Abstract
Language processing requires the orchestrated action of different neuronal populations, and some studies suggest that the role of the basal temporal (BT) cortex in language processing is bilaterally distributed. Our aim was to demonstrate connectivity between perisylvian cortex and both BT areas. We recorded corticocortical evoked potentials (CCEPs) in 8 patients with subdural electrodes implanted for surgical evaluation of intractable epilepsy. Four patients had subdural grids over dominant perisylvian and BT areas, and 4 had electrode strips over both BT areas and left posterior superior temporal gyrus (LPSTG). After electrocortical mapping, patients with grids had 1-Hz stimulation of language areas. Patients with strips did not undergo mapping but had 1-Hz stimulation of the LPSTG. Posterior language area stimulation elicited CCEPs in ipsilateral BT cortex in 3/4 patients with left hemispheric grids. CCEPs were recorded in bilateral BT cortices in 3/4 patients with strips upon stimulation of the LPSTG, and in the LPSTG in the fourth patient upon stimulation of either BT area. This is the first in vivo demonstration of connectivity between LPSTG and both BT cortices. The role of BT cortex in language processing may be bilaterally distributed and related to linking visual information with phonological representations stored in the LPSTG.
Keywords: basal temporal language area, connectivity, cortical mapping, epilepsy, evoked potentials
Introduction
Electrocortical stimulation mapping (ESM) studies have shown that the basal temporal language area (BTLA) is located in the area of the fusiform gyrus, 1–9 cm posterior to the temporal tip (Burnstine et al. 1990;Lüders et al. 1991; Schaffler et al. 1994; Krauss et al. 1996). The language deficits elicited in that area upon electrical stimulation range from complete expressive, receptive, and repetition deficits at higher stimulus intensities to anomia and other aphasic symptoms at lower ones (Lüders et al. 1986, 1991). Some authors have suggested that the precise role of the BTLA is to match a conceptual entity to its phonological representation (Lüders et al. 1991; Usui et al. 2003, 2005), and it has been suggested that the function of BTLA, like declarative memory, may be bilaterally distributed (Lüders et al. 1991). Bilateral representation of the BT language function has been suggested by event-related potentials (Nobre et al. 1994) and functional neuroimaging (Sharp et al. 2004). However, in vivo connectivity between perisylvian and bilateral BT areas has not been demonstrated.
In this corticocortical evoked potential (CCEP) study, we first aimed to investigate connectivity between the posterior language area (PLA) and ipsilateral BT cortex in patients whose language areas were mapped by electrical stimulation. Second, in patients with bilateral subdural strip implantation who did not undergo language mapping, we aimed to study connectivity between the left posterior superior temporal gyrus (LPSTG), a putative receptive language area, and bilateral BT areas.
Materials and Methods
Patients and Clinical Setting
We studied 8 (5 females) patients who were admitted to the Epilepsy Monitoring Unit at Johns Hopkins Hospital after implantation of subdural electrodes for surgical management of intractable focal epilepsy (Table 1). Patients were included who had intracranial electrodes covering the LPSTG, where receptive language cortex was likely to be located, and at least one electrode over BT cortex on either side. Patients with preimplantation IQ of less than 75 and those with cerebral pathology affecting cortical regions of interest for CCEPs were excluded.
Table 1.
