Abstract
Prior research suggests that older adults are less likely than young adults to use effective learning strategies during intentional encoding. This functional magnetic resonance imaging (fMRI) study investigated whether training older adults to use semantic encoding strategies can increase their self-initiated use of these strategies and improve their recognition memory. The effects of training on older adults' brain activity during intentional encoding were also examined. Training increased older adults' self-initiated semantic encoding strategy use and eliminated pretraining age differences in recognition memory following intentional encoding. Training also increased older adults' brain activity in the medial superior frontal gyrus, right precentral gyrus, and left caudate during intentional encoding. In addition, older adults' training-related changes in recognition memory were strongly correlated with training-related changes in brain activity in prefrontal and left lateral temporal regions associated with semantic processing and self-initiated verbal encoding strategy use in young adults. These neuroimaging results demonstrate that semantic encoding strategy training can alter older adults' brain activity patterns during intentional encoding and suggest that young and older adults may use the same network of brain regions to support self-initiated use of verbal encoding strategies.
Keywords: aging, cognitive training, fMRI, intentional encoding, prefrontal cortex
Introduction
Episodic memory is one of the cognitive domains most affected by aging (for reviews, see Kausler 1994; Balota et al. 2000; Jacoby and Rhodes 2006). Older adults' ability to recognize and recall studied information is impaired relative to young adults' when they are instructed to memorize information but are not explicitly told how to do so (i.e., intentional encoding) (Sanders et al. 1980; Hultsch et al. 1990; Witte et al. 1990; Logan et al. 2002). Age-related changes in self-initiated encoding strategy use, potentially resulting from age-related changes in frontal structure and function, may play an important role in these memory impairments. The present study investigated whether training older adults to use semantic encoding strategies can increase their self-initiated use of these strategies and improve their recognition memory. The ability of semantic encoding strategy training to increase older adults' brain activity in prefrontal and temporal regions thought to support self-initiated verbal encoding strategy use in young adults was also examined.
Young and older adults who report using complex elaborative encoding strategies (e.g., sentence generation or visual imagery formation) have better memory for studied information than those who report using relatively simple encoding strategies (e.g., rote repetition or concentration) (Martin et al. 1965; Geiselman et al. 1982; Camp et al. 1983; Hertzog et al. 1998, 2010). According to the production deficiency hypothesis, older adults are less likely than young adults to self-initiate elaborative encoding strategies in learning situations with minimal environmental support such as intentional encoding (Craik and Byrd 1982; Perlmutter and Mitchell 1982). Consistent with this hypothesis, older adults are more likely than young adults to report not using any strategies during intentional encoding (Rowe and Schnore 1971; Devolder and Pressley 1992; Perfect and Dasgupta 1997). When older adults do report using strategies to intentionally encode new information, age differences in self-initiated encoding strategy use still emerge. For example, older adults are less likely than young adults to use elaborative strategies to intentionally encode verbal stimuli (Verhaeghen and Marcoen 1994; Hertzog et al. 1998; Naveh-Benjamin et al. 2007; Hertzog et al. 2010). The results of structural equation modeling studies investigating the relationships among age, self-initiated encoding strategy use, and memory performance suggest that age-related changes in self-initiated encoding strategy use partially mediate age-related changes in episodic memory (Verhaeghen and Marcoen 1994; Hertzog et al. 1998). Thus, prior research suggests that older adults are less likely than young adults to spontaneously use effective encoding strategies during intentional encoding and that this less frequent use of effective encoding strategies contributes to older adults' episodic memory impairments.
Older adults' less frequent self-initiated use of effective encoding strategies may be driven, at least in part, by age-related changes in frontal structure and function. Individuals with frontal lobe lesions due to infarctions, hemorrhages, tumor resections, arteriovenous malformation resections, or traumatic brain injuries use elaborative encoding strategies during intentional encoding less frequently than healthy age-matched controls (Gershberg and Shimamura 1995; Hildebrandt et al. 1998; Baldo et al. 2002; Alexander et al. 2009). However, individuals with frontal lobe lesions can effectively use elaborative encoding strategies when they are explicitly instructed to do so (Hirst and Volpe 1988), suggesting that the frontal lobes play a central role in supporting self-initiated use of elaborative encoding strategies. Structural neuroimaging studies have revealed that the frontal lobes are particularly vulnerable to structural damage in older adults (for reviews, see Raz and Rodrigue 2006; Gunning-Dixon et al. 2009). Older adults have also been shown to have less activity than young adults in left inferior prefrontal regions associated with self-initiated verbal encoding strategy use during intentional encoding (Logan et al. 2002; Kirchhoff and Buckner 2006).
Importantly, prior research has suggested that instructing and training older adults to use elaborative semantic encoding strategies can improve their memory performance. Older adults who are instructed to use semantic strategies while attempting to memorize verbal stimuli use these strategies more frequently than uninstructed older adults (Hulicka and Grossman 1967; Schmitt et al. 1981; Naveh-Benjamin et al. 2007) and are also better able to subsequently recognize verbal stimuli than uninstructed older adults (Naveh-Benjamin et al. 2007). Studies that have trained older adults to use categorization or narrative story encoding strategies have found that these strategies can improve older adults' ability to recall studied words more than placebo training (Hill et al. 1990, 1991). However, older adults in these studies were explicitly told to use the trained strategies during the posttraining memory tests. Therefore, it is unknown whether semantic encoding strategy training can increase older adults' self-initiated use of semantic encoding strategies and improve their recognition memory following unsupported intentional encoding, which are necessary for semantic encoding strategy training to be able to improve older adults' memory functioning in everyday life.
Currently, little is known about the impact that episodic memory cognitive training protocols have older adults' brain structure and function. Prior studies that have examined the effects of episodic memory strategy training on older adults' brain structure and function have trained older adults to use the method of loci mnemonic (Bower 1970) to encode and retrieve serial words lists. This mnemonic requires individuals to visualize a series of familiar locations. Each studied item is then visualized in one of the locations. During retrieval, studied items are recalled by mentally walking through the sequence of locations and retrieving images of the studied items. Engvig et al. (2010) recently reported that relative to age-matched no training controls, middle-aged and older adults who participated in an 8 week method of loci training program exhibited increases in cortical thickness in the right orbitofrontal gyrus, insula, and fusiform gyrus. In addition, training-related changes in participants' ability to remember the presentation order of studied words were positively correlated with training-related changes in cortical thickness in the right orbitofrontal and fusiform gyri. These results suggest that several weeks of memory strategy training may be able to slow age-related reductions in brain volume and thus age-related cognitive decline. Valenzuela et al. (2003) found that 5 weeks of method of loci training increased older adults' creatine and choline signals in the hippocampus in a magnetic resonance spectroscopy study, suggesting that several weeks of memory strategy training may also be able to alter older adults' regional neurochemistry. To date, only one functional neuroimaging study has directly examined the effects of strategy training on older adults' brain activity patterns during encoding of episodic memories (Nyberg et al. 2003). In this study, older adults' brain activity was assessed using positron emission tomography during unsupported intentional encoding before in-scanner method of loci training and immediately after training when they were specifically instructed to use the method of loci mnemonic to encode words. Older adults who had improvements in their memory performance as a result of training had greater activity after relative to before training in occipitoparietal cortex. In contrast, older adults who did not have improvements in their memory performance as a result of training did not have any training-related changes in brain activity patterns. These results suggest that short-duration memory strategy training can alter older adults' brain activity patterns during encoding when training increases their use of elaborative encoding strategies and they are able to effectively implement the trained encoding strategies. Medial superior frontal, left middle and inferior frontal, bilateral orbitofrontal, and left lateral temporal regions are active the most consistently in neuroimaging studies that have investigated the neural correlates of self-initiated, instructed, and trained use of verbal encoding strategies in young adults (Savage et al. 2001; Kirchhoff and Buckner 2006; Miotto et al. 2006; Matsui et al. 2008; for a review, see Kirchhoff et al. forthcoming). These findings suggest that these regions may support self-initiated use of verbal encoding strategies in young adults. In the present study, we examined whether training older adults to use semantic encoding strategies increases their brain activity in these regions to gain a greater understanding of the effects of episodic memory training on older adults' brain activity patterns during encoding.
