Abstract
Structural characterization of a hydrogen sulfate complex with a tren-based urea suggests that the anion is coordinated with six NH ··· O bonds (dN ··· O = 2.857 (3) to 3.092 (3) Å) and one OH ··· O bond (dO ··· O = 2.57 (2) Å) from three receptors; however, in solution the anion is bound within the pseudocavity of one receptor.
Sulfate plays an important role in many biochemical processes.1 The sulfate-binding protein (SBP) was structurally identified in 1985 by Pflugrath and Quiocho, in which the amino acid residues are involved in hydrogen bonding interactions to form a seven-coordinate anion complex.2 In this structure, the sulfate is present in a cleft between the two lobes of SBP and bound by seven hydrogen bonds: five from peptide NH groups (Ala173, Asp11, Gly131, Gly132, Ser45), one from a tryptophan side chain NH group (Trp192), and one from a serine OH group (Ser130). Sulfate is also of significant environmental interest.3 Therefore, an increasing attention has recently been devoted to sulfate binding with synthetic receptors which include polyamines,4 polyamides,5 ureas,6 indoles,7 self-assembled metal–organic hosts,8 and triazolium hosts.9 The oxygen atoms in oxoanions are generally coordinated with two hydrogen bonds, thus sulfate with four oxygens is often found to be octacoordinated with synthetic receptors.5a,10 Custelcean et al. have shown that a tren-based urea linked with Ag2SO4 is capable of binding a sulfate by twelve hydrogen bonds (dN ··· O = 2.8516 to 3.1741 Å),8a which is consistent with Hay’s prediction that each oxygen could be involved in a maximum of three hydrogen bonds.11 Bowman-James et al. have reported a ten-coordinate sulfate complex stabilized in a tetramide-based host with four amides (dN ··· O = 3.06–3.31 Å), two protonated amines (dN ··· O = 2.76 Å), and four H2O molecules (dO ··· O = 2.71 and 2.79 Å).12 Wu et al. have characterized a multiple coordinate sulfate complex with a tris(3-pyridyl)urea showing eleven NH ··· O hydrogen bonds (dN ··· O < 3.2 Å).8b In this communication, we report a heptacoordinated hydrogen sulfate resulting from the coordination of three tren-based ureas (L) via six NH ··· O bonds (dN ··· O = 2.857(3) to 3.092(3) Å) and one OH ··· O bond (dO ··· O = 2.57(2) Å), and its solution binding studies.
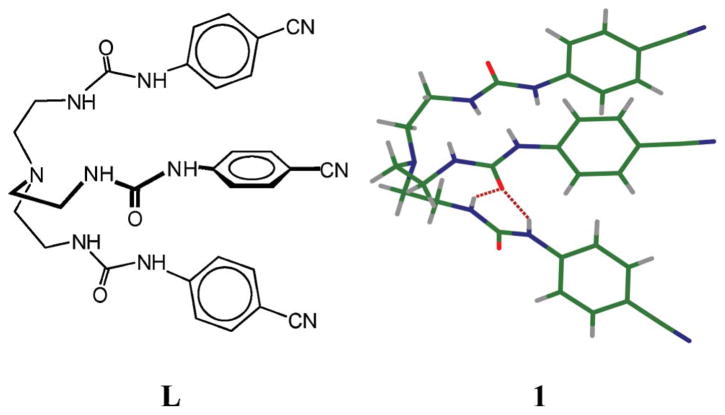
The tripodal urea L was synthesized from the reaction of tren and p-cyanophenyl isocyanate in CHCl3. Crystallographic analysis‡ of the free urea reveals that the ligand forms a pseudo-cavity consisting of three arms (Fig. 1). One oxygen is involved in intermolecular hydrogen bonding interactions with two NH’s of another arm. The bisulfate salt was obtained from the reaction of L with H2SO4 in ethanol at room temperature. The salt crystallizes as [HL · (HSO4)] where the tertiary amine is protonated. The proton on the tertiary nitrogen is pointed inside the tripodal cavity formed by the three arms, and is hydrogen-bonded with one carbonyl oxygen of a urea group (N1 ··· O34 = 2.783(2) Å). The molecule contains a singly-charged hydrogen sulfate to balance the charge of the cationic host. However, the anion is not encapsulated; it is held outside the cavity via two H-bonds of N18 ··· O1 and N21 ··· O1 (dN ··· O = 2.981(2) and 3.020(3) Å, respectively).