Patient characteristics
| Patient # | Age (years), sex | Age of onset | Ictal onset on scalp electroencephalography | Brain MRI | Brain PET | IAP injection side and results | Brain areas included in resective surgery and language outcome | ||
| 1 | 26, F | 14 years | Left frontal–temporal | Normal | Left temporal hypometabolism | L | R | Left TL–AH | |
| O | 5/6 | 5/6 | No language deficits | ||||||
| W | 5/6 | 5/6 | |||||||
| P | 4/4 | 4/4 | |||||||
| T | 4/4 | 3/4 | |||||||
| F | 4/4 | 4/4 | |||||||
| C | 2/4 | 1/4 | |||||||
| R | 2/2 | 1/2 | |||||||
| 2 | 41, F | 35 years | Left temporal | Mild right hippocampal atrophy; normal T2 signal | Nondiagnostic | L* | R | Left TL–AH | |
| O | 6/6 | Residual naming problems, gradually improving | |||||||
| W | 6/6 | ||||||||
| P | 4/4 | ||||||||
| T | 0/4 | ||||||||
| F | 4/4 | ||||||||
| C | 3/4 | ||||||||
| R | 2/2 | ||||||||
| 3 | 42, M | 34 years | Left temporal | Left anterior temporal encephalomalacia secondary to head trauma | Not done | L | R | Left TL–AH | |
| O | 0/6 | 3/6 | Difficulties with word finding, naming, reading, verbal learning, comprehension, fluency, as well as paraphasias and agrammatisms. All these cleared over 3 years | ||||||
| W | 3/6 | 6/6 | |||||||
| P | 2/4 | 4/4 | |||||||
| T | 2/4 | 4/4 | |||||||
| F | 2/4 | 4/4 | |||||||
| C | 0/4 | 1/4 | |||||||
| R | 0/2 | 2/2 | |||||||
| 4 | 40, F | 21 years | Bilateral frontal, left maximum | Mild Left hippocampal atrophy; Normal T2 signal | Left mesial temporal hypometabolism | L | R | Left frontal topectomy | |
| O | 0/6 | 4/6 | No language deficits | ||||||
| W | 2/6 | 6/6 | |||||||
| P | 3/4 | 4/4 | |||||||
| T | 3/4 | 4/4 | |||||||
| F | 3/4 | 4/4 | |||||||
| C | 2/4 | 1/4 | |||||||
| R | 1/2 | 1/2 | |||||||
| 5 | 21, M | 10 years | No clear ictal pattern | Mild Left hippocampal atrophy; Normal T2 signal | Mild right temporal lobe hypometabolism | Not done | Surgery not performed | ||
| 6 | 41, M | Infancy until 7 years, then seizure- free until 33 years | Late frontal-central rhythmic slow | Normal | Right anterior mesial temporal hypometabolism | L | R | Right TL–AH | |
| O | 0/6 | 6/6 | No language deficits | ||||||
| W | 0/6 | 6/6 | |||||||
| P | 0/4 | 4/4 | |||||||
| T | 0/4 | 4/4 | |||||||
| F | 0/4 | 2/4 | |||||||
| C | 1/4 | 4/4 | |||||||
| R | 0/2 | 2/2 | |||||||
| 7 | 37, F | 16 months | No clear ictal pattern | Normal | Nondiagnostic | Not done | Surgery not performed | ||
| 8 | 34, F | 9 years | Right frontal | Normal | Nondiagnostic | L | R* | Left TL–AH | |
| O | 0/6 | No language deficits | |||||||
| W | 0/6 | ||||||||
| P | 0/4 | ||||||||
| T | 1/4 | ||||||||
| F | 3/4 | ||||||||
| C | 0/4 | ||||||||
| R | 2/2 | ||||||||
Note: All patients were right handed. M, Male; F, Female; PET, Positron emission tomography. TL–AH: temporal lobectomy and amygdalohippocampectomy. Key for IAP test portion of the table: O, object naming; W, word reading; P, picture naming; T, token test; F, face judgment; C, calculations; R, repetition. (*) The left side in patient 2 and the right side in patient 8 were not tested due to enlarged posterior communicating arteries and the possibility that amobarbital injections could affect the brainstem.
In 4 patients, subdural grids were implanted to localize seizure onset and map language, and in the other 4, bilateral strips were used to lateralize the seizure focus. Patients were transferred to the Epilepsy Monitoring Unit one day following electrode implantation and underwent continuous video-electroencephalographic monitoring for 5–7 days. The study was approved by the Institutional Review Board at Johns Hopkins University in compliance with the Declaration of Helsinki, and informed consent was obtained from all patients.