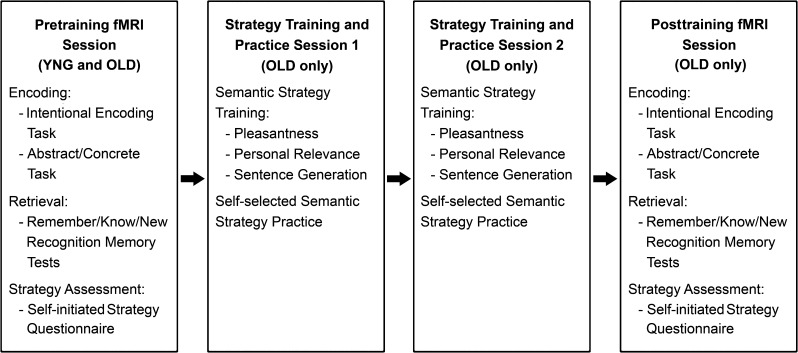
In summary, the primary goals of the present study were 1) to investigate whether semantic encoding strategy training can increase older adults' self-initiated use of semantic encoding strategies and thereby improve their recognition memory following intentional encoding and 2) to investigate the effects of semantic encoding strategy training on older adults' brain activity during intentional encoding. To accomplish these goals, older adults' self-initiated encoding strategy use, recognition memory performance, and brain activity during intentional encoding were examined before and after they were trained to use pleasantness, personal relevance, and sentence generation semantic encoding strategies (Fig. 1). We hypothesized that semantic encoding strategy training would increase older adults' self-initiated use of semantic encoding strategies and improve their recognition memory. We also hypothesized that semantic encoding strategy training would increase older adults' brain activity in medial superior frontal, left middle and inferior frontal, bilateral orbitofrontal, and left lateral temporal regions thought to support self-initiated verbal encoding strategy use in young adults.
Figure 1.
Design of pretraining and posttraining fMRI sessions and semantic encoding strategy training sessions. During the pretraining fMRI session, young and older adults performed intentional encoding and abstract/concrete tasks. Their memory for words presented during these tasks was subsequently assessed by Remember/Know/New recognition memory tests. Young and older adults' self-initiated encoding strategy use during intentional encoding was measured using an encoding strategy questionnaire. During 2 strategy training sessions, older adults were taught to use 3 semantic encoding strategies to intentionally encode words (pleasantness, personal relevance, and sentence generation) and were given extensive practice using each of these strategies. They were also given the opportunity to practice using whichever semantic encoding strategy or combination of semantic encoding strategies that they felt worked best for them at the end of both strategy training sessions. Older adults' brain activity during encoding, recognition memory performance, and self-initiated encoding strategy use was also assessed after strategy training in a posttraining fMRI session with the same experimental procedures as the pretraining fMRI session. YNG = young adults, OLD = older adults.
Materials and Methods
Participants
Seventeen young adults (mean age 22.8, range 18–33) and 16 older adults (mean age 71.9, range 66–81) participated in this study. Participants were recruited from research volunteer pools at Washington University, and informed consent was provided in accordance with Washington University's Human Studies Committee guidelines. All participants were right-handed native English-speakers, had normal or corrected-to-normal vision, reported no significant neurological or psychiatric history and were not taking psychiatric medications or medications known to influence the blood oxygen level–dependent (BOLD) hemodynamic response. Older adults were administered the Short-Blessed (Katzman et al. 1983) to exclude individuals with dementia (all older adults had < 6 errors; mean 0.8, standard deviation [SD] 1.0). Older adults were also screened for glaucoma, significant heart disease, untreated hypertension, diabetes, kidney disease, thyroid conditions, active cancer, previous chemotherapy treatment, and alcoholism. Functional magnetic resonance imaging (fMRI), task performance, and recognition memory data were not available for one pretraining encoding scan of the intentional encoding task for one older adult due to technical difficulties and recognition memory data were not available for 2 posttraining abstract/concrete task recognition word lists for one older adult due to fatigue. Task performance and recognition memory data were excluded from behavioral data analyses for one encoding scan of the abstract/concrete task for one young adult due to excessive motion during scanning. Recognition memory data from one abstract/concrete task recognition word list for one young adult were also not included in data analyses due to an excessive number of trials with no response.
Assessment of Demographic Characteristics, Cognitive Function, and Memory Control Beliefs (Young and Older Adults)
Young and older adults' demographic characteristics, cognitive function, and memory control beliefs were assessed in an experimental session that occurred within 4 weeks of the pretraining fMRI session (Table 1). Older adults completed this session before receiving semantic encoding strategy training. Participants' age and years of education were ascertained using a demographics questionnaire. Participants' cognitive skills in several domains were assessed, including their semantic processing resources (vocabulary and similarities subtests of the Wechsler Adult Intelligence Scale - Third Edition (WAIS-III), Wechsler 1997), verbal fluency (FAS and animal naming, Spreen and Strauss 1991), working memory capacity (listening span, Salthouse and Babcock 1991 and rotation span, Kane et al. 2004), executive function (Wisconsin Card Sorting Test, Heaton 2003 and Simon task, Castel et al. 2007), and processing speed (digit symbol subtest of the WAIS-III, Wechsler 1997). Participants' scores for domains with multiple measures were calculated by computing z-score averages. Memory control beliefs were assessed using the Memory Controllability Inventory (MCI) (Lachman et al. 1995).
Table 1.
Demographic characteristics, cognitive function, and memory control beliefs
| Young (n = 17) | Old (n = 16) | |
| Demographic characteristics | ||
| Female/male | 9/8 | 8/8 |
| Age**** | 22.8 (4.0) | 71.9 (4.1) |
| Education (years) | 15.2 (1.9) | 14.8 (2.7) |
| Cognitive function | ||
| WAIS-III vocabulary* | 54.2 (7.0) | 48.9 (8.2) |
| WAIS-III similarities*** | 28.2 (2.0) | 24.5 (4.2) |
| FAS | 43.2 (10.4) | 38.3 (11.8) |
| Animal naming*** | 24.7 (5.7) | 19.3 (2.0) |
| Listening spana,**** | 0.84 (0.08) | 0.61 (0.14)c |
| Rotation spana,**** | 0.68 (0.13)d | 0.39 (0.18) |
| WCST categories completed** | 5.8 (0.7) | 4.1 (2.5) |
| Simon effectb,*** | 62.5 (39.9) | 142.3 (90.6)c |
| WAIS-III digit symbol**** | 96.4 (14.3) | 66.4 (11.4) |
| Memory control beliefs | ||
| Present ability | 5.5 (1.2) | 5.5 (0.9) |
| Potential improvement | 5.5 (1.0) | 5.1 (0.8) |
| Effort utility | 5.4 (1.1) | 5.2 (1.4) |
| Inevitable decrement | 3.5 (1.3) | 3.3 (1.3) |
Note: Means and SDs (in parentheses) for young and older adults' demographic characteristics, scores on assessments of cognitive function, and memory control belief ratings. Independent sample t-tests were used to examine group differences. WAIS-III, Wechsler Adult Intelligence Scale - Third Edition; WCST, Wisconsin Card Sorting Test.
Partial credit unit scoring (Conway et al. 2005).
Incongruent–congruent trial reaction times.
n = 15.
n = 16.
*P < 0.1, **P < 0.05, ***P < 0.01, ****P < 0.001.
Pretraining fMRI Session (Young and Older Adults)
fMRI Data Acquisition
Scanning was performed using a Siemens 3.0 Tesla Allegra scanner (Erlangen, Germany). An Apple Power Macintosh G4 computer (Apple, Cupertino, CA) and PsyScope software (Cohen et al. 1993) controlled the stimulus display and recorded responses from a magnet-compatible fiber-optic keypress device interfaced with a PsyScope button box. An LCD projector displayed stimuli onto a screen at the head of the magnet bore. Participants viewed the stimuli using a mirror attached to the head coil. Padding and tape minimized head movement and headphones dampened scanner noise. High-resolution structural images (1 × 1 × 1.2 mm) were acquired using a sagittal T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) sequence (time repetition [TR] = 2.3 s, time echo [TE] = 2.83 ms, flip angle = 9°, time to inversion [TI] = 900 ms). Functional images were acquired using T2*-weighted asymmetric spin-echo echo-planar sequences sensitive to BOLD contrast. Four functional scans of 96 whole-brain images (32 4 × 4 × 4 mm contiguous axial slices acquired parallel to the AC–PC plane, TR = 2.5 s, TE = 25 ms, flip angle = 90°) were collected per participant during encoding. The first 4 images in each scan were discarded to allow T1 magnetization to stabilize.