Fig. 1.
Tren-based urea (L) and its X-ray structure (1).
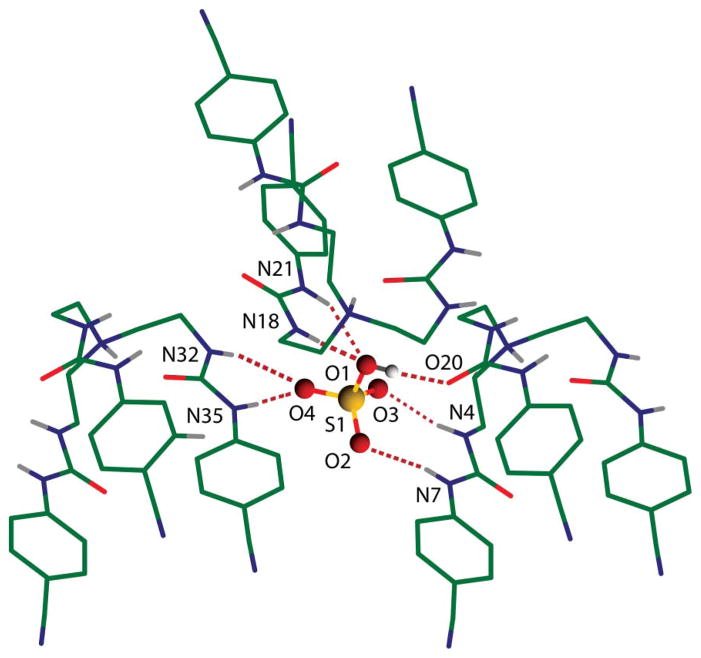
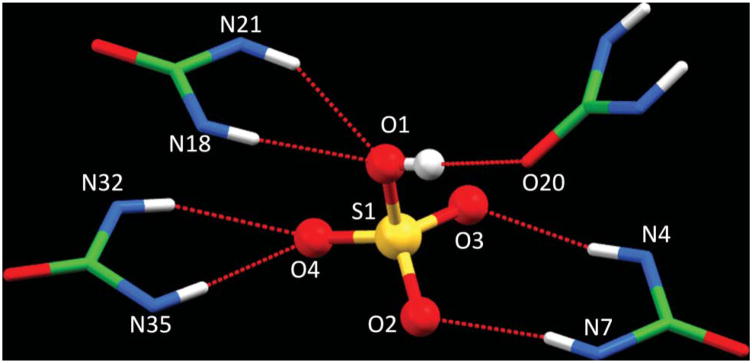
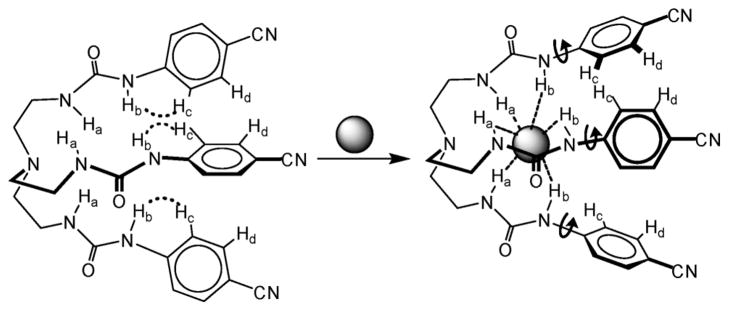
Examining the coordination environment of hydrogen sulfate suggests that it is coordinated with multiple hydrogen bonds comprised of several NH ··· O bonds and one OH ··· O bond in the lattice framework formed by three tren ureas (Fig. 2). The coordination environment of the anion is shown in Fig. 2, and the hydrogen bonding parameters are listed in Table 1. The nature of the hydrogen-bonding interactions involved in the complex can be evaluated from a correlation of H ··· O distance and N–H ··· O angle in the strong hydrogen-bonding interactions region of dN ··· O < 3.2 Å, dH ··· O < 2.5 Å and ∠N–H ··· O > 140°.8b,13 It is clear that all six NH ··· O hydrogen bonds fall within the hydrogen bonding range (see Fig. S6 in ESI). This interpretation is further supported by the work from Ghosh et al. for the 14-coordinate sulfate complex of a pentafluorophenyl-substituted tripodal urea receptor (dN ··· O = 2.852(6) to 3.217(6) Å),6g Custelcean et al. for the 12-coordinate sulfate within metal–organic framework (dN ··· O = 2.8516 to 3.1741 Å),8a and Bowman-James for the sulfate complex of tetramide (N ··· O = 3.06–3.31 A).12 We, therefore, conclude that hydrogen sulfate is coordinated by a total of seven hydrogen bonds comprised of six NH ··· O bonds and one OH ··· O bond (Fig. 3). While each of the three tren ureas contributes two hydrogen-bond donors via NH groups from a single arm, one tren unit acts as a hydrogen-bond acceptor through its carbonyl oxygen.