Except for patients 5 and 7, all patients underwent an intracarotid amobarbital procedure (IAP) to determine language dominance. Our IAP protocol tested separately for receptive, expressive, and naming functions as it included assessment of object naming, picture naming, word reading, token test, face judgment, calculations, and repetition (Table 1). The left hemisphere was dominant for language in 5 patients. Patient 1 appeared to have mixed language dominance by IAP, although functional magnetic resonance imaging (fMRI) had lateralized language to the right hemisphere. The fMRI protocol in that patient included an auditory description decision task, an auditory category (semantic) decision task, listening to stories, and reading stories silently (Gaillard et al. 2007). The patient showed right frontal (right inferior and middle frontal gyral) and right temporal (right middle and superior temporal gyral) activation on all 4 tasks. The expressive language tasks showed right frontal activation. The comprehension paradigms showed right temporal activation.
Subdural Electrode Implantation and Recording
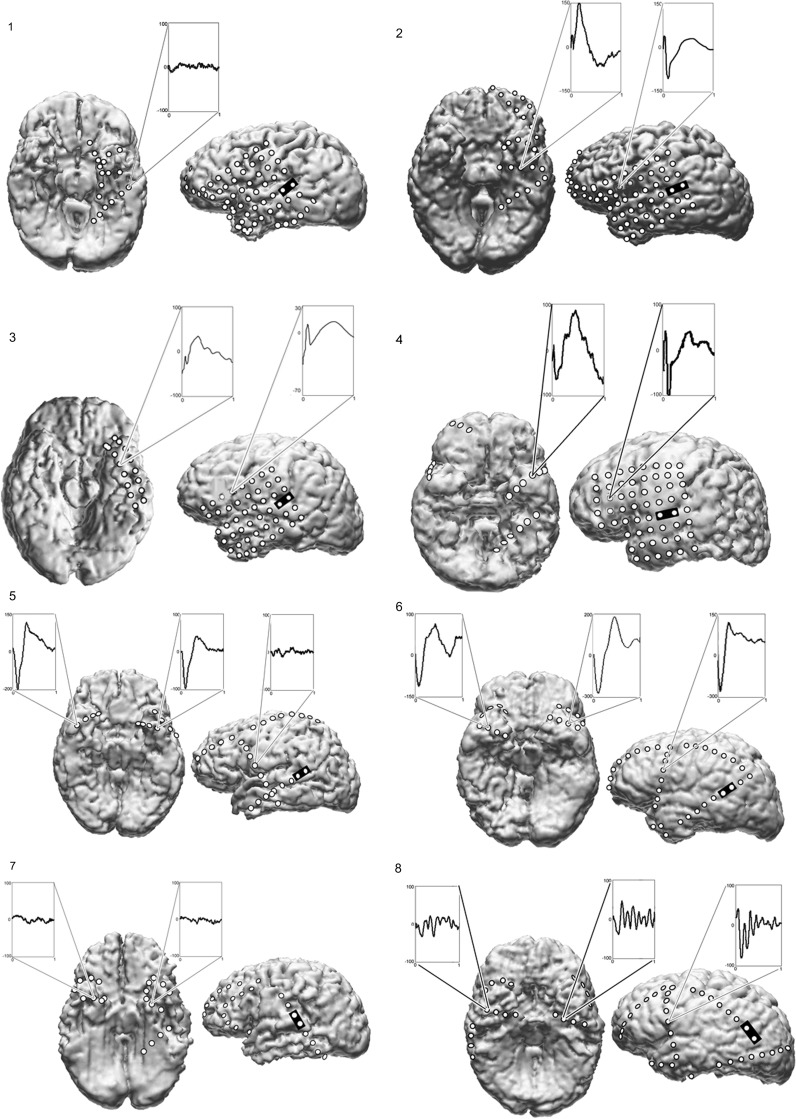
Subdural electrodes embedded in silastic sheets were carefully fitted over the cortical surface and anchored by sutures to overlying dura mater. The electrodes were platinum–iridium disks measuring 4 mm in diameter with 2.3 mm exposed surfaces, evenly spaced at 1 cm center-to-center intervals (Adtech, Racine, WI). Four patients underwent implantation of 6 × 8 subdural grids covering perisylvian cortex, as well as a 2 × 8 grid (patient 3) or two to three 1 × 8 electrode strips over lateral and BT cortex (patients 1, 2, and 4) (Fig. 1). In addition, patient 4 underwent implantation of 2 strips on the right side through a frontal burr hole. In the remaining 4 patients, bilateral strips were placed through frontal and temporal burr holes.