Stimuli
Stimuli for the fMRI sessions were 4–7 letter English words, presented centrally in uppercase letters. Stimulus lists were counterbalanced across tasks and scanning sessions and were matched for word frequency, length, and syllable count. Each word list consisted of half abstract (e.g., love, hope) and half concrete (e.g., table, flower) words.
Encoding
Participants performed an intentional encoding task during the first 2 scans of the pretraining fMRI session. Participants were instructed to study each presented word carefully in anticipation of a later unspecified memory test. To ensure that they were attending to the presented words, they were asked to make a right-handed keypress whenever a word appeared. During the third and fourth scans of the pretraining fMRI session, participants performed an abstract/concrete task. Participants decided whether each word represented an abstract or a concrete entity and made a right-handed keypress to indicate their decision for each word (for encoding task performance results, see Supplementary Table S1). During all encoding scans, 3 blocks of fixation plus signs (30 s) alternated with 2 blocks of words (70 s, 20 words per block). Therefore, a total of 80 words were presented during each task. An additional 10 s of fixation was collected at the beginning of every scan to allow T1 magnetization to stabilize. During word trials, a word was presented for 3250 ms and was followed by a fixation plus sign presented for 250 ms.
Retrieval
Immediately following the last encoding scan, participants' recognition memory for the words studied during intentional encoding and presented during the abstract/concrete task was assessed using Remember/Know/New recognition memory decisions (Tulving 1985; Gardiner 1988) during 6 additional fMRI scans (fMRI data to be presented in a subsequent publication). Older adults who needed to be removed from the scanner before completion of all the recognition memory scans due to fatigue performed recognition memory decisions on the remaining word lists on a laptop outside of the scanner. The retrieval word lists consisted of old word, new word, and fixation plus sign trials. These trials were presented in pseudorandom order so that every trial type was equally likely to be preceded and followed by every other trial type (Buckner et al. 1998). There were a total of 80 old words from the intentional encoding task, 80 old words from the abstract/concrete task, and 160 new words presented in these retrieval word lists. During fixation trials, a plus sign was presented for 3000 ms. During old and new word trials, individual words were presented for 2775 ms and were followed by fixation plus signs presented for 225 ms. Participants were instructed to make a Remember response if they recognized that a word had been encountered during the encoding scans and were able to consciously recollect aspects of its prior presentation. They were instructed to make a Know response if they recognized that a word had been encountered during the encoding scans but could not consciously recollect aspects of its prior presentation. They were also instructed to make a New response if they thought they had not seen the word during the encoding scans. Participants indicated their responses by pressing keys on a magnet-compatible fiber-optic keypress device or keyboard.
Strategy Questionnaire
After performing the Remember/Know/New recognition memory decisions, participants completed a self-initiated encoding strategy questionnaire outside of the scanner. Participants were asked to rate how often they used 24 possible encoding strategies during the intentional encoding scans, including the pleasantness (“Thought about whether each word was pleasant or unpleasant”), personal relevance (“Thought about the personal relevance of each word”), and sentence generation (“Constructed phrases, sentences, and/or stories that contained one studied word”) strategies that older adults were trained to use in this study (see below), and how often they used no encoding strategy (“Read each word but did not use any particular strategy to try to remember the words”). Participants rated the frequency of their use of these strategies using a scale of never, rarely, sometimes, usually, or always. These ratings were converted into numerical values for statistical analyses (1 = never, 2 = rarely, 3 = sometimes, 4 = usually, and 5 = always).
Semantic Encoding Strategy Training (Older Adults Only)
After the pretraining neuroimaging session, older adults completed 2 semantic encoding strategy training sessions (for a detailed protocol description, see Supplementary Methods). During the first training session, older adults were taught to use 3 semantic encoding strategies to memorize studied words. Older adults were taught to decide whether each presented word was pleasant or unpleasant and to think about why they felt that way (pleasantness strategy), to think about how each word was personally relevant to them (personal relevance strategy), and to form a sentence that contained each presented word (sentence generation strategy). After older adults were given extensive practice using each of these semantic encoding strategies, they were instructed to study additional word lists using whichever semantic encoding strategy or combination of semantic encoding strategies that they felt worked best for them. During the second training session, older adults were given additional practice using each of the semantic encoding strategies and then were again allowed to choose whichever semantic encoding strategy or strategies they wanted to use to study additional word lists. We allowed older adults to choose which semantic encoding strategy or strategies to practice at the end of the cognitive training sessions instead of training them to use just one semantic encoding strategy throughout training because we thought they would be most likely to self-initiate self-selected semantic encoding strategy(ies) during the posttraining fMRI session.
Posttraining fMRI Session (Older Adults Only)
The day after the second training session, older adults participated in a posttraining fMRI scanning session. This session occurred approximately 2 weeks after the pretraining scan session (range 8–18 days). The structural and functional scan parameters, encoding and retrieval task design, and strategy questionnaire were the same for both scanning sessions, but new lists of words were used for the encoding and retrieval tasks. Participants were not explicitly told to use the strategies that they learned in the training sessions during the posttraining fMRI session, so participants' brain activity during performance of the intentional encoding task and strategy use reports reflect self-initiated encoding strategy use.
Data Analyses
Training-Related Changes in Self-Initiated Encoding Strategy Use and Memory Performance
To examine age differences in self-initiated encoding strategy use, young adults' and older adults' pretraining and posttraining strategy use ratings were compared using independent sample t-tests. Paired sample t-tests were used to examine the effects of training on older adults' self-initiated use of encoding strategies. Age-related changes in recognition memory (Hits–False Alarms, Remember and Know responses; for d′ estimates of recognition memory discrimination, see Supplementary Results and Supplementary Table S2) and the effectiveness of semantic encoding strategy training were investigated using Task (intentional encoding, abstract/concrete) × Age (young, old) and Task (intentional encoding, abstract/concrete) × Training (pre, post) analyses of variance (ANOVAs) and t-tests.
fMRI Data Analysis
fMRI data were preprocessed to remove noise and artifacts (for method details, see Maccotta et al. 2001). Preprocessing included adjustment for slice timing differences using ideal sinc interpolation, correction for odd–even slice intensity differences, mode normalization, and motion–correction using a rigid-body rotation and translation correction. fMRI data were resliced into 3 mm isotropic voxels and transformed into the stereotaxic atlas space of Talairach and Tournoux (1988) using a template constructed from 16 young adult and 16 older adult T1-weighted MP-RAGE scans acquired on the MRI scanner used in this study (Snyder et al. 2002). Functional data were analyzed using the general linear model implemented in an in-house analysis and display package (Miezin et al. 2000). Brain activity during performance of the intentional encoding and abstract/concrete tasks was modeled as an extended gamma function (Boynton et al. 1996) and scaled to percent signal change. Run mean and slope were coded as effects of no interest. Data were smoothed using a 2 voxel isotropic Gaussian filter. Functional data were overlaid onto structural images from the combined young/older adult anatomical template to facilitate localization of significant brain activations.
The Effects of Semantic Encoding Strategy Training on Brain Activity During Intentional Encoding
The effects of semantic encoding strategy training on brain activity during intentional encoding were investigated using a whole-brain voxel-based Task (intentional encoding, abstract/concrete) × Training (pre, post) ANOVA (Monte-Carlo multiple comparison correction, P < 0.05) to identify brain regions that had training-related changes in activity during intentional encoding but not during performance of the abstract/concrete task. An automated algorithm identified activation peaks in the Task × Training interaction functional activation map. Regions of interest (ROIs) were grown that included all significant contiguous voxels within 12 mm of an activation peak. Magnitude estimates of percent signal change during performance of the intentional encoding and abstract/concrete tasks were calculated by averaging percent signal change across all voxels within these ROIs. Paired sample t-tests were then performed using these magnitude estimates to examine the effects of training on brain activity during the intentional encoding and abstract/concrete tasks in these ROIs. Young adults' and older adults' pretraining and posttraining brain activity patterns during intentional encoding and the abstract/concrete task were also compared in these ROIs using independent sample t-tests.
