Abstract
Motion processing regions apart from V5+/MT+ are still relatively poorly understood. Here, we used functional magnetic resonance imaging to perform a detailed functional analysis of the recently described cingulate sulcus visual area (CSv) in the dorsal posterior cingulate cortex. We used distinct types of visual motion stimuli to compare CSv with V5/MT and MST, including a visual pursuit paradigm. Both V5/MT and MST preferred 3D flow over 2D planar motion, responded less yet substantially to random motion, had a strong preference for contralateral versus ipsilateral stimulation, and responded nearly equally to contralateral and to full-field stimuli. In contrast, CSv had a pronounced preference to 2D planar motion over 3D flow, did not respond to random motion, had a weak and nonsignificant lateralization that was significantly smaller than that of MST, and strongly preferred full-field over contralateral stimuli. In addition, CSv had a better capability to integrate eye movements with retinal motion compared with V5/MT and MST. CSv thus differs from V5+/MT+ by its unique preference to full-field, coherent, and planar motion cues. These results place CSv in a good position to process visual cues related to self-induced motion, in particular those associated to eye or lateral head movements.
Keywords: CSv, eye movements, flow, fMRI, pursuit, self-motion
Introduction
Visual motion is one of the best-studied attributes in neuroscience. However, the vast majority of studies has focused on the complex of areas denoted V5+/MT+ that comprises several areas, including V5/MT and MST, and whose lesion leads to akinetopsia or the inability to perceive motion (Zeki et al. 1991; Born and Bradley 2005). One region that has received comparably little attention but whose activation is apparent in a number of studies using visual motion cues resides in the dorsal posterior cingulate cortex (dPCC) (Sunaert et al. 1999; Braddick et al. 2001; Dieterich et al. 2003; Orban et al. 2003; Antal et al. 2008; Wall and Smith 2008). Two recent functional magnetic resonance imaging (fMRI) studies focused on this region in particular. Antal et al. (2008) found preferential responses to complex motion stimuli (expansion flow and rotation vs. planar motion), while Wall and Smith (2008) observed increased activation to an expanding optic flow stimulus in comparison to an array of multiple flow patches. Wall and Smith (2008) suggested the region to encode visual egomotion cues, similar to area MST and to parietal areas that have also been reported to respond preferentially to self-motion cues (Morrone et al. 2000; Bartels et al. 2008), and referred to the region as to the cingulate sulcus visual area (CSv) (Wall and Smith 2008). In the following, we will refer to this region by its functional term “CSv” coined by Wall and Smith (2008), as this is more accurate in context of this functional study compared with the anatomically more broad term dPCC that comprises CSv. Cytologically, CSv is located within the dPCC, which in turn includes areas 23d, d23a/b/c, and adjacent area 31 (Vogt et al. 2006). In the non-human primate, electrophysiology and anatomy suggest a link of dPCC to eye movements. Neurons in area 23 of the PCC have been shown to code for orbital eye position and to respond to large textured visual fields (Olson et al. 1996; Vogt and Laureys 2005; Vogt et al. 2006). Their projections to various premotor and cingulate motor areas have suggested a role in the visual orientation of oneself and one’s body (Dean et al. 2004; Dean and Platt 2006), including visual feedback of limb movements (Vogt 2005; Vogt et al. 2006) and predictability of self-generated actions in the human (Blakemore et al. 1998; Vogt et al. 2006). In line with this, neural responses in dPCC have been shown to occur immediately following the onset of eye movements (Dean et al. 2004) and visually guided saccades (Olson et al. 1996), and fMRI has shown its involvement in optokinetic nystagmus (Dieterich et al. 2003). However, in the human, there is so far only little evidence that CSv or dPCC is involved in the processing of eye movement-related signals and hence its role in integrating visual with nonvisual cues during self-induced motion remains unclear (Petit et al. 1993; Culham et al. 1998; Dieterich et al. 2003). Also, since human CSv is a relatively small region, its link to the above non-human and human studies (apart from those of Antal et al. [2008] and Wall and Smith [2008]) is only tentative and relies on its anatomical location.
In contrast to CSv, V5/MT and MST have been extremely well studied. Both respond to visual motion stimuli of almost any kind, with MST having larger receptive fields (RFs) than V5/MT and preferring more complex and coherent motion types compared with V5/MT (Morrone et al. 2000; Huk et al. 2002; Smith et al. 2006; Bremmer et al. 2010; Maciokas and Britten 2010; Yu et al. 2010). Both regions receive input from FEFs as well as efferent copies of eye movements (Desimone and Ungerleider 1986; Boussaoud et al. 1990), and MST additionally receives vestibular input critical for self-motion processing (Gu et al. 2007). MST can thus combine visual self-motion signals, such as optic flow with vestibular signals to enhance heading discrimination (Gu et al. 2006; Chowdhury et al. 2009). MST is also known to contain particularly high fractions of “real-motion” cells that respond preferentially to stimuli moving in the external world, regardless of presence or absence of retinal motion (Sakata et al. 1985; Erickson and Thier 1991; Galletti and Fattori 2003), consistent with imaging data showing enhanced integration of eye position with retinal flow in MST (Goossens et al. 2006). Thus, many properties established for V5/MT and MST have not been examined in CSv, and hence the functional differences between CSv on one hand and of MST and V5/MT on the other are far from clear. This concerns response characteristics to distinct types of motion stimuli, preference to stimulus size and lateralization, and also the capability to integrate eye-movement signals with retinal motion to extract real motion estimates. In this fMRI study, we therefore sought to systematically investigate the functional responses of CSv and compare them with those of MST and V5/MT. We functionally identified each of these regions in both hemispheres of each of 14 human participants and examined their responses to 2D planar motion, 3D flow, trajectory-matched random motion, and hemifield stimulation while subjects performed a central distractor task. In addition, we compared signals during visual pursuit and nonpursuit, with the stimuli designed such that pursuit conditions were matched in retinal motion content to nonpursuit conditions, allowing us to obtain estimates of each area’s response to 1) retinal motion, 2) eye movements, and 3) objective motion signals. We found that CSv differed dramatically from both V5/MT and MST in nearly all aspects we examined, suggesting a role in full-field processing of visual cues related to eye movements, with an enhanced capability to extract objective motion compared with V5/MT and MST.
Materials and Methods
Subjects and Stimuli
A total of 14 volunteers with normal or corrected to normal vision (6 males, aged 23–35, 2 left-handed) participated in this study after signing an informed consent form. The study was conducted in accord with the joint ethics committee of the Max Planck Institute and University Hospital, Tübingen, Germany. Prior to scanning, all subjects were instructed about the experimental procedures and performed a test run with the experimental task and stimuli.
All visual stimuli consisted of random dot patterns of black and white dots (size ranging from 0.1 to 1.1°) on a gray (90 cd/m2) background, presented at 100% contrast. The 320 visible dots yielded an average density of 0.75 dots/degree2 on a visual display subtending 24 × 18°, viewed at 82 cm distance. The stimuli were back projected using a gamma-corrected projector onto a screen positioned behind the observers’ head and viewed via a front-surfaced mirror mounted on the head coil, with 640 × 480 pixels resolution at 60 Hz. Stimuli were presented using Cogent Graphics (http://www.vislab.ucl.ac.uk/cogent_graphics.php) developed by John Romaya from the Wellcome Department of Imaging Neuroscience and run on MATLAB 7.3 (Mathworks Inc.) on a windows PC.
Three experiments were conducted. In experiments 1 and 2, 11 volunteers participated, and in experiment 3, 7 volunteers participated. Experiment 1 had 7 conditions, each was presented 6 times in each of 4 scanning sessions. Experiments 2 and 3 were nearly identical, with differences outlined in a subsequent section. Both had 8 conditions, each was presented 4 times in each of 6 scanning sessions. All experiments were block design experiments with trials lasting 12 s presented in pseudorandom trial sequences designed such that each condition was preceded equally often by all other conditions.
Fixation Task
During all trials (of all 3 experiments), subjects performed a fixation task that controlled eye movements and ensured a balanced attentional load during all conditions. Subjects were asked to indicate character repeats during a continuous serial display of randomly assembled alphabetical characters (n = 26) by pressing a key on a button box. The characters were presented in red color (1.6° height) on a gray fixation annulus (2° width, 72 cd/m2). Display times for the characters varied randomly between 1 and 2.16 s and characters repeated randomly every 3 to 8 character presentations.
Eye Tracking
We performed eye tracking during experiment 3 inside the scanner using a video-based infrared eye-tracker with long-range optics (Eye-Trac6, Applied Science Laboratories, MA). This allowed us to obtain horizontal and vertical eye positions at 60 Hz in 7 subjects. After blink removal, drifts due to changes in head and/or eye position were removed, and data were smoothed using a Gaussian filter. The eye-velocity was calculated using a 6-point running average-and-differentiating filter, and saccades were identified at a velocity threshold of >21 degree/s. Following saccade removal, data points were linearly interpolated. Fixation accuracy was quantified by calculating 1) the root-mean-square (RMS) error of the actual eye position relative to the fixation cross and 2) the RMS error of the eye velocity compared with that of the fixation cross, for each stimulus condition separately, across sessions (n = 6) and subjects (n = 7).