Figure 1.
Cortical surface rendering of preoperative MRIs of all patients, with the location of subdural electrodes from a postoperative CT scan superimposed, allowing 3D visualization of the implanted electrodes in relation to gyral anatomy. The coregistration is done with Curry software Compumedics Neuroscan, El Paso, TX. In patients 1–4, grid electrodes were implanted over left frontal and temporal lobes covering the perisylvian cortex, and strip electrodes sampled BT cortex. In patients 5–8, bilateral strip electrodes were placed through burr holes in order to help lateralize the seizure focus. Three or 4 strips were placed through each of the frontal and temporal burr holes on each side. For the purposes of this study, only left lateral and basal views are shown. Black rectangles mark electrode pairs where single pulse stimulation was applied within the PLAs in patients 1–4 and the LPSTG in patients 5–8. The most robust CCEPs are shown. These were recorded very focally, with the surrounding electrodes showing no CCEPs or ones with significantly lower voltage.
Intracranial electrode locations were verified by coregistration of presurgical volumetric brain MRI (1- to 1.8-mm coronal slice thickness) with postsurgical volumetric brain CT (1-mm axial slice thickness) according to anatomic fiducials (Curry, Compumedics Neuroscan, El Paso, TX) as shown in Figure 1. Electrocorticorticographic (ECoG) recordings were made using the Stellate Harmonie system (Montreal, Canada). ECoG channels were amplified, filtered (0.1–300 Hz, 60 Hz notch), and digitally recorded at 1000 Hz per channel.
Language Mapping
Patients 1 through 4 underwent ESM of language as clinically indicated for their care and as described elsewhere (Lesser et al. 1987). Language mapping was performed with picture naming, sentence comprehension, paragraph reading, and spontaneous speech. Picture naming was performed with a set of black and white pictures from the Boston naming test. Electrical stimulation was applied immediately before picture onset and lasted no longer than 5 s. Mapping of language was confirmed only if language impairment, clearly different from the patient's baseline performance, was noticed during at least 2 stimulus trains. An electrode site was considered to lie within the anterior language area (ALA—“Broca’s area”) if it was within 1 cm of the posterior inferior frontal gyrus (pIFG) and if ESM at the site resulted in language deficits. Likewise, the PLA (“Wernicke’s area”) was operationally defined as the area within 2 cm of the posterior third of the STG where language was mapped. In patients 1–4, attempts to map language in left BT cortex resulted in ipsilateral facial discomfort or twitching that distracted the patient and prevented language mapping, as described previously (Lesser et al. 1985).
Corticocortical Evoked Potential Recording
Similar to other authors (Matsumoto et al. 2004), we applied square wave pulses of 0.3 ms duration and alternating polarity through 2 contiguous electrodes in a bipolar fashion. The frequency of stimulation was 1 Hz, and the starting current was 2.5 mA, increased in 1 mA increments to a maximum of 8.5 mA (100–200 trials per current level). We digitally recorded markers for stimulus onset during the CCEP study on an auxiliary channel in the ECoG file. We generated a computer software to average CCEP time-locked to single-pulse stimulus onset. In patients 1–4, we applied single pulse cortical stimulation to ALA and PLA, as determined by ESM. In the remaining patients, we stimulated putative receptive language cortex in the LPSTG. Single pulse electrical stimulation in both BT areas in patients 5, 6, and 8 resulted in uncomfortable facial twitching necessitating cessation of stimulation, whereas patient 7 tolerated single pulse stimulation in both BT cortices.