To investigate the neural correlates of individual differences in older adults' ability to benefit from semantic encoding strategy training, a whole-brain voxel-based Pearson Product Moment correlation analysis was conducted between older adults' training-related changes (posttraining–pretraining) in brain activity during intentional encoding and training-related changes in recognition memory performance (Monte-Carlo multiple comparison correction, P < 0.05). An automated algorithm identified activation peaks in this activation map, and ROIs were grown that included all significant contiguous voxels within 12 mm of an activation peak. Pearson Product Moment correlation analyses were performed within these ROIs between magnitude estimates (percent signal change averaged across all voxels within these ROIs) of training-related changes in brain activity during intentional encoding and training-related changes in older adults' recognition of words studied during intentional encoding to calculate r values and generate scatter plots.
Results
Semantic Encoding Strategy Training Increased Older Adults' Self-Initiated Use of Semantic Encoding Strategies
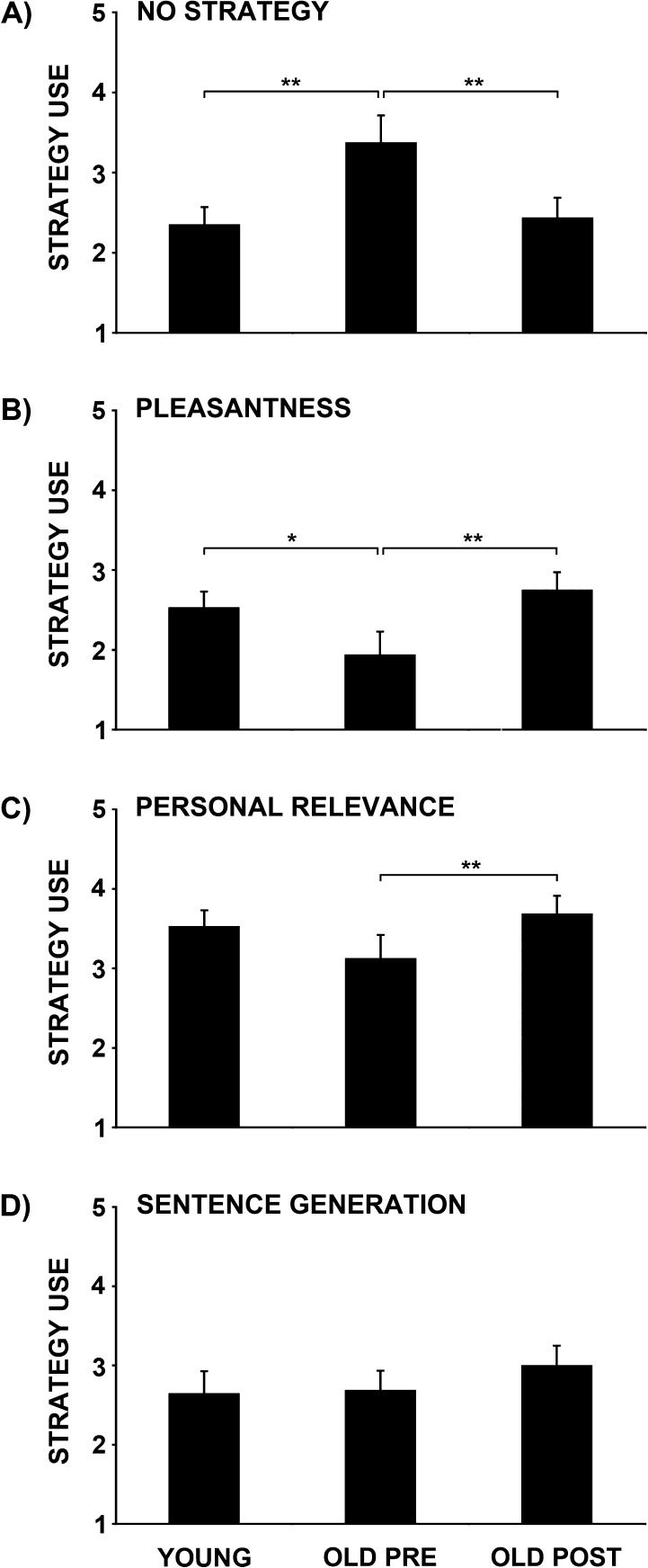
Prior to training, older adults reported not using encoding strategies during intentional encoding more frequently than young adults (t31 = 2.7, P < 0.05) (Fig. 2). There was also a trend toward older adults using the pleasantness strategy less frequently than young adults (t31 = −1.8, P < 0.1). Importantly, training decreased the frequency of older adults' reports of not using an encoding strategy (t15 = −2.5, P < 0.05) and increased older adults' self-initiated use of the pleasantness (t15 = 2.2, P < 0.05) and personal relevance strategies (t15 = 2.3, P < 0.05). After training older adults' ratings of no encoding strategy use and ratings of use of the pleasantness, personal relevance, and sentence generation strategies did not significantly differ from young adults’ (ts < 1, Ps > 0.1).
Figure 2.
Semantic encoding strategy training increased older adults' self-initiated use of semantic encoding strategies. Young adults' and older adults' pretraining and posttraining ratings of use of (A) no encoding strategy and (B) pleasantness, (C) personal relevance, and (D) sentence generation strategies during intentional encoding. Prior to training, older adults reported not using any encoding strategy more frequently than young adults, and there was a trend toward older adults using the pleasantness strategy less frequently than young adults. Training significantly decreased the frequency with which older adults' reported not using any encoding strategy. It also increased older adults' self-initiated use of the pleasantness and personal relevance strategies. Strategy use ratings: 1 = never, 2 = rarely, 3 = sometimes, 4 = usually, and 5 = always; *P < 0.1, **P < 0.05.
Semantic Encoding Strategy Training Improved Older Adults' Ability to Recognize Intentionally Encoded Words
Before older adults' training sessions, recognition memory performance was higher for young than for older adults (F1,31 = 7.6, P < 0.05) and for words presented during the abstract/concrete task than for words studied during intentional encoding (F1,31 = 43.8, P < 0.001) (Table 2). No interaction occurred between task and age (F1,31 = 1.5, P > 0.1). Analyses of the effects of training on older adults' recognition memory revealed a significant Task × Training interaction (F1,15 = 8.0, P < 0.05). Training improved older adults' memory for words studied during intentional encoding (t15 = 6.3, P < 0.001) but not for words from the abstract/concrete task (t15 = 1.1, P > 0.1). This selective training effect reveals that older adults' improvements in recognition memory following intentional encoding were driven by their being trained to use semantic encoding strategies and not by practice effects or performance feedback given during training. Consistent with this, there was a trend toward a positive correlation between older adults' training-related changes in self-initiated use of the sentence generation strategy and recognition memory following intentional encoding (see Supplementary Results). Analyses of the effects of training on older adults' recognition memory also revealed that recognition of words from the abstract/concrete task was greater than recognition of words studied during intentional encoding before (t15 = −4.5, P < 0.001) but not after training (t15 = 0.4, P > 0.1). Following older adults' strategy training, there was a significant Task × Age interaction (F1,31 = 12.8, P < 0.01). While older adults continued to recognize fewer words from the abstract/concrete task than young adults (t31 = −2.1, P < 0.05), age differences in recognition of words studied during intentional encoding were eliminated (t31 = 0.7, P > 0.1).
Table 2.
Recognition memory performance for young adults and older adults before and after semantic encoding strategy training
| Intentional encoding | Abstract/concrete | |||||
| Hits | FA | Hits–FA | Hits | FA | Hits–FA | |
| Young (n = 17) | 0.66 (0.16) | 0.27 (0.14) | 0.40 (0.18) | 0.87 (0.12) | 0.29 (0.16) | 0.58 (0.19) |
| Old pretraining (n = 16) | 0.60 (0.16) | 0.34 (0.21) | 0.26 (0.16) | 0.75 (0.16) | 0.36 (0.21) | 0.39 (0.19) |
| Old posttraining (n = 16) | 0.76 (0.20) | 0.31 (0.19) | 0.45 (0.25) | 0.73 (0.21) | 0.30 (0.18) | 0.43 (0.20) |
Note: Means and SDs (in parentheses) for recognition memory performance. Hits = proportion of old words receiving Remember or Know responses, False Alarms (FA) = proportion of new words receiving Remember or Know responses.