Experiment 1
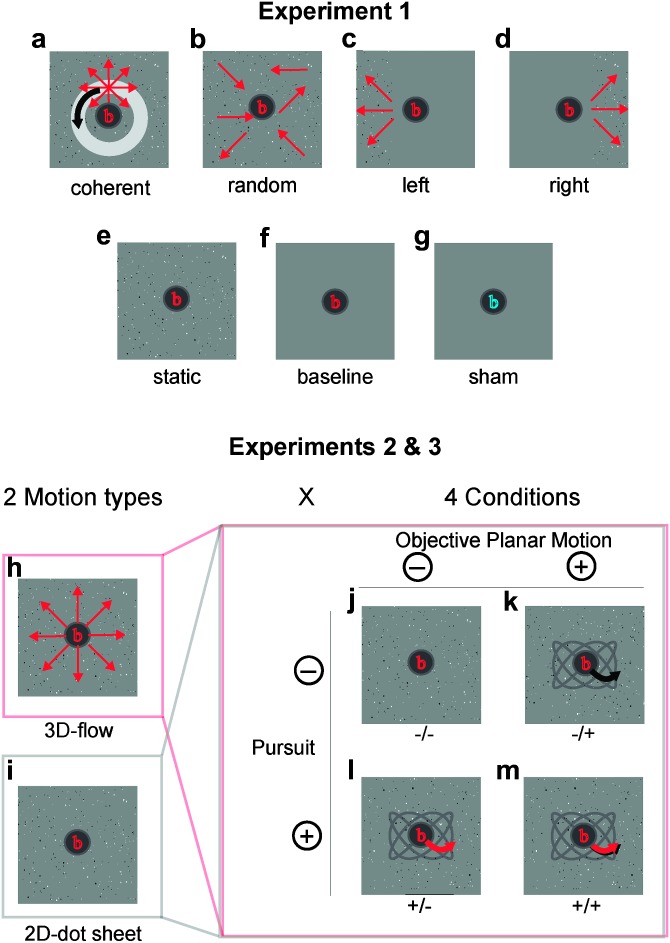
This experiment contained 7 conditions that allowed us 1) to localize regions of interest (ROIs) and 2) to characterize the responses that were not used to localize them. The fixation task was present at all times, except for one (baseline) condition. The conditions were as follows (Fig. 1a–g)
Figure 1.
Stimuli used for experiments 1 to 3. (a–g) Stimuli used in Experiment 1. (a) Full-field coherent motion of a random dot pattern with 3D flow (expansion/contraction) and added circular planar motion (including the focus of expansion). (b) Trajectory-matched random motion. (c and d) Left/right hemifield 3D motion in the left/right thirds of the screen. (e) Static condition. (f and g) Baseline conditions with and without attention task. (h–m) Stimuli of Experiment 2 (and 3). (h and i) A 3D expanding/contracting flow field and an equivalent 2D dot sheet (lacking the expansion flow component) were used as baseline stimuli. (j–m) A 2 × 2 factorial design with the factors pursuit (i.e., movement of the fixation task on the screen on a sinusoidal path [red arrow on a gray path]) and “objective planar motion” (i.e., planar motion of the dot-cloud on the screen [black arrow on the gray path]). In expt. 3, pursuit and motion were limited to the x-dimension. When both factors were “on” (i.e., +/+ condition), the motion of the fixation path locked to the planar motion of the dot-cloud. In the ±/± notation of conditions, the first sign refers to the presence (+) or absence (−) of pursuit, the second to that of objective planar motion. A 1-back character matching task was presented on the fixation disk at all times in all experiments.
(1) 3-D full-field motion (coherent motion): The 3D flow pattern was generated by modeling the forward and backward movement of dots that were uniformly (randomly) distributed in 3D space (with no lateral limits), with the depth of visibility ranging from 0.4 to 2.40 m distance to the observer. The dot size was scaled (inversely) as a function of distance, within 0.1 and 1.1°. The resulting 3D motion created a strong feeling of self-motion. Forward and backward flow alternated with a period of 12 s (=trial duration) such that both occurred within each trial. The velocity varied with the function of abs(sin(t))(1/3) to ensure both, fast yet not abrupt transitions between forward and backward motion. The 1/3 power for the speed function had the consequence that dots were faster than half of the maximal speed during 92% of the time (compared with 66% of the time using a normal sine function) and faster than 75% of the maximal speed during 72% of the time (compared with 46% with the sine). The maximal (mean) speed of forward and backward motion in the simulated 3D space was 1.80 (1.48) m/s (6.5 (5.3) km/h). This resulted in dot speeds on the display ranging between 2.3 and 32.5 degree/s with a mean (median) of 8.6 (8.1) degree/s, depending on the time of the alternating velocity trajectory and on the 3D dot position within the simulated 3D cloud. In addition, translational motion was added by moving the focus of expansion on a fixed circular path (as described below in experiment 2).
(2) Random motion: a trajectory- (and speed-) matched random motion stimulus was created by scrambling dot positions and randomly mirroring x- and y-motion directions for every dot of the coherent motion stimulus.
(3, 4) left/right hemifield motion: to separate MST from V5/MT on the basis of MST’s (but not V5/MT’s) response to ipsilateral motion, we displayed the left third or the right third of the coherent motion stimulus in conditions (3) and (4), with the remainder of the screen consisting of static dots, similar to procedures reported before (Huk et al. 2002; Smith et al. 2006). The static dots were displayed as in condition (5).
(5) static: the static stimulus contained stationary dots taken from a snapshot of condition (1), but every frame 4% of the dots were redrawn at random positions to match the rate of appearance/disappearance of dots of the 3D flow stimulus. Note that this redrawing frequency did not induce any percept of motion or apparent motion.
(6, 7) Baseline conditions: two baseline conditions were added. (6) was a gray screen with the central fixation task. (7) was the same as (6) but without the subject carrying out the fixation task. Here, the central character was displayed in a distinct color (blue instead of red) to indicate that subjects did not have to carry out the serial character back-matching task but simply fixate.
Experiment 2: 2D Versus 3D Motion and Pursuit, Retinal, and Objective Motion
The aim of this experiment were 2-fold: first, to compare responses to 3D flow (expansion/contraction of the dot pattern) with responses to 2D planar motion; second, to segregate responses to planar “objective” motion (i.e., planar motion on the screen) from those to planar “retinal” motion. To achieve the second aim, we used objective planar motion that was or was not nulled (in retinal terms) with smooth pursuit (see Fig. 1j–m). The 4 possible combinations of objective planar motion (on or off) with smooth pursuit (on or off) allowed us to induce retinal planar motion in the presence or absence of objective planar motion and, vice versa, to induce objective planar motion in the presence or absence of retinal motion. On its own, this constituted a 2 × 2 factorial design with the factors planar objective motion (on or off) and pursuit (on or off). To achieve the first aim, a third factor was introduced by either adding or not adding 3D flow to all conditions, thus yielding a 2 × 2 × 2 factorial design with 8 conditions (Fig. 1h,i). This allowed us to independently estimate responses to planar retinal or planar objective motion, in the presence or absence of 3D flow. Additionally, this allowed us to use a subset of the conditions in order to compare responses to pure objective 2D planar motion with responses to pure 3D flow and to combinations of both (in the absence of pursuit).
It is important to note that the factor “planar objective motion” and “planar retinal motion” (the latter being the interaction of planar objective motion with pursuit) were entirely controlled and free of confounds, as both contained equally many pursuit and nonpursuit conditions. The factor “pursuit” was also counterbalanced with respect to the other factors, but it included several potential contributors (like in most studies involving pursuit): nonretinal signals such as efferent copies of eye movements and potentially also far peripheral visual planar motion signals originating from off-screen residual light in the scanner bore during visual pursuit. We report results relating to all factors for completeness but concentrate on planar retinal motion and planar objective motion, emphasizing here the multiple sources contributing to “eye movements.”
The 3D flow was the same as described above, superimposed on the planar component. Pursuit was controlled by moving the fixation task within a third of width and height of the screen with an unequal number of cycles of sinusoidal displacement per trial in horizontal and vertical axes, respectively (randomly assigned 3 or 4 cycles, each with random initial phases). This resulted in planar motion of the fixation task with smooth transitions between all directions, with speeds ranging from 0.1 to 11.5 degree/s, with a mean (median) speed of 3.8 (3.8) degree/s. Planar objective motion of the dot field was governed by the same variables, resulting in planar motion of the 2D dot field or in a planar displacement (including the center of expansion) of the 3D dot cloud. When both pursuit and planar objective motion were “on,” the 2 were coupled, such that the fixation task moved locked together with the dots, resulting ideally in zero planar retinal motion in case of the 2D conditions and in pure 3D flow in case of the 3D conditions.
The main effects of the 2 factors and their interaction allowed us to disentangle responses associated to 3 types of motion: eye movements (pursuit on vs. off), “objective motion” (planar objective motion on vs. off), and planar retinal motion (interaction of the above factors: planar retinal motion was present in (−/+) and (+/−) conditions but absent in (−/−) and (+/+) conditions) in context of a general linear model (GLM) analysis (calculated separately for 2D and 3D stimuli).