Fig. 2.
X-ray structure of the hydrogen sulfate complex with L showing seven hydrogen bonds (six NH ··· O and one OH ··· O bonds).
Table 1.
H-bonding parameters (Å, °) for the bisulfate complex of L
| D—H ··· O | H ··· O | D ··· O | ∠DHO |
|---|---|---|---|
| O1—H1A ··· O20a | 1.79(3) | 2.573(2) | 178(3) |
| N4—H4 ··· O3b | 2.15(3) | 2.979(3) | 169(2) |
| N7—H7 ··· O2b | 2.12(3) | 2.936(3) | 171(2) |
| N18—H18 ··· O1 | 2.23(2) | 2.981(2) | 159(2) |
| N21—H21 ··· O1 | 2.20(2) | 3.020(3) | 153(2) |
| N32—H32 ··· O4a | 2.40(2) | 3.092(3) | 142(2) |
| N35—H35 ··· O4a | 2.06(3) | 2.857(3) | 170(2) |
,
.
Fig. 3.
The coordination environment of bisulfate showing seven hydrogen bonds from three ureas.
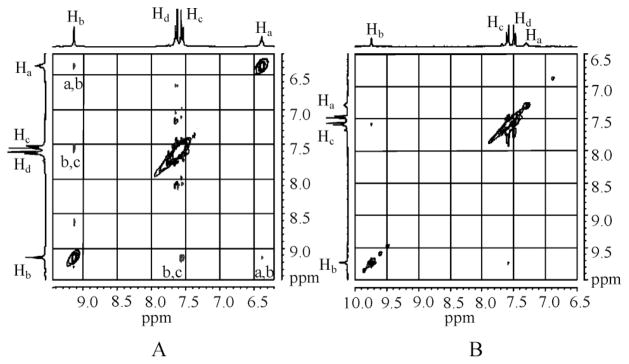
In order to evaluate the binding affinity of L for anions, 1H NMR titration studies were performed in DMSO-d6. The addition of n-Bu4NHSO4 to L resulted in a significant downfield shift of both NH signals (Δδmax = 0.87 and 0.60 ppm), suggesting an interaction of NH groups with the anion. The change in the chemical shift of NH resonances of NMR spectra, as recorded with an increasing amount of anion solution at room temperature, gave the best fit for a 1 : 1 binding model, yielding a binding constant of log K = 3.0 (Fig. 4).14 The observed binding constant is much higher than that observed for singly-charged bisulfate in an amide-based cryptand (log K =1.83(3)).4a The solution binding mode was further confirmed by 2D NOESY NMR experiments of both free ligand and hydrogen sulfate complex in DMSO-d6 (Fig. 5) As shown in Fig. 5A, the free ligand shows two strong NOESY contacts between Hb ··· Hc and Ha ··· Hb, an observation which is also supported by single crystal structure of L showing distances of Hb ··· Hc = 2.30 and Ha ··· Hb = 2.08 Å. Upon the addition of bisulfate, Hc proton on aromatic ring shifts more downfield as compared to Hd, leading the reversal of the relative position of these two protons (see Fig S9a in ESI). The NOESY between Ha and Hb is absent in the complex, while the contact between Hb and Hc becomes significantly weaker; indicating a conformational change of the ligand due to encapsulation of a bisulfate anion (Fig. 6). Similar changes were reported by Schneider et al. in the optimized structure of chloride complex of a tren-based urea ligand.15 The 1 : 1 binding stoichiometry in DMSO-d6 was further verified by the Job’s plot showing a maximum at the 0.5 mole fraction of L (see ESI).