To further characterize the effects of semantic encoding strategy training on older adults' recognition memory, the effects of training on Remember and Know responses were examined (see Supplementary Results and Supplementary Table S3). The results of those analyses suggest that training selectively improved older adults' ability to consciously recollect words studied during intentional encoding as assessed by training-related changes in their Remember responses.
Exploratory analyses of the contributions of individual differences in older adults' age, education, cognitive function, and memory control beliefs to individual differences in their ability to benefit from semantic encoding strategy training were also conducted (see Supplementary Results and Supplementary Table S4). The results of those analyses suggest that individual differences in older adults' semantic processing resources, executive function, and memory control beliefs may play an important role in individual differences in their ability to benefit from training.
Semantic Encoding Strategy Training Increased Older Adults' Brain Activity During Intentional Encoding in the Frontal Lobes and the Left Caudate
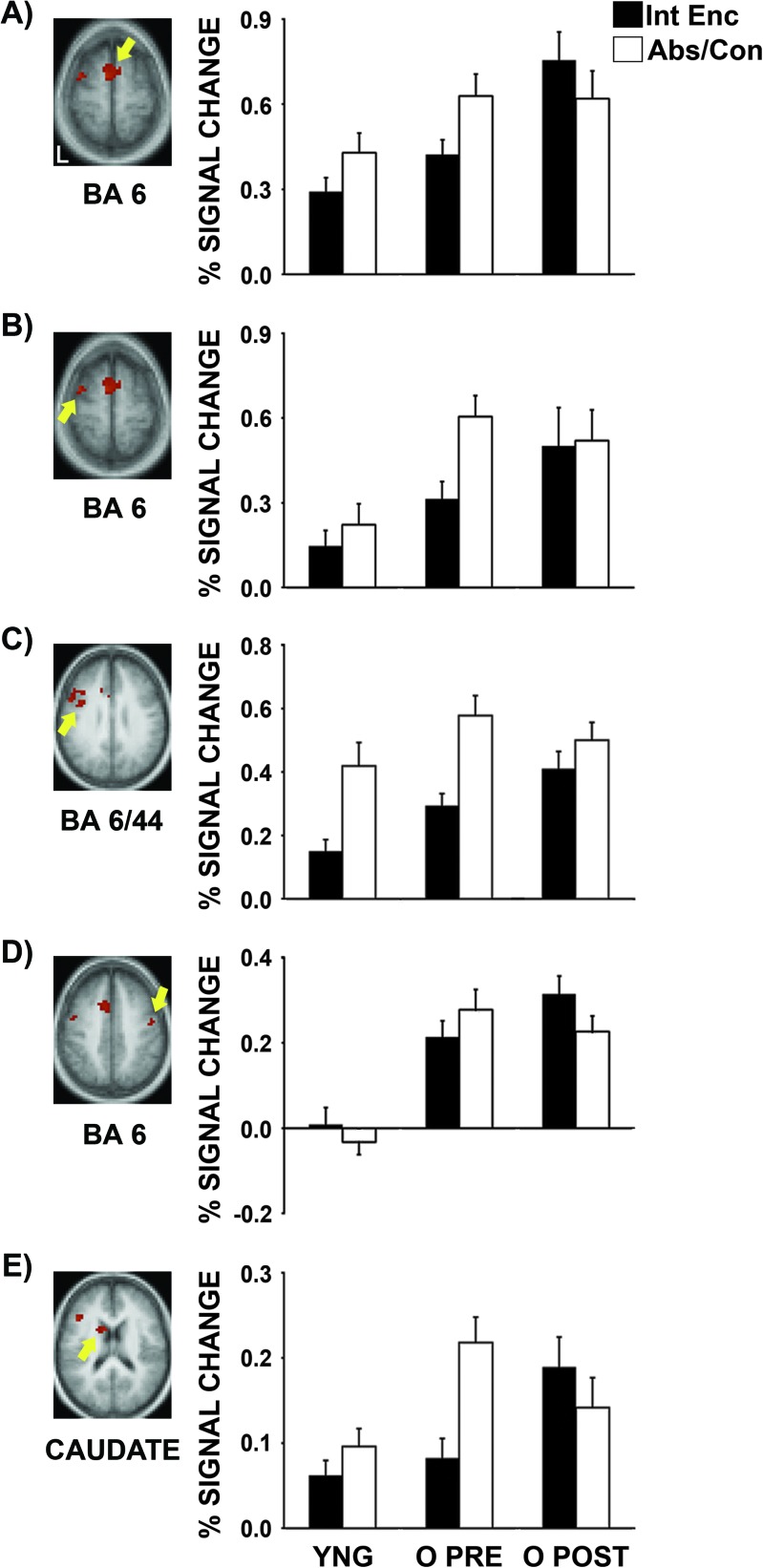
A whole-brain voxel-based Task (intentional encoding, abstract/concrete) × Training (pre, post) ANOVA was conducted to examine the effects of semantic encoding strategy training on older adults' brain activity during intentional encoding. Five regions, including the medial superior frontal gyrus (Brodmann's area [BA] 6; −7, 2, 64), left middle frontal gyrus (BA 6; −36, 1, 60), left posterior inferior frontal gyrus (BA 6/44; −39, 3, 29), right precentral gyrus (BA 6; 47, −8, 42), and left caudate (−12, 8, 19) had significant Task × Training interactions (Fig. 3). Training significantly increased older adults' brain activity during intentional encoding in the medial superior frontal gyrus (t15 = 4.3, P < 0.01), right precentral gyrus (t15 = 2.6, P < 0.05), and left caudate (t15 = 3.1, P < 0.01). There were also trends toward greater activity after than before training in the left middle frontal (t15 = 2.0, P < 0.07) and posterior inferior frontal gyri (t15 = 2.1, P < 0.06). In contrast, there were no significant training-related changes in activity during performance of the abstract/concrete task in the medial superior frontal (t15 = −0.2, P > 0.1), left middle frontal (t15 = −1.1, P > 0.1), left posterior inferior frontal (t15 = −1.7, P > 0.1), and right precentral gyri (t15 = −1.2, P > 0.1) and training decreased activity during performance of the abstract/concrete task in the left caudate (t15 = −2.4, P < 0.05). These selective increases in brain activity during intentional encoding strongly suggest that increases in activity during intentional encoding after relative to before training in these regions are due to semantic encoding strategy training and not to changes in scanner signal across scanning sessions, task practice effects, or performance feedback during training.
Figure 3.
Semantic encoding strategy training increased brain activity during intentional encoding in the frontal lobes and left caudate. (A) Medial superior frontal, (B) left middle frontal, (C) left posterior inferior frontal, (D) right precentral, and (E) left caudate regions with significant Task (intentional encoding, abstract/concrete) × Training (pre, post) brain activity interactions (Monte-Carlo multiple comparison corrected, P < 0.05). Semantic encoding strategy training selectively increased older adults' brain activity during intentional encoding in these regions. Int Enc = intentional encoding task, Abs/Con = abstract/concrete task, YNG = young adults, O PRE = older adults pretraining, O POST = older adults posttraining.
Young adults' and older adults' pretraining and posttraining brain activity patterns during intentional encoding and performance of the abstract/concrete task were also compared in the regions with significant Task × Training interactions. In this study, older adults were considered to have “overactivated” a brain region whenever they had greater activity within a brain region during encoding than young adults. Prior to training, older adults overactivated the left middle frontal (t31 = 2.1, P < 0.05), left posterior inferior frontal (t31 = 2.8, P < 0.01), and right precentral (t31 = 3.9, P < 0.01) gyri during intentional encoding (Fig. 3). There was also a trend toward older adults having greater brain activity than young adults in the medial superior frontal gyrus (t31 = 1.9, P < 0.07). After training, older adults overactivated all 5 regions with significant Task × Training interactions (medial superior frontal gyrus: t31 = 4.4, P < 0.001; left middle frontal gyrus: t20 = 2.5, P < 0.05; left posterior inferior frontal gyrus: t31 = 4.2, P < 0.001; right precentral gyrus: t31 = 5.5, P < 0.001; left caudate: t22 = 3.3, P < 0.01). During the abstract/concrete task, older adults overactivated the left middle frontal gyrus (t31 = 3.7, P < 0.01), right precentral gyrus (t31 = 5.8, P < 0.001), and left caudate (t31 =3.5, P < 0.01) before training. There was also a trend toward older adults having greater brain activity than young adults in the medial superior frontal gyrus (t31 = 2.0, P < 0.06). Older adults continued to overactivate the left middle frontal (t31 = 2.4, P < 0.05) and right precentral (t31 = 5.8, P < 0.001) gyri following training. This pattern of results demonstrates that older adults overactivated prefrontal cortex during both encoding tasks before training when their subsequent recognition memory was worse than young adults' and continued to overactivate prefrontal cortex during intentional encoding after training when their subsequent recognition memory no longer differed from young adults'.