Experiment 3: Replication of Experiment 2
In the pursuit, experiment 2D planar motion and 3D flow were each independently made visually salient to optimize analyses within 2D or within 3D conditions. For direct comparisons between selected 2D versus selected 3D conditions, there would, however, be 2 potential confounds: 1) 2D and 3D mean dot speeds were not precisely matched and 2) 2D motion was circular, therefore presenting any given screen location with many different motion directions over time, while 3D flow alternated between forward and backward motion, therefore presenting only 2 motion directions at a given screen location, thus potentially exerting different loads on mechanisms of neural adaptation in the 2 conditions. In order to control for this, 7 subjects (3 males, 4 females, age 24–35 years) were tested in experiment 3 that was identical to experiment 2 but with matched motion parameters between 2D and 3D motion. Here, 2D planar motion alternated between left-right motion, with the same speed trajectory used for the 3D forward–backward flow. Within one trial of 12 s, 2D and/or 3D motion alternated with 4 cycles. The mean (median) dot speeds for 3D flow were 3.2 (2.3) degree/s and for 2D planar motion 3.3 (2.3) degree/s. In addition, the starting direction was randomized for each stimulus type and each trial. Note that 3D flow was considerably (about 4×) slower in experiment 3 compared with experiment 2.
Image Acquisition
Anatomical T1-Weighted Images as well as Functional Gradient-Echo Echoplanar
-weighted images (EPI) with blood oxygen level–dependent contrast were acquired on a Siemens TIM 3-T scanner with a 12-channel phased-array head coil (Siemens, Erlangen, Germany). The EPI sequence had a repetition time of 2300 ms, an echo time of 40 ms, a flip angle of 90°, a field of view of 192 × 192 mm, and a matrix size of 64 × 64 pixels. Each functional image consisted of 32 slices, with a thickness of 2.6 mm and 0.4 mm gap, resulting in a voxel size of 3 × 3 × 3 mm. Sessions of experiment 1 consisted of 226 functional volumes and lasted 8.4 min, sessions of experiments 2 and 3 consisted of 176 functional volumes and lasted 6.4 min. The first 4 images of each scanning session were discarded as dummy volumes to allow for equilibration of T1 signal. A high-resolution anatomical scan was also obtained for each observer with a T1-weighted magnetization prepared rapid gradient echo sequence of 1 × 1 × 1 mm resolution.
fMRI Data Preprocessing
All data were processed using the SPM5 software package from the Wellcome Department of Imaging Neuroscience (www.fil.ion.ucl.ac.uk/spm/). Prior to statistical analysis, functional images were resliced to correct for the acquisition time lags and realigned to the first image to compensate for head motion. The structural image was coregistered to the mean functional image and then segmented for purposes of bias correction, spatial normalization, and tissue classification. The normalization parameters were used to spatially normalize the functional images. Functional images were convolved with a Gaussian Kernel of 6-mm full-width at half-maximum for single-subject whole-brain analyses, and ROI data were extracted from single-subject analyses carried out on nonsmoothed images.
Statistical Analysis
Each subject was analyzed separately using the GLM. The data was high-pass filtered using a 128 s cutoff to remove low-frequency signal drifts. The design matrix included one regressor for each condition, modeled using a boxcar function convolved with the canonical hemodynamic response function. Button presses were modeled separately as events, and the 6 realignment parameters obtained from the motion correction were included to remove variance explainable by head motion.
We report single-subject results as voxel-wise statistical maps, thresholded at P < 0.05 FWE corrected, as well as random effects group level (RFX) statistics of beta estimates extracted from each ROI and averaged across subjects, with analyses of variance (ANOVAs) and t-tests performed across n hemispheres.
In order to extract the relative contribution of the responses to the 3 types of motion (planar retinal motion, planar objective motion, and eye movements) contained in the 2 × 2 conditions of experiments 2 and 3, we entered the 4 beta estimates extracted from each ROI into a second-level GLM with the following regressors (notation: (pursuit/objective motion), see also Fig. 1j–m): retinal motion: (+/−) and (−/+) versus (−/−) and (+/+), real motion: (−/+) and (+/+) versus (−/−) and (+/−), and eye movement: (+/−) and (+/+) versus (−/−) and (−/+).
Definition of ROIs
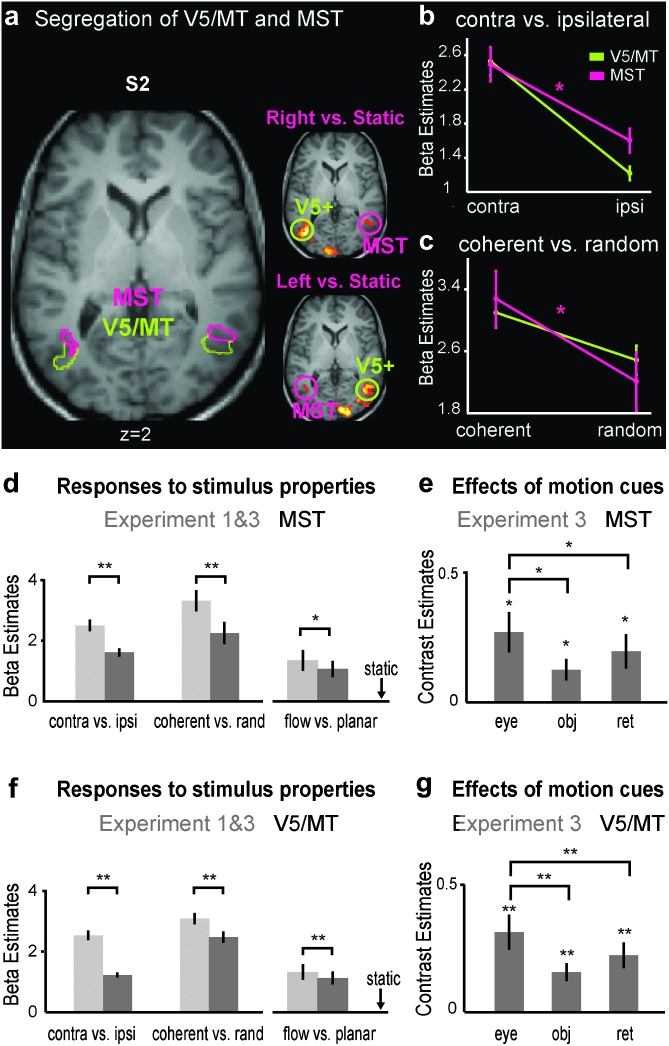
We used experiment 1 to identify ROIs in each subject separately as follows. MST was defined as the ipsilateral response in lateral occipital cortex near the ascending limb of the inferior temporal sulcus (Dumoulin et al. 2000) during hemifield coherent motion presentation (compared with static dots) as described before (Huk et al. 2002; Smith et al. 2006). Region MT/V5 was defined as contiguous set of voxels in the same region, activated in the contrast of coherent motion versus the static condition, excluding voxels belonging to MST (see Fig. 2). CSv was localized comparing responses to coherent versus random motion, similar to the method of Wall and Smith (2008) (see Fig. 3). The mean coordinates of all ROIs ± standard deviation across subjects are given in Table 1.
Figure 2.
Localization and responses of areas V5/MT and MST. (a) Single-subject example of ipsilateral and contralateral responses to hemifield stimuli, used to segregate MST from V5/MT in experiment 1 (P < 0.05 FWE corrected) (right MST is located in a protrusion of gray matter). (b) Contra- and ipsilateral responses in V5/MT and MST (note that the difference in ipsilateral responses between V5/MT and MST is a result of the ROI definition) (ANOVA, interaction: F1,19 = 7.88, P = 0.01). (c) Responses to coherent versus trajectory-matched random motion. This contrast reveals a segregation of V5/MT from MST (2-way ANOVA, interaction: F1,19 = 6.15, P = 0.023). (d and f) Responses to distinct motion conditions after subtraction of the baseline conditions (static condition for conditions of experiment 1 or 2D (−/−) for conditions of experiment 3). The x-axis is broken before the flow versus planar conditions to indicate the distinct experiment. (e and g) Mean contribution of eye movements, objective motion, and retinal motion in experiment 3 (i.e., main effects and interaction of the 2 factors pursuit and planar motion, see Materials and Methods), averaged across 2D and 3D conditions. See Supplementary Figure S1b for similar results in experiment 2. All results are shown as mean ± standard error; n = 20 hemispheres (for e and g: 14 hemispheres); *: P < 0.05; **: P < 0.001.