Fig. 4.
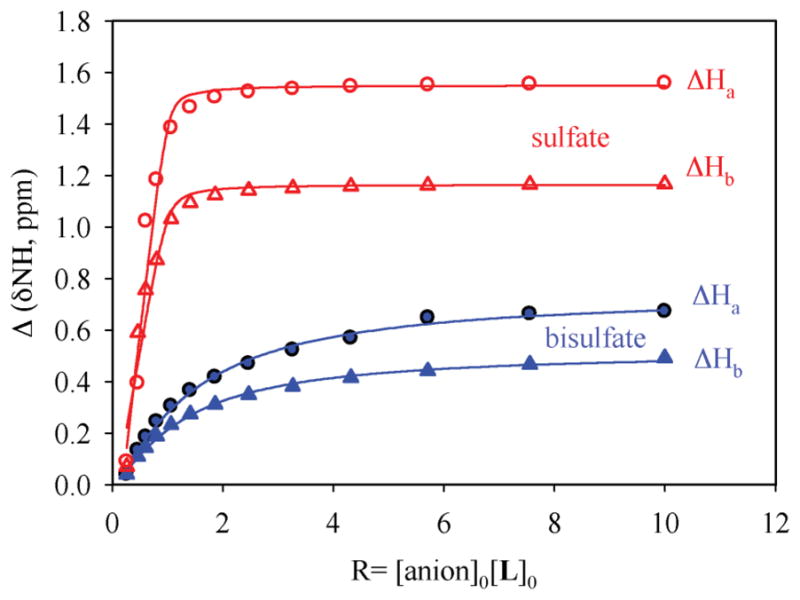
1H NMR titration curves of L (2 mM) with n-Bu4 NHSO4 (●, ▲) and ZnSO4 (○, △) in DMSO-d6. Net changes in the chemical shifts of NH are shown against the increasing amount of anion (20 mM). H1 = CH2 NHCO and H2 = CONHAr.
Fig. 5.
2D NOESY NMR experiments of L in absence (A) and presence (B) of hydrogen sulfate (5 equiv.) in DMSO-d6.
Fig. 6.
Proposed binding mode of L for hydrogen sulfate in solution.
We also performed 1H NMR titrations for other oxoanions: SO42−, H2PO4−, ClO4− and NO3− in DMSO- d6.16 The results show that the host forms a strong 1 : 1 complex with SO42− giving the binding constant of log K = 4.7 which is comparable to that observed for a pentafluorophenyl-substituted tripodal urea (K = 4.72)6h or nitrophenyl-substituted tripodal urea log K = 4.97).6c Clearly, an additional charge on SO42− compared to HSO4− plays a role for stronger electrostatic interactions, resulting in a stronger binding of SO42−. The ligand also binds H2PO4− strongly with the binding constant of log K = 4.2. On the other hand, the addition of ClO4− and NO3− did not result in any appreciable change in the NMR resonances (see ESI). Therefore, the binding largely depends on the relative basicity of the anions included in this study and is in accordance with the Hofmeister series.17
In summary, we have presented a seven coordinate complex of hydrogen sulfate formed by three tren-based receptors in solid state. The anion is coordinated with six NH ··· O bonds and one OH ··· O hydrogen bond. In contrast, the ligand was found to encapsulate a single anion within its cavity in solution, suggesting an obvious discrepancy of binding mode from that observed in solid state. While many examples exist for seven-coordinate complexes with metal ions,18 to the best of our knowledge, there is just one structure of seven-coordinate anion complex reported by Bowman-James and coworkers, where one sulfate is encapsulated by an amide-based cryptand with four hydrogen-bonds to the cryptand and three additional hydrogen-bonds to the crystalline water molecules.19 The seven coordinate complex in our case has been resulted from the packing influence of the crystal, which represents a rare example of a heptacoordinated anion with a synthetic receptor.