Older Adults' Training-Related Changes in Recognition Memory and Brain Activity Were Strongly Correlated in Prefrontal and Left Lateral Temporal Cortex
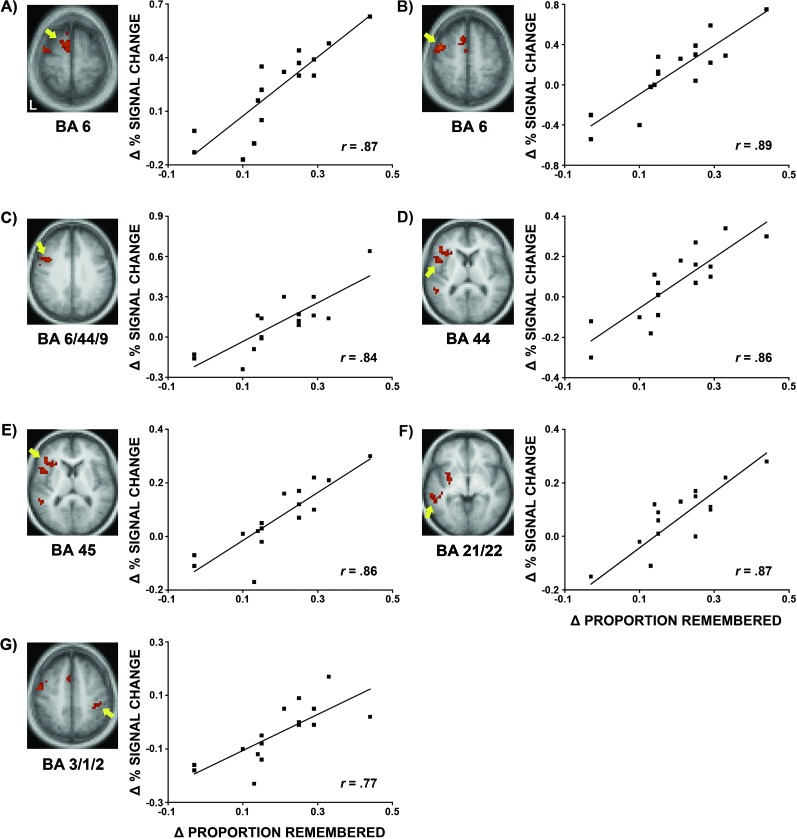
The neural correlates of individual differences in older adults' ability to benefit from semantic encoding strategy training were investigated using a whole-brain voxel-based Pearson Product Moment correlation analysis. Strong positive correlations were found between training-related changes in recognition memory and brain activity during intentional encoding in the medial superior frontal (BA 6; −6, −12, 64), left middle frontal/precentral (BA 6; −41, 1, 55), left dorsal posterior inferior frontal (6/44/9; −46, 7, 35), left ventral posterior inferior frontal (BA 44; −47, 6, 6), left anterior inferior frontal (BA 45; −36, 21, 8), left middle/superior temporal (BA 21/22; −53, −39, −5), and right postcentral gyri (BA 3/1/2; 38, −28, 43) (Fig. 4). In these regions, individuals who had the greatest training-related improvements in recognition memory had the greatest training-related increases in brain activity during intentional encoding. Older adults' training-related changes in brain activity during intentional encoding were also correlated with their semantic processing resources, executive function, memory control beliefs, and/or training-related changes in self-initiated use of the sentence generation encoding strategy in these regions (see Supplementary Results and Supplementary Table S5).
Figure 4.
Neural correlates of individual differences in older adults' ability to benefit from semantic encoding strategy training. Older adults' training-related changes (posttraining–pretraining) in recognition memory and brain activity during intentional encoding were strongly correlated in the (A) medial superior frontal, (B) left middle frontal/precentral, (C) left dorsal posterior inferior frontal, (D) left ventral posterior inferior frontal, (E) left anterior inferior frontal, (F) left middle/superior temporal, and (G) right postcentral gyri (Monte-Carlo multiple comparison corrected, P < 0.05).
Discussion
In this study, semantic encoding strategy training increased older adults' self-initiated use of semantic encoding strategies and substantially improved their ability to recognize words following intentional encoding. Training increased older adults' mean brain activity in the medial superior frontal gyrus, right precentral gyrus, and left caudate during intentional encoding. Training-related changes in recognition memory were also strongly positively correlated with training-related changes in brain activity in prefrontal and left lateral temporal regions engaged by young adults during semantic processing and/or self-initiated use of verbal encoding strategies. The implications of these results are discussed below.
Cognitive Training Can Increase Older Adults' Self-Initiated Use of Semantic Encoding Strategies
Semantic encoding strategy training decreased the frequency of older adults' reports of not using any encoding strategy during intentional encoding and increased the frequency of their use of personal relevance and pleasantness strategies. Critically, these results demonstrate that semantic encoding strategy training can alter older adults' self-initiated strategic behavior in an experimental setting. These findings are encouraging because they suggest that semantic encoding strategy training could have a lasting impact on older adults' memory performance. Future research is needed to determine how long these training effects can be maintained and whether semantic encoding strategy training can lead to improvements in older adults' real world memory function.
Semantic Encoding Strategy Training Can Improve Older Adults' Recognition of Intentionally Encoded Words
Semantic encoding strategy training significantly improved older adults' ability to recognize intentionally encoded words, and after training older adults' ability to recognize intentionally encoded words no longer differed from that of young adults. To begin to assess the mechanisms of these training-related changes in older adults' memory performance, we examined the effects of training on older adults' ability to recognize words based on conscious recollection versus feelings of familiarity (see Supplementary Results and Supplementary Table S3). Older adults have greater impairments in the ability to consciously recollect previously encountered information than in the ability to recognize it based on a feeling of familiarity (Craik and Jennings 1992; Hay and Jacoby 1999; Jacoby et al. 2001). For example, Perfect and Dasgupta (1997) found that older adults were less likely to recollect words and pseudowords studied during intentional encoding than young adults, but their ability to recognize words and pseudowords based on feelings of familiarity did not differ from young adults. Older adults were also less likely to use elaborative strategies to encode pseudowords than young adults in their study. However, when age differences in self-initiated encoding strategy use were controlled by comparing young and older adults' memory for words and pseudowords that were encoded with elaborative strategies, there were no longer significant age differences in recollection. This pattern of results suggests that age-related changes in self-initiated encoding strategy use make an important contribution to age-related changes in recollection. Consistent with this proposal, semantic encoding strategy training selectively increased older adults' corrected Remember responses and eliminated pretraining age differences in corrected Remember responses for words studied during intentional encoding in the present study. Semantic encoding strategy training likely improves older adults' recollection by facilitating their ability to form detailed and distinctive memory traces during encoding, which they are subsequently able to recollect during memory retrieval.
Effects of Semantic Encoding Strategy Training on Older Adults' Brain Activity During Intentional Encoding
Semantic encoding strategy training increased older adults' mean brain activity during intentional encoding in the medial superior frontal gyrus, right precentral gyrus, and left caudate. Individual differences in older adults' ability to benefit from training were also associated with individual differences in their training-related changes in brain activity during intentional encoding in prefrontal, right postcentral, and left lateral temporal regions. These individual difference analyses focused on the combination of Remember and Know Hits–False Alarms. Theoretically, one might have expected the results to be strongest for Remember responses alone, as these were most impaired in older adults before training. However, we found stronger relationships between training-related changes in performance and brain activity for overall recognition memory performance rather than for Remember responses alone. We did find that many of the same brain regions identified in the overall recognition memory analysis also showed positive correlations between training-related changes in Remember Hits–False Alarms and brain activity. However, the magnitude of these correlations was smaller. One possible explanation for the weaker correlations between training-related changes in Remember Hits–False Alarms and brain activity during intentional encoding is that there were substantially fewer Hit and False Alarm trials for Remember responses than for Remember and Know responses combined, especially before training.