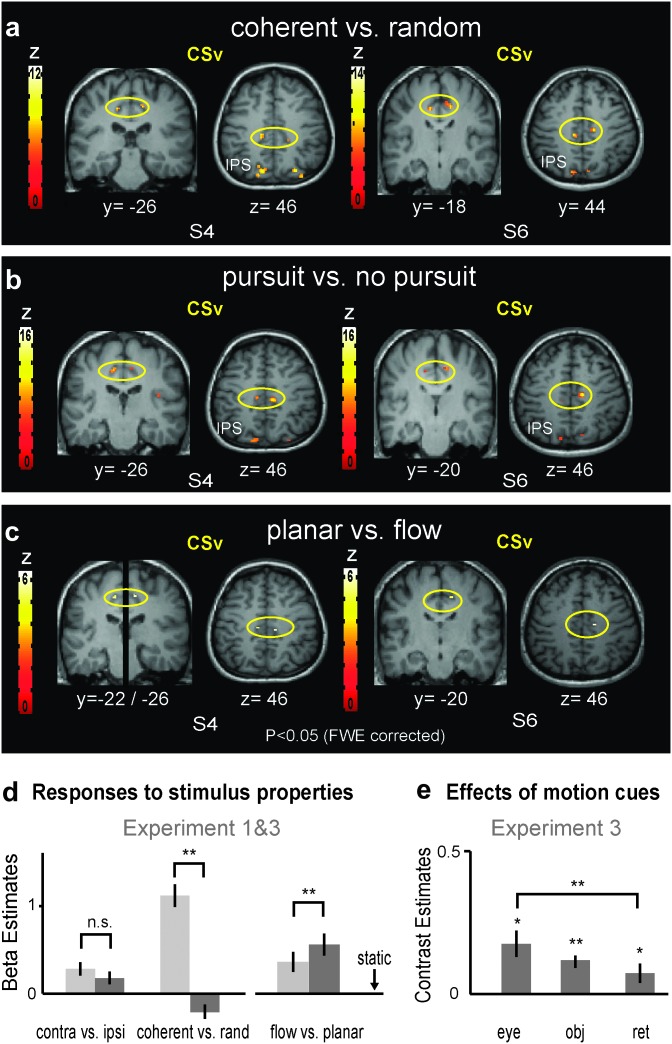
Figure 3.
Localization and responses of the CSv. (a–c) Localization of CSv by different functional contrasts, shown in coronal and axial views for 2 single subjects (P < 0.05, FWE corrected). (a) Contrast of coherent versus random motion. (b) Contrast of pursuit conditions (+/−) and (+/+) versus nonpursuit conditions (−/−) and (−/+), averaged for 2D and 3D stimuli. (c) Contrast of 2D planar motion versus 3D flow during fixation (i.e., 2D−/+ vs. 3D−/−). (d) Mean parameter estimates of CSv for conditions of experiments 1 and 2. CSv had a weak response to hemifield stimulation, lacked a significant preference to contralateral stimulation and preferred 2D planar motion to 3D expansion flow in experiment 3. Note that this ROI was localized in the contrast coherent versus random: for this contrast, the beta-estimates thus serve as illustration only. However, all results remained unchanged when CSv was localized using pursuit versus no pursuit (not shown). All responses are shown after subtraction of the baseline (static condition or 2D(−/−), as described for Fig. 2d). (e) Mean contribution of eye movements, objective motion, and retinal motion as measured in experiment 3 (see Supplementary Fig. S1b for similar results in experiment 2). Mean ± standard error; n = 21 hemispheres (flow vs. planar and panel e: 13 hemispheres); *: P < 0.05; **: P < 0.001.
Table 1.
Mean Montreal Neurological Institute coordinates of the ROIs identified in this study (±SD across subjects, n = 11)
| Areas | Left | Right | ||||
| MST | −48 ± 6 | −68 ± 4 | 5 ± 4 | 50 ± 6 | −64 ± 3 | 4 ± 4 |
| MT | −45 ± 8 | −69 ± 4 | 7 ± 6 | 48 ± 6 | −66 ± 3 | 6 ± 5 |
| CSv | −13 ± 3 | −26 ± 5 | 42 ± 3 | 13 ± 3 | −26 ± 8 | 45 ± 3 |
Results
In the following, we compare the degree to which V5/MT, MST, and CSv responded to coherent versus random motion, 3D flow and 2D planar motion, hemifield stimulation, pursuit signals, planar retinal motion, and planar objective motion. It is important to note that in cases where stimuli were used to localize regions (such as hemifield responses separating MT/V5 from MST), the corresponding contrasts are shown for illustration only, as noted in the text.
Eye Movements
During scanning, all subjects performed the central fixation task near ceiling and there were no differences of performance between conditions, suggesting that attention and fixation were balanced across conditions. The eye tracking results from experiment 3 were consistent across subjects, with all subjects maintaining high fixational accuracy in all conditions (see Table 2). Three-way ANOVAs with factors pursuit (on/off), planar objective motion (on/off), and motion type (2D/3D) were carried out for eye-position and eye-velocity signals. There was no significant effect for any interaction (notably not for the one corresponding to planar retinal motion), nor for the factor objective motion, nor for “stimulus type” (2D vs. 3D), neither within nor across subjects, for either of the measures (eye position or eye velocity). There was however a small but significant increase of the RMS error for eye position during pursuit compared with nonpursuit conditions (see Table 2; F1,41 = 113.878; P < 0.001). This was not observed for eye velocity. In view of the functional data presented below, estimates of planar retinal motion and planar objective motion were therefore not affected by eye-movement differences. Note also that both were balanced in pursuit- and nonpursuit conditions. Also, estimates for 2D versus 3D conditions were not affected by eye movements. Thus, the only estimate influenced by fixational accuracy was the estimate for eye movements (i.e., pursuit vs. nonpursuit conditions). We nevertheless report cortical responses for the factor eye-movements for completeness (even though this is not central to this manuscript) and emphasize that this factor—in contrast to the others—includes several potential contributors: effects of nonretinal signals such as efferent copies, retinal signals from motion in the periphery outside the display (due to weak but unavoidable residual light in the scanner bore), and retinal signals due to the above small increase in fixational jitter. Since all these effects are also associated to eye movements in real-world situations (or in most experimental settings using open-eye pursuit), cortical responses to eye-movements should be taken to reflect all the above components and not only nonretinal pursuit signals.
Table 2.
Fixation accuracy expressed as RMS deviation between eye position and fixation cross and as RMS deviation between eye velocity and stimulus velocity for different experimental conditions of experiment 3 (in visual degrees ± standard error of the mean, n = 7 subjects)
| RMS | −/−: fixation, no obj | −/+: fixation, obj | +/−: pursuit, no obj | +/+: pursuit, obj |
| 2D:distance [degree] | 1.023 ± 0.078 | 1.097 ± 0.077 | 1.329 ± 0.063 | 1.341 ± 0.042 |
| 2D:velocity [degree/s] | 2.820 ± 0.1819 | 3.000 ± 0.1860 | 2.832 ± 0.103 | 2.899 ± 0.090 |
| 3D:distance [degree] | 1.096 ± 0.085 | 1.086 ± 0.062 | 1.351 ± 0.065 | 1.268 ± 0.054 |
| 3D:velocity [degree/s] | 2.953 ± 0.192 | 2.899 ± 0.153 | 2.691 ± 0.108 | 2.700 ± 0.088 |
V5/MT and MST: Segregation in Lateralization, Coherence, and Pursuit
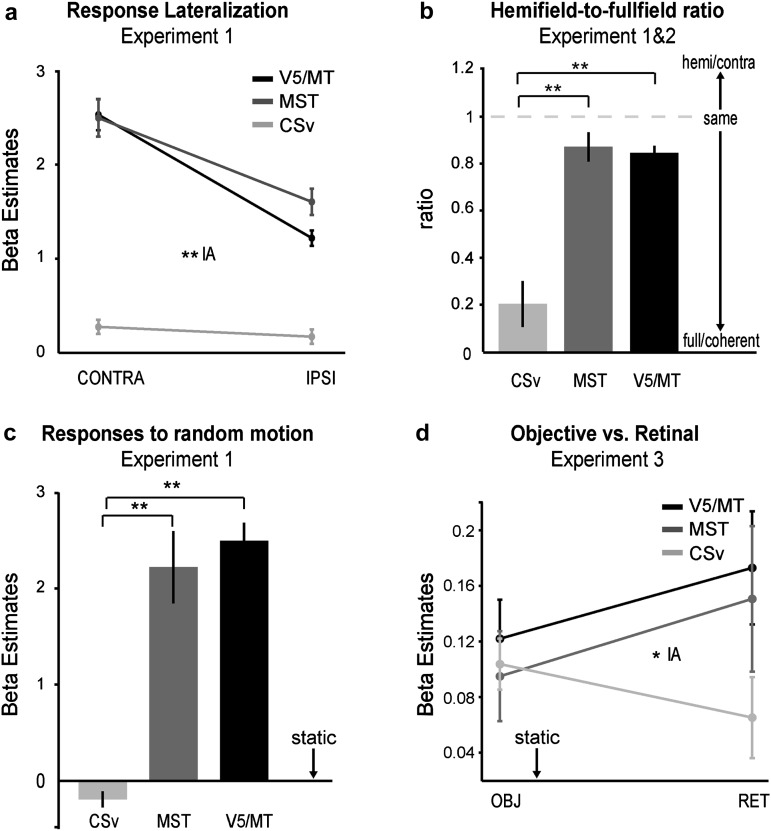
First, we examine results obtained for V5/MT and MST in context of experiment 1, which primarily confirmed prior findings related to these 2 regions. The hemifield stimulation allowed us to segregate V5/MT from MST based on the stronger ipsilateral response of MST(d) in 20 of 22 hemispheres in 11 subjects (Huk et al. 2002; Smith et al. 2006) (see also Materials and Methods). Figure 2a illustrates the spatial segregation of V5/MT and MST for a single subject, and Figure 2b shows the mean responses to contralateral and ipsilateral stimulation in the 2 ROIs across all hemispheres. Despite the selection of MST for a high ipsilateral response, both V5/MT and MST had a strong preference for contralateral compared with ipsilateral motion stimuli (V5/MT: t19 = 10.63, P < 0.001; MST(d): t19 = 7.21, P < 0.001). Similarly, despite the selection of V5/MT on the basis of its preference to coherent motion over static dots, area MST had a greater “increase” to coherent motion compared with V5/MT (Fig. 2c), which confirms prior work (Morrone et al. 2000; Smith et al. 2006). This was reflected in an interaction between area and stimulus (coherent vs. random) in a 2-way ANOVA (F1,19 = 6.15, P = 0.023). Note though that both areas preferred coherent versus trajectory-matched random motion (V5/MT: t19 = 5.032, P < 0.001; MST(d): t19 = 4.930, P < 0.001) and that both showed a strong response to random motion compared with the static baseline (V5/MT: t19 = 12.72, P < 0.001; MST(d): t19 = 6.60, P < 0.001).
