosing method. In another study, oral gavage of rats was shown to significantly increase plasma corticosterone (Brown et al., 2000). Corticosterone is secreted by the adrenal gland as a result of activating the hypothalamic-pituitary-adrenal axis by stress. In our study, we found that oral gavage of mice significantly induced fecal corticosterone metabolites 6–10 hr after dosing. Touma et al. (2004) have established that measuring fecal corticosterone metabolites 6–10 hr after a stress event is an accurate and noninvasive technique for monitoring increases in stress hormones. The use of this noninvasive technique allowed us to compare different potential stressors in the same animals and reduce the total number of animals needed for study.
One unexpected result of our study was the increase in MAP in the first hour after pill administration. We hypothesize that this resulted from the significant increase in activity that was also observed in the first hour after pill administration. After a pill is placed into a cage, the mouse quickly investigates it, handles and smells it, and often buries it in the bedding within the first few minutes. The mouse returns to it frequently, moves it from one spot to another, and finally eats it in about 30 min. Thus, the significant increase in activity could easily account for the modest increase in MAP observed during the first hour. In support of this, both activity and MAP returned to control levels in the next two hours, suggesting that the increases in these two parameters maybe interrelated. In contrast, the increase in MAP and heart rate observed following oral gavage cannot be explained by an increase in activity. In fact, activity levels of mice receiving oral gavage were comparable to controls at all time points measured.
We have applied this pill formulation method successfully to incorporate both therapeutic drugs and xenobiotics into pills, including the angiotensin converting enzyme inhibitor, captopril (Zhang et al., 2010); the endothelin A receptor antagonist, PD155080 (de Frutos et al., 2010; Zhang et al., 2010), and the environmental pollutant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Kopf et al., 2010). We have used various solvents to dissolve the drugs or xenobiotics, including water, dimethly sulfoxide, p-dioxane, and corn oil, and no problems were observed when incorporating any of these solvents into the transgenic dough. In the case of PD155080, we needed to use a large amount of water due to the limited solubility of the drug. As a result, we modified our procedure by adding pregelatinized corn starch to thicken the dough mixture and coating the pill molds with 5% (w/w) magnesium stearate in ethanol suspension to reduce sticking (Zhang et al., 2010). It is worth noting that the final concentration of chemical achieved in the pill formulation should be confirmed, particularly when incorporating a volatile pharmaceutical or xenobiotic, since loss could occur during the pill drying phase. Certainly, one disadvantage of this method would be if the xenobiotic or solvent left a residual unpleasant odor or taste that would discourage voluntary consumption. We have not observed this in the studies we have conducted.
Additionally, results from our previously published studies demonstrate that therapeutic drugs and xenobiotics are bioavailable after being incorporated into pills and consumed orally by mice. For example, PD155080 reached a therapeutic concentration in the plasma and exhibited significant efficacy in reducing blood pressure (Zhang et al., 2010). For TCDD, hepatic accumulation was not significantly different when mice were exposed via pills (6.3 ± 0.7 ng TCDD/g, n=12) versus oral gavage (6.8 ± 1.6 ng TCDD/g, n=4) (Kopf et al., 2008) and both routes of exposure resulted in a similar degree of increase in blood pressure (Kopf et al., 2008; Kopf et al., 2010). Interestingly, however, TCDD administered by oral gavage resulted in oscillatory increases in blood pressure that eventually plateaued in a hypertensive phenotype (Kopf et al., 2008). In contrast, TCDD administration using pills resulted in a very uniform increase in blood pressure that reached a hypertensive plateau and did not change further. These differences in the pattern of blood pressure changes could reflect differences in the stress response of mice to the dosing regimens.
This pill formulation method also has advantages over other routes of oral delivery such as incorporation into the food or drinking water. Only small amounts of drug are needed and this can be particularly important when drugs are expensive or when limited amounts are available from chemical synthesis. Further, the drugs do not need to be water soluble, which allows for considerable flexibility in the types of drugs that can be incorporated into a pill form. Other benefits include that oral administration is the preferred route of delivery or exposure to mimic that in humans and is preferred for administration of chemicals in transplacental studies. Further, this method allows for the specific amounts of the drug consumed by individual animals to be calculated rather than estimated. Although this would require animals to be housed individually, it would be feasible to separate the animals by use of a cage divider for a short period (~10–15 min) during administration and consumption of the pills.
Our approach is similar to that reported by others where a drug or xenobiotic was pipetted onto a food wafer (Schantz and Bowman, 1989; Ferguson and Boctor, 2009); however, our method uses an established rodent diet formulation, ensures uniform incorporation of the drug or xenobiotic throughout the pill, and establishes a uniform size of the pill. Finally, although the pill process was developed for mice, it could easily be adapted to make smaller or larger pills suitable for other species since pill molds of various sizes are commercially available. In conclusion, we have developed a pill formulation that mice will quickly and voluntarily consume and that fails to induce any significant stress responses, compared to the traditional method of oral gavage.
Highlights.
Development of a novel oral dosing method using a pill that mice will readily consume
Measure stress response using changes in blood pressure, heart rate, and fecal corticosterone metabolites following dosing
Demonstrate that our pill dosing is significantly less stressful on the mice when compared to oral gavage
Acknowledgments
We sincerely thank Edith Klobetz-Rassam for performing the EIA analysis of fecal corticosterone metabolites and Larry N. Agbor for his technical assistance. This work was supported by NIH grant HL078914 to M.K.W.
Footnotes
Conflict of Interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragon AC, Kopf PG, Campen MJ, Huwe JK, Walker MK. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol Sci. 2008;101:321–330. doi: 10.1093/toxsci/kfm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Bonnichsen M, Dragsted M, Hansen AK. The welfare impact of gavaging laboratory rates. Anim Welfare. 2005;14:223–227. [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17–21. [PubMed] [Google Scholar]
- de Frutos S, Caldwell E, Nitta CH, Kanagy NL, Wang J, Wang W, Walker MK, Gonzalez Bosc LV. NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice. Am J Physiol Heart Circ Physiol. 2010;299:H356–H363. doi: 10.1152/ajpheart.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Boctor SY. Use of food wafers for multiple daily oral treatments in young rats. J Am Assoc Lab Anim Sci. 2009;48:292–295. [PMC free article] [PubMed] [Google Scholar]
- Irvine RJ, White J, Chan R. The influence of restraint on blood pressure in the rat. J Pharmacol Toxicol Methods. 1997;38:157–162. doi: 10.1016/s1056-8719(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Huwe JK, Walker MK. Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc Toxicol. 2008;8:181–193. doi: 10.1007/s12012-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117:537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K, Voss HP, Grimbergen JA, Mills PA, Huetteman D, Zwiers L, Brockway B. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab Anim. 2000;34:272–280. doi: 10.1258/002367700780384663. [DOI] [PubMed] [Google Scholar]
- Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev Environ Health. 2008;23:167–202. doi: 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo- p -dioxin. Neurotoxicol Teratol. 1989;11:13–19. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Mostl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Zhang N, Agbor LN, Scott JA, Zalobowski T, Elased KM, Trujillo A, Duke MS, Wolf V, Walsh MT, Born JL, Felton LA, Wang J, Wang W, Kanagy NL, Walker MK. An activated renin-angiotensin system maintains normal blood pressure in aryl hydrocarbon receptor heterozygous mice but not in null mice. Biochem Pharmacol. 2010;80:197–204. doi: 10.1016/j.bcp.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]