Finding that semantic encoding strategy training can alter older adults' brain activity in prefrontal cortex is encouraging because it suggests that cognitive training could be an effective treatment for the age-related changes in prefrontal function that are reflected in age differences in brain activity during episodic memory encoding and retrieval (for reviews, see Cabeza 2002; Rajah and D'Esposito 2005; Reuter-Lorenz and Lustig 2005; Persson and Nyberg 2006; Spreng et al. 2010). However, the results of this study also suggest that some age-related changes in brain activity in older adults may be resistant to cognitive training-based treatments. Before training, older adults overactivated the left middle frontal, left posterior inferior frontal, and right precentral gyri during intentional encoding, and there was also a trend toward overactivation in the medial superior frontal gyrus. The pretraining overactivation of these regions may reflect the recruitment of additional neural resources to compensate for inefficient cognitive processing within these regions or regions functionally connected to them due to age-related changes in brain structure or neurochemistry (Reuter-Lorenz and Cappell 2008). Following training, older adults continued to overactivate these regions during intentional encoding when their memory performance no longer differed from young adults'. Therefore, training increased older adults' self-initiated recruitment of these regions but was not able to improve processing efficiency in these regions. An important future research direction would be to examine the effects of semantic strategy training on young and older adults' brain activity patterns during encoding within the same research study. The current study provided training only to older adults. Thus, we do not know whether the changes in brain activity following training were unique to older adults or would also have occurred in young adults. The results of this study combined with a prior semantic encoding strategy training study in young- and middle-aged adults (Miotto et al. 2006) suggest that semantic encoding strategy training can increase brain activity in prefrontal cortex across the life span. However, little is currently known regarding whether prefrontal cortical plasticity increases, decreases, or remains the same as we age.
Prior research suggests that medial superior frontal, left middle and inferior frontal, bilateral orbitofrontal, and left lateral temporal regions may support young adults' self-initiated use of verbal encoding strategies (Savage et al. 2001; Kirchhoff and Buckner 2006; Miotto et al. 2006; Matsui et al. 2008). The results of this study suggest that this same network of brain regions may also support older adults' self-initiated use of verbal encoding strategies. Specifically, semantic encoding strategy training significantly increased older adults' mean brain activity during intentional encoding in the medial superior frontal gyrus, and there were trends toward significant increases in the left middle and posterior inferior frontal gyri. Further, older adults' ability to benefit from semantic encoding strategy training was strongly correlated with training-related changes in brain activity in medial superior frontal, left middle frontal/precentral, left inferior frontal, and left lateral temporal cortex. There was also a positive correlation between older adults' training-related changes in self-initiated use of the sentence generation strategy and training-related changes in brain activity in the left middle frontal/precentral gyrus and trends toward positive correlations in the medial superior and left inferior frontal gyri. We did not find training-related changes in brain activity in orbitofrontal cortex in this study. However, this region is difficult to image with fMRI due to susceptibility artifacts (Ojemann et al. 1997). Thus, we may have not have found training effects in orbitofrontal cortex due to low signal in this region.
Semantic encoding strategy training also significantly increased older adults' mean brain activity during intentional encoding in the right precentral gyrus, which was overactivated by older adults during both encoding tasks before and after training. Older adults' overactivation of this region is consistent with prior research demonstrating more bilateral activation patterns in frontal cortex in older than in young adults during performance of cognitive tasks (Reuter-Lorenz et al. 2000; Logan et al. 2002; Cabeza 2002). Frontal cortex in the vicinity of the right posterior inferior frontal gyrus and precentral gyrus is preferentially engaged during encoding of visual versus verbal stimuli in young adults (Kelley et al. 1998; Wagner et al. 1998; Kirchhoff et al. 2000). In addition, significant correlations between young adults' brain activity in right posterior inferior prefrontal cortex and their self-initiated visual encoding strategy use have been reported (Kirchhoff and Buckner 2006). Therefore, older adults' overactivation of the right precentral gyrus before training could reflect greater visualization of word meaning by older than by young adults and training-related increases in brain activity during intentional encoding in this region could reflect an increase in older adults' visualization of word meaning following training. Alternatively, increased activity in the right precentral gyrus in older adults after relative to before training could indicate that older adults recruited this region to support self-initiated processing of word meaning per se, and therefore that the neural correlates of self-initiated verbal encoding strategy use are altered in older adults. Single session functional neuroimaging studies of verbal memory encoding have reported greater activation in right frontal cortex in older adults with good memory performance relative to older adults with poor memory performance (Rosen et al. 2002) and greater reliance on right frontal regions for successful encoding in older adults than in young adults (Morcom et al. 2003; Dennis et al. 2007). These findings suggest that the relative overactivation of right frontal cortex by older adults during verbal processing compensates for age-related changes in brain structure and supports older adults' cognitive function (Reuter-Lorenz et al. 2000; Cabeza 2002). The training-related increases in mean brain activity in the right precentral gyrus in this study are consistent with this compensation view of right frontal overactivation in older adults. Interestingly, semantic categorization strategy training has been shown to increase brain activity in the right middle and inferior frontal gyri in young- and middle-aged adults (Miotto et al. 2006). This suggests that semantic strategy training results in bilateral increases in brain activity in frontal cortex regardless of age. Future research is needed to elucidate the specific role of right posterior inferior frontal/precentral cortex in self-initiated encoding strategy use in older adults, and the factors that contribute its overactivation by older adults.
Older Adults' Semantic Processing Resources, Executive Function, and Memory Control Beliefs Influence Their Ability to Benefit from Semantic Encoding Strategy Training
Exploratory analyses of individual differences in older adults' ability to benefit from semantic encoding strategy training revealed that older adults' semantic processing resources, executive function, and memory control beliefs contributed to their training-related changes in recognition memory and brain activity in prefrontal cortex (see Supplementary Results and Supplementary Tables S4 and S5). Older adults' scores on assessments of semantic processing resources and executive function were positively correlated with their training-related changes in recognition memory, demonstrating that older adults with the greatest semantic processing resources and executive function capabilities are the most likely to benefit from semantic encoding strategy training. These associations indicate that older adults with the highest scores on assessments of semantic processing resources and executive function may have had the largest training-related increases in self-initiated use of semantic encoding strategies and/or were able to implement the trained strategies most effectively. Consistent with these possibilities, older adults' semantic processing and executive function scores were positively correlated with their training-related changes in brain activity in regions previously shown to be engaged during semantic processing by young and older adults (Logan et al. 2002; Daselaar et al. 2003) and/or engaged during self-initiated verbal encoding strategy use by young adults (Kirchhoff and Buckner 2006).
Older adults' scores on the Effort Utility and Inevitable Decrement subscales of the MCI were negatively correlated with their training-related changes in recognition memory, demonstrating that older adults' perceptions of their ability to control their memory influence their ability to benefit from semantic encoding strategy training. The negative correlations between older adults' ratings on these subscales and their training-related changes in memory performance reveal that older adults who are least likely to endorse a “use it or lose it” theory of memory performance and the belief that their memory will inevitably get worse as they get older prior to semantic encoding strategy training are the most likely to benefit from training. A possible explanation for this pattern of results is that older adults who are least likely to endorse these theories prior to semantic encoding strategy training are the most likely to increase their self-initiated semantic encoding strategy use as a result of semantic encoding strategy training. Consistent with this possibility, older adults' scores on the Effort Utility and Inevitable Decrement subscales of the MCI were negatively correlated with their training-related changes in brain activity in prefrontal and left lateral temporal regions previously shown to be engaged during semantic processing by young and older adults (Logan et al. 2002; Daselaar et al. 2003) and/or engaged during self-initiated verbal encoding strategy use by young adults (Kirchhoff and Buckner 2006). Prior research has also shown that self-initiated encoding strategy use at least partially mediates positive associations between memory control beliefs and free recall in older adults (Hertzog et al. 1998; Lachman and Andreoletti 2006). The results of this study extend these findings by suggesting that individual differences in training-related changes in self-initiated semantic encoding strategy use can at least partially mediate positive associations between older adults' memory control beliefs and training-related changes in recognition memory. The significant correlations among memory control beliefs and training-related changes in memory performance and brain activity in this study also suggest that including procedures designed to strengthen older adults' beliefs that they have control over their memory in cognitive training protocols could enhance the effectiveness of training.