In order to compare responses to 3D flow with those to 2D planar motion, we compared 2 conditions from experiment 3, both of which involved central fixation without pursuit. These were 3D flow (i.e., 3D(−/−)) and 2D planar motion (i.e., 2D(−/+)), which were carefully matched in dot speeds and alternation profiles. Three-dimensional flow involved alternating expansion/contraction from the fixation point and 2D planar motion involved rigid translation alternating between left- and rightwards directions (see Fig. 1). Figure 2d,f show that both V5/MT and MST significantly preferred 3D flow over 2D planar motion (t-test: MST: t13 = 2.82, P = 0.014; MT: t13 = 3.09, P = 0.008). In experiment 2 (where dot speeds were not precisely matched between the 2 conditions), there was no significant response difference between these 2 motion types (see Supplementary Fig. S3).
Finally, we examined the responses of each ROI to eye movements, planar objective motion, and planar retinal motion (i.e., the main effects and their interaction in experiments 2 and 3, see Materials and Methods). Figure 2e,g show that both V5/MT and MST responded significantly to all 3 types of motion and with the same relative preferences to the different motion cues (shown for experiment 3). Both ROIs responded most to eye movements, followed by planar retinal motion, with the smallest response to planar objective motion. The same was true in experiment 2 (that had more subjects), with responses of both regions being significantly larger for planar retinal motion compared with planar objective motion (V5/MT: t19 = 3.04, P = 0.007; MST(d): t19 = 2.43, P = 0.025; see Supplementary Fig. S1b). This was true for averaged 2D and 3D analyses, as well as for the separate 3D conditions in both regions, and for 2D in V5/MT, with MST having the same trend (V5/MT 2-D: t19 = 2.41, P = 0.026; 3D: t19 = 3.15, P = 0.005; MST(d) 3D: t19 = 2.12, P = 0.047; see Supplementary Fig. S1 for separate plots of 2D and 3D conditions).
CSv: Preference for Objective Motion and Planar Motion
CSv was localized in 21 of 22 hemispheres (with P < 0.001 uncorrected) in experiment 1 on the basis of its preferred response to coherent motion compared with trajectory-matched random motion (like V5/MT above), thus confirming its functional preference to coherent motion as described earlier (Antal et al. 2008; Wall and Smith 2008). Its coordinates (−13 ± 3, −26 ± 5, 42 ± 3; 13 ± 3, −26 ± 8, 45 ± 3; n = 21) overlap with those described by Antal et al. (2008) (−12, −24, 39; 10, −28, 42; n = 10) and with the anatomical position shown by Wall and Smith (2008) (coordinates not given). Figure 3a shows examples for 2 subjects (P < 0.05, FWE corrected). The responses of CSv to the various different motion types are quantified in Figure 3d across all subjects.
First, and in contrast to V5/MT and MST, we found that CSv did not significantly prefer contralateral stimulation over ipsilateral stimulation, even though there was a trend (see Fig. 3d). This indicates that CSv has either a nonretinotopic organization or that its RFs are very large, spanning across both hemifields. Note that the hemifield stimuli were identical in nature (but restricted to the lateral outer third of the full field) to those used to localize CSv in this as well as in previous studies. This makes it unlikely that the stimuli were simply suboptimal in nature for CSv. Its highly distinct organization in terms of retinotopy or RF field size was additionally apparent in the much-enhanced response to full-field stimuli in comparison to contralateral hemifield stimuli (Fig. 3d).
The particularities of the spatial stimulus preferences of CSv were accompanied by particularities in its preference for stimulus type (see Fig. 3d). CSv lacked any significant response to random motion, which was also reported by Antal et al. (2008). Note that this cannot be accounted for by random motion being part of the contrast defining this ROI, as it also held true when CSv was defined using other contrasts such as pursuit versus no-pursuit (not shown).
Secondly, using data of experiment 3 whose stimulus parameters were carefully matched between 2D planar motion and 3D flow, we found that while CSv responded well to both stimuli, it showed a strong preference for our 2D planar objective motion stimuli (i.e., 2D(−/+)) compared with 3D flow (i.e., 3D(−/−)) (both in the absence of pursuit): t12 = 5.732, P < 0.001 (see Fig. 3d). CSv responses were 40% higher to 2D planar objective motion compared with 3D flow. This preference was even stronger in experiment 2 (where stimulus properties were not precisely matched), with a significance of t20 = 7.75, P < 0.001 (see Supplementary Fig. S3). Again this finding cannot be explained by the localizer contrast that was used to define CSv, as the localizer contained 3D flow and planar motion, was recorded in a separate session, and was the same used to localize V5/MT and MST that did not share this preference, neither in experiment 2 or 3. CSv’s preference for 2D planar motion was sufficiently pronounced in every subject so that the contrast 2D planar motion versus 3D flow activated CSv in each hemisphere with P < 0.001 (uncorrected), allowing for a reliable localization of CSv in 13 of 14 hemispheres in experiment 3 and in 21 of 22 hemispheres in experiment 2 (see Figs 3c and 5b for single subject examples).
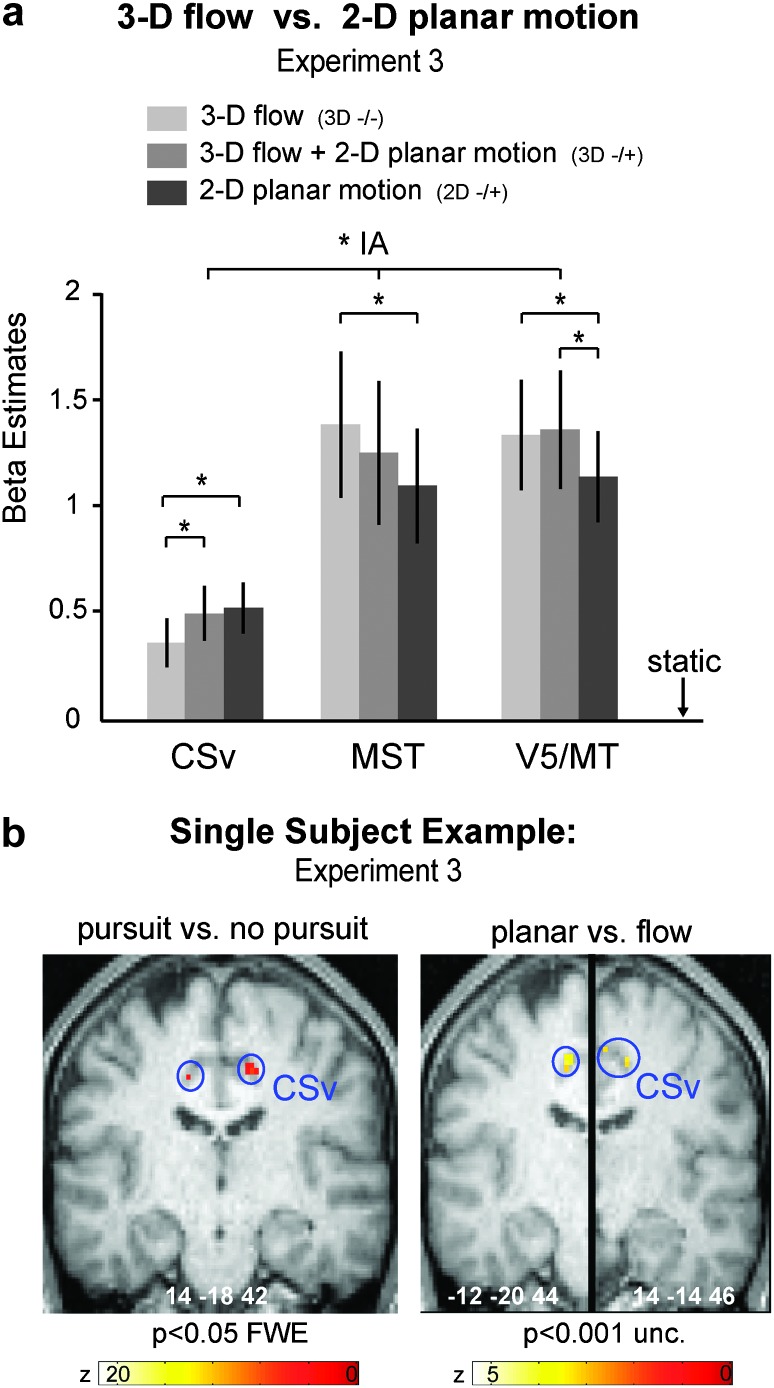
Figure 5.