BT, TH and KT were supported by DOE under the Faculty and Student Teams (FaST) Program at ORNL hosted by Dr. Bruce A. Moyer. Participation of PVB and MAH was sponsored by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, U.S. Department of Energy. This work was supported by National Institutes of Health, Division of National Center for Research Resources, under Grant Number G12RR013459 (MAH). This material is based upon work supported by the National Science Foundation under CHE-0821357 (500 MHz NMR). The authors thank the National Science Foundation (CHE-0130835) and the University of Oklahoma for funds to acquire the diffractometer used in this work. National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to MAH.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Synthesis and NMR studies. CCDC reference numbers 795639 and 814969. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ob05052d
Crystal data for L: C30 H30 N10 O3, M = 578.64, triclinic, a = 8.7312(11) Å, b = 12.8400(17) Å, c = 13.6820(18) Å, α = 91.989(3)°, β = 107.888(2)°, γ = 100.753(2)°, V = 1427.1(3) Å3, T = 100(2)K, space group P 1̄, Z = 2, 16034 reflections measured, 7004 independent reflections (Rint = 0.0320). The final R1 values were 0.0495 (I > 2σ(I)). The final wR(F2) values were 0.1166 (I > 2σ(I)). CCDC 814969. Crystal data for HL+·HSO4−: C30 H31 N10 O3 ·HO4S, M = 676.72, monoclinic, a = 12.696(2) Å, b = 12.411(2) Å, c = 20.491(4) Å, β = 103.912(9)°, V = 3134.1(9) Å3, T = 100(2)K, space group P21/n, Z = 4, 29878 reflections measured, 7849 independent reflections (Rint = 0.0716). The final R1 value was 0.0587 (I > 2σ(I)). The final wR(F2) value was 0.1188 (I > 2σ(I)). CCDC 795639.
Notes and references
- 1.(a) Antonisse MMG, Reinhoudt DN. Chem Commun. 1998:443–448. [Google Scholar]; (b) Atwood JL, Holman KT, Steed JW. Chem Commun. 1996:1401–1407. [Google Scholar]; (c) Gale PA. Coord Chem Rev. 2003;240:191–221. [Google Scholar]; (d) Cormode DP, Murray SS, Cowley AR, Beer PD. Dalton Trans. 2006:5135–5140. doi: 10.1039/b609817g. [DOI] [PubMed] [Google Scholar]
- 2.Pflugrath JW, Quiocho FA. Nature. 1985;314:257–260. doi: 10.1038/314257a0. [DOI] [PubMed] [Google Scholar]
- 3.Moyer BA, Delmau LH, Fowler CJ, Ruas A, Bostick DA, Sessler JL, Katayev E, Pantos GD, Llinares JM, Hossain MA, Kang SO, Bowman-James K. Adv Inorg Chem. 2007;59:175–204. [Google Scholar]
- 4.(a) Kang SO, Hossain MA, Powell D, Bowman-James K. Chem Commun. 2005:328–330. doi: 10.1039/b411904e. [DOI] [PubMed] [Google Scholar]; (b) Mani G, Guchhait T, Kumar R, Kumar S. Org Lett. 2010;12:3910–3913. doi: 10.1021/ol101598e. [DOI] [PubMed] [Google Scholar]; (c) Mendy JS, Pilate M, Horne T, Day VW, Hossain MA. Chem Commun. 2010;46:6084–6086. doi: 10.1039/c0cc01699c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saeed MA, Fronczek FR, Powell DR, Hossain MA. Tetrahedron Lett. 2010;51:4233–4236. doi: 10.1016/j.tetlet.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Hossain MA, Llinares JM, Powell D, Bowman-James K. Inorg Chem. 2001;40:2936–2937. doi: 10.1021/ic015508x. [DOI] [PubMed] [Google Scholar]; (b) Kang SO, Day VW, Bowman-James K. Org Lett. 2009;11:3654–3657. doi: 10.1021/ol9014249. [DOI] [PubMed] [Google Scholar]; (c) Lakshminarayanan PS, Suresh E, Ghosh P. Inorg Chem. 2006;45:4373–4380. doi: 10.1021/ic052159o. [DOI] [PubMed] [Google Scholar]