Funding
National Institute on Aging at the National Institutes of Health (R01 AG13845 to L.L.J. and K01 AG031301 to B.A.K.); McDonnell Center for Higher Brain Function at Washington University in St. Louis (D.M.B. and B.A.K.).
Supplementary Material
Acknowledgments
We thank Carlee Hawkins, Danielle Hirschfeld, Shari Steinman, Naomi Yodkovik, Jennifer Staplins, Christy Meier, and Joe Hilgard for assistance with data collection and/or data analyses and Carol McKenna for assistance with participant recruitment. We also thank David Balota, Randy Buckner, David McCabe, Denise Head, and Martha Storandt for helpful discussions, Abraham Snyder and Mark McAvoy for development of MR analysis procedures, Carole Jacoby for administrative assistance, and Staci Smith for assistance with manuscript preparation. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Conflict of Interest : None declared.
References
- Alexander MP, Stuss D, Gillingham S. Impaired list learning is not a general property of frontal lesions. J Cogn Neurosci. 2009;21:1422–1434. doi: 10.1162/jocn.2009.21094. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test-II: findings from patients with focal frontal lesions. J Int Neuropsychol Soc. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy older adults. In: Tulving E, Craik FIM, editors. The oxford handbook of memory. New York: Oxford University Press; 2000. pp. 395–409. [Google Scholar]
- Bower GH. Analysis of a mnemonic device. Am Sci. 1970;58:496–510. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional–anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Camp CJ, Markley RP, Kramer JJ. Naive mnemonics: what the “do-nothing” control group does. Am J Psychol. 1983;96:503–511. [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: evidence for disproportionate selection impairments in the simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user’s guide. Psychon Bull Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: the role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale (NJ): Lawrence Erlbaum Associates; 1992. pp. 51–110. [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol Aging. 2003;24:1005–1011. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Devolder PA, Pressley M. Causal attributions and strategy use in relation to memory performance differences in younger and older adults. Appl Cogn Psychol. 1992;6:629–642. [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Functional aspects of recollective experience. Mem Cognit. 1988;16:309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Geiselman RE, Woodward JA, Beatty J. Individual differences in verbal memory performance: a test of alternative information-processing models. J Exp Psychol Gen. 1982;111:109–134. [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JF, Jacoby LL. Separating habit and recollection in young and older adults: effects of elaborative processing and distinctiveness. Psychol Aging. 1999;14:122–134. doi: 10.1037//0882-7974.14.1.122. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin card sorting test: computer version 4. Lutz (FL): Psychological Assessment Resources; 2003. [DOI] [PubMed] [Google Scholar]
- Hertzog C, McGuire CL, Horhota M, Jopp D. Does believing in “use it or lose it” relate to self-rated memory control, strategy use and recall? Int J Aging Hum Dev. 2010;70:61–87. doi: 10.2190/AG.70.1.c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, McGuire CL, Lineweaver TT. Aging, attributions, perceived control, and strategy use in a free recall task. Aging Neuropsychol Cogn. 1998;5:85–106. [Google Scholar]
- Hildebrandt H, Brand A, Sachsenheimer W. Profiles of patients with left prefrontal and left temporal lobe lesions after cerebrovascular infarcations on California Verbal Learning Test-like indices. J Clin Exp Neuropsychol. 1998;20:673–683. doi: 10.1076/jcen.20.5.673.1119. [DOI] [PubMed] [Google Scholar]
- Hill RD, Allen C, McWhorter P. Stories as a mnemonic aid for older learners. Psychol Aging. 1991;6:484–486. doi: 10.1037//0882-7974.6.3.484. [DOI] [PubMed] [Google Scholar]
- Hill RD, Storandt M, Simeone C. The effects of memory skills training and incentives on free recall in older learners. J Gerontol. 1990;45:P227–P232. doi: 10.1093/geronj/45.6.p227. [DOI] [PubMed] [Google Scholar]
- Hirst W, Volpe BT. Memory strategies with brain damage. Brain Cogn. 1988;8:379–408. doi: 10.1016/0278-2626(88)90060-7. [DOI] [PubMed] [Google Scholar]
- Hulicka IM, Grossman JL. Age-group comparisons for the use of mediators in paired-associate learning. J Gerontol. 1967;22:46–51. [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychol Aging. 1990;5:356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Debner JA, Hay JF. Proactive interference, accessibility bias, and process dissociations: valid subject reports of memory. J Exp Psychol Learn Mem Cogn. 2001;27:686–700. [PubMed] [Google Scholar]
- Jacoby LL, Rhodes MG. False remembering in the aged. Curr Dir Psychol Sci. 2006;15:49–53. [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kausler DH. Learning and memory in normal aging. San Diego (CA): Academic Press; 1994. [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Smith SE, Luntz JD. Prefrontal cortex and self-initiated encoding strategy use in healthy younger and older adults. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; Forthcoming. [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Andreoletti C. Strategy use mediates the relationship between control beliefs and memory performance for middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61:P88–P94. doi: 10.1093/geronb/61.2.p88. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Bandura M, Weaver SL, Elliott E. Assessing memory control beliefs: the memory controllability inventory. Aging Cogn. 1995;2:67–84. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Zacks JM, Buckner RL. Rapid self-paced event-related functional MRI: feasibility and implications of stimulus versus response-locked timing. Neuroimage. 2001;14:1105–1121. doi: 10.1006/nimg.2001.0912. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Boersma FJ, Cox DL. A classification of associative strategies in paired-associate learning. Psychon Sci. 1965;3:455–456. [Google Scholar]
- Matsui M, Suzuki M, Zhou SY, Takahashi T, Kawasaki Y, Yuuki H, Kato K, Kurachi M. The relationship between prefrontal brain volume and characteristics of memory strategy in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1854–1862. doi: 10.1016/j.pnpbp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MGM, Iaki S, Amaro E Jr. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Hum Brain Mapp. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychol Aging. 2007;22:202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Bäckman L. Neural correlates of training-related memory improvement in adulthood and aging. Proc Natl Acad Sci U S A. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Perlmutter M, Mitchell DB. The appearance and disappearance of age differences in adult memory. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press; 1982. pp. 127–144. [Google Scholar]
- Perfect TJ, Dasgupta ZRR. What underlies the deficit in reported recollective experience in old age? Mem Cognit. 1997;25:849–858. doi: 10.3758/bf03211329. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Altered brain activity in healthy seniors: what does it mean? Prog Brain Res. 2006;157:45–56. doi: 10.1016/s0079-6123(06)57004-9. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rowe EJ, Schnore MM. Item concreteness and reported strategies in paired-associate learning as a function of age. J Gerontol. 1971;26:470–475. doi: 10.1093/geronj/26.4.470. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- Sanders RE, Murphy MD, Schmitt FA, Walsh KK. Age differences in free recall rehearsal strategies. J Gerontol. 1980;35:550–558. doi: 10.1093/geronj/35.4.550. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Murphy MD, Sanders RE. Training older adult free recall rehearsal strategies. J Gerontol. 1981;36:329–337. doi: 10.1093/geronj/36.3.329. [DOI] [PubMed] [Google Scholar]
- Snyder AZ, Morris JC, Williams L, Buckner RL. Automated whole-brain atrophy assessment: effects of aging and dementia status. Neurobiol Aging. 2002;23:1337. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 1991. [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, Sachdev P. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport. 2003;14:1333–1337. doi: 10.1097/01.wnr.0000077548.91466.05. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A. Production deficiency hypothesis revisited: adult age differences in strategy use as a function of processing resources. Aging Cogn. 1994;1:323–338. [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- Witte KL, Freund JS, Sebby RA. Age differences in free recall and subjective organization. Psychol Aging. 1990;5:307–309. doi: 10.1037//0882-7974.5.2.307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.