Double dissociation between CSv, V5/MT, and MST in their responses to 2D planar motion and to 3D flow. (a) Responses of CSv, V5/MT, and MST in experiment 3 for the following conditions: 3D flow (−/−), 3D flow with superimposed 2D linear translation 3D(−/+), and pure linear 2D planar translation (2D−/+) (all without pursuit). As shown in Figure 3d, CSv preferred 2D planar motion to 3D flow (P = 0.016, Bonferroni corrected). Its activation was lowest for pure 3D flow and increased when planar motion was added (t12 = 2.21, P < 0.05). In contrast, V5/MT and MST preferred 3D flow to 2D planar motion (P < 0.05, Bonferroni corrected). The area × stimulus interaction was significant (2-way ANOVA: F4,48 = 6.14, P = 0.012). The same was true for experiment 2 (see Supplementary Fig. S3; F4,76 = 20.63, P = 0.001). (b) Single-subject example for localization of CSv using the contrast pursuit versus nonpursuit (left) and 2D planar translation versus 3D flow (right). *: P < 0.05, Bonferroni corrected.
Figure 3e shows the responses of CSv to the 3 motion types in experiment 3: eye movements, planar objective motion, and planar retinal motion (pooled across 2D and 3D conditions that each showed a similar response profile, shown in Supplementary Fig. S1b). Similar to V5/MT and MST, CSv responded most to eye movements, but it differed quantitatively and qualitatively from the V5+/MT+ complex in that its second strongest response was that to planar objective motion, with the least response to planar retinal motion. These results were very similar in experiment 2 (with more subjects) as illustrated in Supplementary Figure S1b. Here, CSv’s response preference for planar objective motion compared with planar retinal motion was significant for the 2D conditions (t20 = 2.75, P = 0.012) and reached a trend for the combined 2D and 3D conditions (t20 = 1.97, P = 0.061) (see Supplementary Fig. S1b for separate plots). We conclude from these findings that CSv has a preference for full-field 2D planar motion, with a small preference for planar objective (real) motion compared with planar retinal motion.
Figure 3b shows single-subject examples illustrating that CSv could also be localized (and significantly activated, with P < 0.001 uncorrected) in 19 of 21 hemispheres by contrasting conditions involving pursuit versus nonpursuit, for example, (+/−) and (+/+) versus (−/−) and (−/+) in experiment 2. The same was true for 13 of 14 hemisphere in experiment 3.
Direct Comparisons between V5/MT, MST, and CSv
The above results indicate substantial differences between CSv compared with V5/MT and MST across virtually all properties we examined. In the following, we compare these regions directly.
First, we compared the degree of lateralization (i.e., preference for contralateral stimulation vs. ipsilateral stimulation) between CSv, MST, and V5/MT (Fig. 4a). Since MST was defined using its ipsilateral response, there may be a selection bias toward a stronger ipsilateral response in MST, that is, toward less lateralization. Despite this, a 2-way ANOVA with the factors area (MST, V5/MT, and CSv) and stimulation side (ipsi and contra) revealed a significant interaction, indicating that each, V5/MT and MST, had a stronger preference for contralateral stimuli than CSv (F2,38 = 37.35, P < 0.001). In addition to the lack of lateralization, CSv also had a stronger preference for full-field stimuli. Figure 4b shows that MST and V5/MT had nearly equal responses to full field and to contralateral hemifield stimuli, while CSv responded only a fourth in magnitude to hemifield stimuli compared with full-field stimuli. Plotted is the ratio of the responses to contralateral hemifield motion to those of full-field coherent motion, after subtraction of the baseline response. CSv had thus a stronger preference for full-field stimuli compared with V5/MT (2-sample t-test: t39 = 5.72, P < 0.001) and with MST(d) (t39 = 6.17, P < 0.001).
Figure 4.
Functional comparisons between CSv, V5/MT, and MST. (a) CSv responses were significantly less lateralized than those of V5/MT and MST. (b) CSv had a more pronounced preference for full-field stimuli compared with V5/MT and MST, here shown as ratio of responses for contralateral hemifield to full-field stimulation. (c) Responses to random motion in CSv, V5/MT, and MST. (d) Dissociation between CSv versus V5/MT and MST in their responses to retinal planar motion and to objective planar motion, averaged for 2D and 3D stimuli (2-way ANOVA with factors area and motion type), shown for experiment 3. Interaction: F2,24 = 5.72, P = 0.02. The same held true in experiment 2, shown in Supplementary Figure S2a: F2,38 = 8.35, P = 0.003. Mean ± standard error; n = 21 (a–c) or n = 13 (d) hemispheres; *: P < 0.05, **: P < 0.001.
While the above comparisons concerned preferences to stimulus size and location, we thirdly compared the responses to distinct motion types.
Figure 4c shows that CSv had a significantly reduced response to random motion compared with MST and V5/MT (V5/MT vs. CSv: t39 = 13.22,P < 0.001; MST(d) vs. CSv: t39 = 6.42, P < 0.001—note that this also held true when CSv was localized independently, using pursuit vs. no pursuit).
Fourth, we tested whether the respective preferences to planar objective motion (CSv) or to planar retinal motion (V5/MT and MST) led to an interaction between our ROIs and motion type in experiment 3 (see Fig. 4d). We thus performed a two-way ANOVA on the factors area (CSv, V5/MT, MST) and motion type (planar objective motion vs. planar retinal motion). The ANOVA revealed a significant interaction between area and motion type, with opposing preferences in CSv versus V5/MT and MST (see Fig. 4d; F2,24 = 5.72, P = 0.02). The same was true for experiment 2, shown in Supplementary Figure S2a (F2,38 = 8.35, P = 0.003). This shows that CSv has an enhanced capability to take eye movements into account compared with the V5+/MT+ complex, thus enabling it to respond more to planar objective motion and to discard more of the planar retinal motion. In fact, also simple comparisons revealed this. For example, comparing pursuit on a stationary background versus pursuit on a comoving background (i.e., 2D+/− vs. 2D+/+) essentially pins retinal motion without objective motion (2D+/−) versus objective motion without retinal motion (2D+/+), both during pursuit. Supplementary Figure S2b shows for experiment 2 that CSv responded more to objective motion, and both, V5/MT and MST more to retinal motion, leading to a significant interaction between area and condition (F2,38 = 5.08, P = 0.019).
Finally, we compared responses to 2D planar motion with those to 3D flow (both in the absence of pursuit) across CSv, MST, and V5/MT. Figure 5 reports these results for experiment 3 in which low-level features such as dot speed, direction reversals, and reversal rate were matched between 2D and 3D motion stimuli. Figure 5a plots responses of each ROI to pure 3D flow (i.e., 3D −/−), to 3D flow with added planar motion (i.e., 3D −/+), and to pure planar motion (i.e., 2D −/+). There is a clear double dissociation in that CSv responds most strongly to 2D planar motion and least for 3D motion (with combined 2D + 3D motion in the middle), while the opposite was true for both V5/MT and MST. A 2-way ANOVA with the factors area (CSv, V5/MT, MST) and stimulus (3D, 2D + 3D, 2D) confirmed this double dissociation statistically in form of a significant interaction between area and stimulus (F4,48 = 6.14, P = 0.012). Post hoc comparisons confirmed the stronger response of CSv for 2D planar motion compared with 3D flow and vice versa for V5/MT and MST (P = 0.0163 for CSv, P = 0.04 for MST, and P = 0.032 for V5/MT, all Bonferroni corrected), as reported for each area separately in Figures 2d,f and 3d. Supplementary Figure S3 reports the corresponding results for experiment 2, also revealing a significant interaction between area and motion type (F4,76 = 20.63, P = 0.001), with CSv preferring 2D motion over 3D flow (P < 0.001, Bonferroni corrected) while V5/MT and MST showed no preference between 2D and 3D stimuli. For CSv, the combined 2D and 3D stimuli evoked significantly more activity than pure 3D flow (t20 = 7.523, P < 0.01) and significantly less activity than 2D planar motion (t20 = 7.75, P < 0.001). Figure 5b shows single-subject examples for the localization of CSv using the contrast planar 2D motion versus 3D flow in experiment 3 and Figure 5c for the contrast of pursuit conditions versus nonpursuit conditions (corresponding to those of experiment 2 shown in Fig. 3b,c).
Discussion
In this study, we characterized the functional responses of CSv to a set of fundamental visual motion cues, including those related to visual pursuit and compared them with those of the well-established areas V5/MT and area MST(d). We found that CSv differed in every aspect so substantially from both V5/MT and MST that the functional properties of the latter 2 appeared very similar by comparison.