- 6.(a) Wu B, Yang X-J, Janiak C, Gerhard Lassahn P. Chem Commun. 2003:902–903. doi: 10.1039/b211616b. [DOI] [PubMed] [Google Scholar]; (b) Wu B, Huang X, Xia Y, Yang X-J, Janiak C. CrystEngComm. 2007;9:676–685. [Google Scholar]; (c) Jose DA, Kumar DK, Ganguly B, Das A. Inorg Chem. 2007;46:5817–5819. doi: 10.1021/ic062466+. [DOI] [PubMed] [Google Scholar]; (d) Zhuge F, Wu B, Liang J, Yang J, Liu Y, Jia C, Janiak C, Tang N, Yang X. Inorg Chem. 2009;48:10249–10256. doi: 10.1021/ic9012685. [DOI] [PubMed] [Google Scholar]; (e) Custelcean R, Sellin V, Moyer BA. Chem Commun. 2007:1541–1543. doi: 10.1039/b616761f. [DOI] [PubMed] [Google Scholar]; (f) Custelcean R, Bosano J, Bonnesen PV, Kertesz V, Hay BP. Angew Chem, Int Ed. 2009;48:4025–4029. doi: 10.1002/anie.200900108. [DOI] [PubMed] [Google Scholar]; (g) Ravikumar I, Ghosh P. Chem Commun. 2010;46:1082–1084. doi: 10.1039/b915661e. [DOI] [PubMed] [Google Scholar]; (h) Ravikumar I, Lakshminarayanan PS, Arunachalam M, Suresh E, Ghosh P. Dalton Trans. 2009:4160–4168. doi: 10.1039/b820322a. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kim J, Juwarker H, Liu X, Lah MS, Jeong KS. Chem Commun. 2010;46:764–766. doi: 10.1039/b919519j. [DOI] [PubMed] [Google Scholar]; (b) Gale PA, Hiscock JR, Jie CZ, Hursthouse MB, Light ME. Chem Sci. 2010;1:215–220. [Google Scholar]
- 8.(a) Custelcean R, Moyer BA, Hay BP. Chem Commun. 2005:5971–5973. doi: 10.1039/b511809c. [DOI] [PubMed] [Google Scholar]; (b) Wu B, Liang J, Yang J, Jia C, Yang X-J, Zhang H, Tangb N, Janiak C. Chem Commun. 2008:1762–1764. doi: 10.1039/b719019k. [DOI] [PubMed] [Google Scholar]
- 9.Schulze B, Friebe C, Hager MD, Günther W, Köhn U, Jahn BO, Görls H, Schubert US. Org Lett. 2010;12:2710–2713. doi: 10.1021/ol100776x. [DOI] [PubMed] [Google Scholar]
- 10.Bowman-James K. Acc Chem Res. 2005;38:671–678. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]
- 11.Hay BP, Firman TK, Moyer BA. J Am Chem Soc. 2005;127:1810–1811. doi: 10.1021/ja043995k. [DOI] [PubMed] [Google Scholar]
- 12.Kang SO, Day VW, Bowman-James K. Org Lett. 2009;11:3654–3657. doi: 10.1021/ol9014249. [DOI] [PubMed] [Google Scholar]
- 13.Pirard B, Baudoux G, Durant F. Acta Crystallogr, Sect B: Struct Sci. 1995;B51:103–107. [Google Scholar]
- 14.Schneider HJ, Kramer R, Simova S, Schneider U. J Am Chem Soc. 1988;110:6442–6448. [Google Scholar]
- 15.Werner F, Schneider HJ. Helv Chim Acta. 2000;83:465–478. [Google Scholar]
- 16.Titrations with Na2SO4 were hampered due to the solubility problem in DMSO-d6. We, therefore, used ZnSO4 which was previously used by other groups in DMSO-d6.8b.
- 17.Hofmeister F. Naunyn-Schmiedebergs Arch Pharmacol. 1888;24:247–260. [Google Scholar]
- 18.(a) Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 5. Wiley; New York: 1988. pp. 10–22. [Google Scholar]; (b) Duan L, Fischer A, Xu Y, Sun L. J Am Chem Soc. 2009;131:10397–10399. doi: 10.1021/ja9034686. [DOI] [PubMed] [Google Scholar]
- 19.Kang SO, Powell D, Bowman-James K. J Am Chem Soc. 2005;127:13478–13479. doi: 10.1021/ja054332l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.