There was a dramatic difference in the size and location of preferred stimuli for CSv and V5+/MT+. While both V5/MT and MST strongly preferred contralateral stimulation, CSv showed only a marginal and nonsignificant preference for contralateral stimulation. In addition, CSv responded several-fold stronger to full field compared with contralateral hemifield stimulation, while V5+/MT+ regions responded about equally strong to both. CSv also differed in its stimulus preference. CSv responded exclusively to coherent motion types, with a strong preference for 2D planar objective motion compared with 3D flow stimuli, while V5+/MT+ showed the opposite preference in experiment 3 and no preference in experiment 2. CSv’s preference for 2D stimuli was robust, in that it persisted across both experiments 2 and 3 that differed in speeds and trajectories of the 2D and 3D stimuli and in that it was observed in every single subject. Adding 3D flow to 2D motion actually led to a decrease in CSv’s response while it increased that of V5/MT and MST. CSv showed no response to random motion, while V5+/MT+ responded substantially. Finally, CSv appeared to be able to integrate eye movements with retinal motion better than V5+/MT+, as it showed a greater response to planar objective motion compared with planar retinal motion, while the opposite was true for V5/MT and MST. In all, the findings suggest that CSv is functionally entirely distinct from V5/MT and MST.
We interpret these findings to be compatible with the idea that CSv is involved in integrating eye movements with planar retinal motion (Olson et al. 1996; Bremmer et al. 2001; Konen and Kastner 2008) that together allow the brain to infer real motion or one’s own position in a stable environment, respectively (Gibson 1954; Galletti and Fattori 2003). CSv appears particularly selective to visual stimuli of the type evoked by eye movements or by lateral head- or body motion.
CSv: Self-motion Processing and Pursuit-Related Cues
Our findings are consistent with previous evidence showing a preference of coherent motion types in CSv (Sunaert et al. 1999; Braddick et al. 2001; Dieterich et al. 2003; Orban et al. 2003; Antal et al. 2008; Wall and Smith 2008). Similar to previous studies is also the striking lack of response to random motion stimuli compared with static controls (Antal et al. 2008). However, our results expand prior knowledge of the CSv region considerably. The lack of lateralization and the preference to full-field stimuli are striking differences when compared with other motion responsive regions such as V5/MT and MST.
One possible explanation could be that CSv contains very large RFs spanning across both hemifields. An alternative account could be that CSv contains small RFs but that it lacks a retinotopic organization, such that RFs falling into either hemifield are located in either hemisphere. The latter account appears unlikely given that no known visually responsive cortical or subcortical area has such properties. The first account appears optimal for processing the type of motion that is induced by pursuit eye movements or by lateral head movements: both induce full-field planar motion. This interpretation is compatible with evidence from macaque neurons in PCC that are responsive to eye movements and whose response latencies indicate a role in visual-sensory feedback rather than control of eye movements (Olson et al. 1996).
Two- Versus Three-Dimensional Flow
Equally striking as the spatial response properties was the strong preference for 2D compared with 3D stimuli in CSv in all experiments, even though we note that CSv responded robustly to both types of motion. V5/MT showed either the opposite preference (experiment 3 with matched 2D and 3D low-level features) or no preference (experiment 2 with semicircular 2D motion and faster 3D dot speed). The 2D preference is also compatible with the above potential role of CSv.
Wall and Smith (2008) showed that CSv responded more to a spiral expansion motion stimulus compared with a panel of 9 smaller versions of the same stimulus, while controlling for attention using a central fixation task, similar to our study. Their spiral expansion stimulus may come nearest to our coherent flow stimulus of the localizer, which combined 3D flow with a circular planar component, and their 9-subfield stimulus to our random motion stimulus, even though our random stimulus was certainly even less coherent. Given these rough correspondences, our results nevertheless allow for a qualitative reproduction of their findings, in that CSv responded much stronger to the coherent motion stimulus compared with random motion, while the difference was not nearly as strong in V5/MT or MST, similar to the results reported by Wall and Smith. Our study extends their findings in suggesting that it was the planar (and not the 3D) component of the coherent motion stimulus that appeared to be the main contributor to activity in CSv. Correspondingly, our interpretation also supports the role of self-motion processing, but our results argue for a specialization to planar self-motion types in CSv.
Antal et al. (2008) reported a preference to 3D motion compared with 2D planar motion in PCC but apparently this preference held only true when the number of activated voxels was tested and not when the response magnitude was examined, as was done here. Importantly, Antal et al. (2008) used a passive viewing paradigm that did not control the level of attention as was done here. Attention may have boosted the responses to 3D motion in the study of Antal et al. (2008), as 3D flow tends to draw considerably more attention compared with other types of stimuli (Franconeri and Simons 2003). Stimulus-driven attention in passive viewing designs has been shown previously to account for apparent responses in motion processing that disappeared after introduction of a distractor task (Huk et al. 2001). As pointed out by Antal et al. (2008), PCC has previously been shown to be modulated by attention to radial motion (Buchel et al. 1998). Given the lack of a response-magnitude difference between planar and flow stimuli in the Antal et al. (2008) study, the potential attentional boost of their 3D-related activity may account for their observation.
The dissociation of response preferences between CSv and V5+/MT+ for 2D and 3D stimuli, replicated across the different motion trajectories and velocities in experiments 2 and 3, suggests a certain robustness of CVs’s preference for 2D stimuli. Furthermore, this preference was not limited to the comparison of 2D versus 3D motion. The responses of all ROIs to the combined 2D plus 3D stimuli tended to lie in-between the responses to pure 2D or 3D stimuli (for CSv in both, experiments 2 and 3), indicating that adding a nonpreferred feature to the preferred feature deprecated the overall response, while adding the preferred feature to the nonpreferred boosted it—all in opposite directions in CSv on one hand and V5/MT and MST on the other hand. This also speaks against CSv being driven by speed differences across the stimuli (or between fovea and periphery in case of the 3D stimuli), as the faster dot speeds resulting from combined 2D and 3D stimuli (compared with the isolated stimuli) resulted in both experiments in responses intermediate to those of the isolated stimuli. These results can only be accounted for by a genuine preference of CSv for planar stimuli over 3D flow, which was demonstrated here in direct comparison to V5/MT and MST for a relatively broad set of stimulus conditions used across both experiments.
An Hypothetical Model for Receptive Fields in CSv
If we assume for a moment that CSv does contain large RFs, the following model may account for the response profile observed here. If large RFs of CSv result from combining inputs from earlier visual motion processing stages, it is conceivable that dissimilar directions inhibit each other or that RFs are built by combining congruent directions only. The reduced response to 3D motion in CSv would then be a consequence of the differential motion directions inherent to 3D motion stimuli. This would also explain why adding 3D flow to 2D planar motion would lead to a reduction in response. The same model would also account for the lack of responses to random motion stimuli, where no coherent directions are present. This model would also be compatible with the spatial response properties observed in CSv (no or weak lateralization, strong preference for full-field stimuli) and suggest that inputs from both hemifields (at least within the 24° stimulated here) sum with nearly equal weight into the response of CSv in each hemisphere. In contrast, RFs in V5/MT and MST are small enough to cover only part of our stimuli, and MST is known to contain not only directionally selective neurons but also neurons responsive to expansion flow, thus likely accounting for the response preference for 3D flow (Desimone and Ungerleider 1986; Tanaka et al. 1986; Duffy and Wurtz 1991; Erickson and Thier 1991; Graziano et al. 1994; Page and Duffy 1999; Thiele et al. 2002). For flow selective neurons in MST, a similar model has been tested using electrophysiology, with the difference that RFs of such neurons in MST are derived of smaller RFs with response preference to planar stimuli of differing, not the same, directions (Yu et al. 2010). Of course the above reasoning and our data do not exclude the possibility that CSv may also contain units responsive to 3D flow, but if so, our data suggest them to form a considerably smaller fraction compared with those in MST and compared with those responsive to planar motion.
Integration of Eye-Movement Signals with Retinal Signals in CSv
Our result of CSv’s enhanced response to planar objective motion relative to planar retinal motion in comparison to MST and V5/MT can only be explained by an enhanced integration of extraretinal signals with visual signals in CSv. Our results do however not resolve whether this integration is performed within CSv or whether it reflects results from upstream processing stages. In the latter case, it is unlikely that these upstream stages are V5/MT or MST, as they showed less integration with extraretinal signals. To answer this question, it would therefore be informative to know whether CSv does receive extraretinal signals. Our study cannot answer this conclusively, since our pursuit versus nonpursuit contrast that activated CSv cannot disentangle extraretinal signals from those potentially induced by peripheral visual ones or from those arising from the small increased fixational jitter during pursuit. Our pursuit results are merely consistent with the non-human findings referred to above and in accord with human fMRI studies investigating pursuit of isolated targets that also reported activity in posterior cingulate cortex, with coordinates overlapping those of CSv (Kimmig et al. [2008]: −12, −22, 38 [n = 12]; Berman et al. [1999]: ±11, −21, 41 [n = 11]). However, Kimmig et al. (2008) took care to eliminate residual light in the scanner bore and provided evidence for activation of CSv by oculo-motor activity alone, in the absence of peripheral retinal motion. Their findings thus suggest that CSv indeed receives (directly or indirectly) nonvisual signals related to eye movements and that these likely also contributed to our less well-controlled pursuit contrast versus nonpursuit contrast. These extraretinal signals may thus provide a basis for our result of CSv’s enhanced response to planar objective motion, indicating that CSv at least has the ingredients to perform the observed integration of retinal with nonretinal signals. The integration of internal position signals with external ones is also consistent with navigational deficits observed in patients having lesions in PCC (Cammalleri et al. 1996; Katayama et al. 1999; Maguire 2001).
Our findings therefore run counter to the suggestion that CSv is primarily concerned with self-motion in depth (Wall and Smith 2008) and support the alternative notion also suggested by previous non-human primate studies that CSv is involved in processing eye movements and related planar visual motion cues (Dean et al. 2004).
V5/MT and MST: Mid-Level Motion Processing
Our results confirmed response properties of and differences between V5/MT and MST with regard to their differential ipsilateral responses (Dukelow et al. 2001; Huk et al. 2002; Smith et al. 2006), the higher response increase to coherent motion in MST compared with V5/MT (Morrone et al. 2000; Goossens et al. 2006; Becker et al. 2008) and the greater modulation of MST by pursuit signals compared with V5/MT (Sakata et al. 1985; Komatsu and Wurtz 1988; Newsome et al. 1988; Goossens et al. 2006). However, the differences between V5/MT and MST were dwarfed in comparison to their differences to CSv. V5+/MT+ had a stronger contralateral response bias, nearly equally strong responses to full field and to contralateral hemifield stimuli, robust responses not only to 2D but also to 3D stimuli, and a response preference to 3D over 2D stimuli, robust response also to random motion, and a higher response to retinal than to objective motion. The multitude and degree of differences suggest that V5/MT and MST are part of a motion processing pathway that either precedes or runs parallel to that encompassing CSv and that they are less specialized in the array of motion types they are responsive to compared with CSv, yielding signals useful for estimates of speed and heading (Duhamel et al. 1998; Zhang et al. 2004; Born and Bradley 2005; Bartels et al. 2008; Konen and Kastner 2008).
Conclusions
Our results suggest that CSv and V5+/MT+ are involved in different aspects of motion processing. While V5/MT and MST responded equally to different types of coherent motion and also to incoherent motion, CSv responded with high selectivity to full-field coherent motion stimuli with a particular preference to planar motion. CSv was strongly modulated by pursuit signals and had an enhanced capability of responding to planar objective rather than planar retinal motion when compared with V5+/MT+. Our results suggest that CSv is specialized to process self-induced full-field motion, in particular planar motion induced by lateral head movements or by eye movements.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Centre for Integrative Neuroscience, Tübingen; Max Planck Society, Germany.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Antal A, Baudewig J, Paulus W, Dechent P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis Neurosci. 2008;25:17–26. doi: 10.1017/S0952523808080024. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S, Logothetis NK. Natural vision reveals regional specialization to local motion and to contrast-invariant, global flow in the human brain. Cereb Cortex. 2008;18:705–717. doi: 10.1093/cercor/bhm107. [DOI] [PubMed] [Google Scholar]
- Becker HG, Erb M, Haarmeier T. Differential dependency on motion coherence in subregions of the human MT+ complex. Eur J Neurosci. 2008;28:1674–1685. doi: 10.1111/j.1460-9568.2008.06457.x. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18:7511–7518. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O'Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30:61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Pekel M, Hoffmann KP, Lappe M. Visual selectivity for heading in monkey area MST. Exp Brain Res. 2010;200:51–60. doi: 10.1007/s00221-009-1990-3. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion. A functional MRI study. Brain. 1998;121(Pt 7):1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- Cammalleri R, Gangitano M, D'Amelio M, Raieli V, Raimondo D, Camarda R. Transient topographical amnesia and cingulate cortex damage: a case report. Neuropsychologia. 1996;34:321–326. doi: 10.1016/0028-3932(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Takahashi K, DeAngelis GC, Angelaki DE. Does the middle temporal area carry vestibular signals related to self-motion? J Neurosci. 2009;29:12020–12030. doi: 10.1523/JNEUROSCI.0004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Dean HL, Crowley JC, Platt ML. Visual and saccade-related activity in macaque posterior cingulate cortex. J Neurophysiol. 2004;92:3056–3068. doi: 10.1152/jn.00691.2003. [DOI] [PubMed] [Google Scholar]
- Dean HL, Platt ML. Allocentric spatial referencing of neuronal activity in macaque posterior cingulate cortex. J Neurosci. 2006;26:1117–1127. doi: 10.1523/JNEUROSCI.2497-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Stephan T, Yousry TA, Brandt T. fMRI signal increases and decreases in cortical areas during small-field optokinetic stimulation and central fixation. Exp Brain Res. 2003;148:117–127. doi: 10.1007/s00221-002-1267-6. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol. 1991;65:1329–1345. doi: 10.1152/jn.1991.65.6.1329. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. J Neurophysiol. 2001;86:1991–2000. doi: 10.1152/jn.2001.86.4.1991. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CLJ, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cerebral Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Erickson RG, Thier P. A neuronal correlate of spatial stability during periods of self-induced visual motion. Exp Brain Res. 1991;86:608–616. doi: 10.1007/BF00230534. [DOI] [PubMed] [Google Scholar]
- Franconeri SL, Simons DJ. Moving and looming stimuli capture attention. Percept Psychophys. 2003;65:999–1010. doi: 10.3758/bf03194829. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P. Neuronal mechanisms for detection of motion in the field of view. Neuropsychologia. 2003;41:1717–1727. doi: 10.1016/s0028-3932(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The visual perception of objective motion and subjective movement. Psychol Rev. 1954;61:304–314. doi: 10.1037/h0061885. [DOI] [PubMed] [Google Scholar]
- Goossens J, Dukelow SP, Menon RS, Vilis T, van den Berg AV. Representation of head-centric flow in the human motion complex. J Neurosci. 2006;26:5616–5627. doi: 10.1523/JNEUROSCI.0730-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA, Andersen RA, Snowden RJ. Tuning of MST neurons to spiral motions. J Neurosci. 1994;14:54–67. doi: 10.1523/JNEUROSCI.14-01-00054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10:1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Watkins PV, Angelaki DE, DeAngelis GC. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci. 2006;26:73–85. doi: 10.1523/JNEUROSCI.2356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001;32:161–172. doi: 10.1016/s0896-6273(01)00452-4. [DOI] [PubMed] [Google Scholar]
- Katayama K, Takahashi N, Ogawara K, Hattori T. Pure topographical disorientation due to right posterior cingulate lesion. Cortex. 1999;35:279–282. doi: 10.1016/s0010-9452(08)70801-3. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Ohlendorf S, Speck O, Sprenger A, Rutschmann RM, Haller S, Greenlee MW. fMRI evidence for sensorimotor transformations in human cortex during smooth pursuit eye movements. Neuropsychologia. 2008;46:2203–2213. doi: 10.1016/j.neuropsychologia.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci. 2008;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciokas JB, Britten KH. Extrastriate area MST and parietal area VIP similarly represent forward headings. J Neurophysiol. 2010;104:239–247. doi: 10.1152/jn.01083.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 2000;3:1322–1328. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Olson CR, Musil SY, Goldberg ME. Single neurons in posterior cingulate cortex of behaving macaque: eye movement signals. J Neurophysiol. 1996;76:3285–3300. doi: 10.1152/jn.1996.76.5.3285. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia. 2003;41:1757–1768. doi: 10.1016/s0028-3932(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Page WK, Duffy CJ. MST neuronal responses to heading direction during pursuit eye movements. J Neurophysiol. 1999;81:596–610. doi: 10.1152/jn.1999.81.2.596. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Salamon G, Mazoyer B, Berthoz A. PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulate cortical involvement. J Neurophysiol. 1993;69:1009–1017. doi: 10.1152/jn.1993.69.4.1009. [DOI] [PubMed] [Google Scholar]
- Sakata H, Shibutani H, Kawano K, Harrington TL. Neural mechanisms of space vision in the parietal association cortex of the monkey. Vision Res. 1985;25:453–463. doi: 10.1016/0042-6989(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Smith AT, Wall MB, Williams AL, Singh KD. Sensitivity to optic flow in human cortical areas MT and MST. Eur J Neurosci. 2006;23:561–569. doi: 10.1111/j.1460-9568.2005.04526.x. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp Brain Res. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie Y, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Henning P, Kubischik M, Hoffmann KP. Neural mechanisms of saccadic suppression. Science. 2002;295:2460–2462. doi: 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MB, Smith AT. The representation of egomotion in the human brain. Curr Biol. 2008;18:191–194. doi: 10.1016/j.cub.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Yu CP, Page WK, Gaborski R, Duffy CJ. Receptive field dynamics underlying MST neuronal optic flow selectivity. J Neurophysiol. 2010;103:2794–2807. doi: 10.1152/jn.01085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Heuer HW, Britten KH. Parietal area VIP neuronal responses to heading stimuli are encoded in head-centered coordinates. Neuron. 2004;42:993–1001. doi: 10.1016/j.neuron.2004.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.