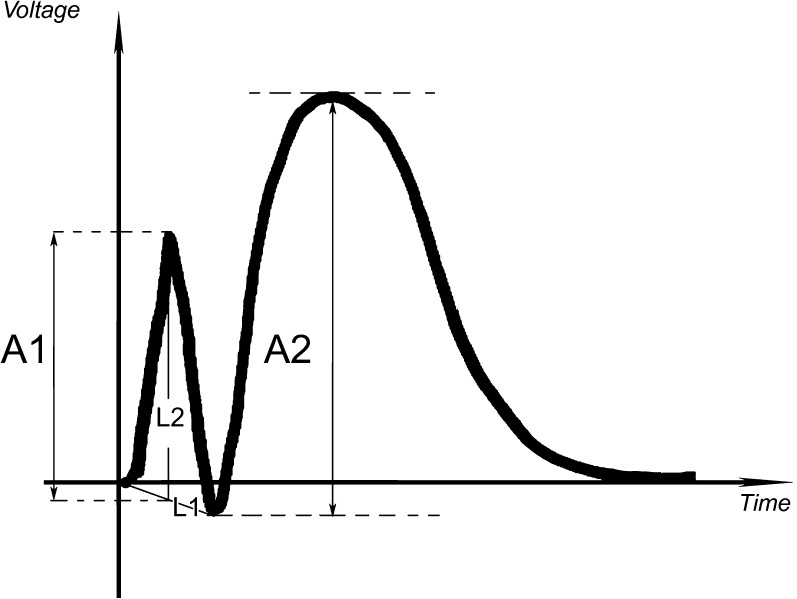
We measured the voltage of the CCEPs in a manner identical to Matsumoto et al. (2004) (Fig. 2). The CCEP morphology in patients 5 and 6 was different (Fig. 1), and the voltages of the initial positive phase (A1) and second negative phase (A2) simply corresponded to the vertical line joining the horizontal tangents drawn through the beginning and end of these phases. We used the paired t-test to compare latencies and amplitudes of different evoked potentials.
Figure 2.
An illustration of the method used to measure the voltage of the peaks of the CCEPs as in Matsumoto et al. (2004). The voltage of the initial component (A1) corresponds to the vertical segment drawn between a horizontal tangent to the initial peak and the intersection of L1 (joining the onset of the ascending phase of the first peak and the aftergoing positive trough) and L2 (a vertical line drawn through the initial peak). The voltage of second component (A2) corresponds to the vertical segment drawn between a horizontal tangent to the second peak and another horizontal tangent to the positive trough between the 2 peaks.
Results
Stimulation of PLAs
In patients 2–4, in whom IAP revealed left language dominance and the ALA and PLA were confirmed by ESM to be in the left hemisphere, single pulse electrical stimulation of a pair of electrodes within the PLA resulted in 2 fields of CCEPs: one in the ALA and one in left BT cortex. Figure 1 shows the most robust CCEPs recorded within the ALA (mapped by electrical stimulation) and BT cortex (not mapped secondary to discomfort as mentioned above), and Table 2 shows the latencies and amplitudes of the CCEP components. The CCEPs consisted of an early negative peak followed by a positive trough and a slower negative wave. In all of these 3 subjects, the latency of the initial negative component of the CCEP recorded over the ALA was earlier than that recorded over the left BT area (P < 0.05). Although the latency of the second component appeared to occur earlier over the left BT than the ALA, the difference was not significant (P = 0.204). The CCEPs recorded over the ALA and left BT area were seen focally over 1 or 2 electrodes, with their voltage dropping by more than 50% in neighboring electrodes within a distance of 1 cm.
Table 2.
Latencies and voltages of CCEPs in all patients.
| Patient | Stimulated area | Recorded area | First component | Second component | ||
| Latency (ms) | Amplitude (μV) | Latency (ms) | Amplitude (μV) | |||
| 1 | ALA | L BT | 74 | 255.5 | 438 | 344.4 |
| 2 | PLA | ALA | 23.92 | 61.54 | 564.6 | 144.87 |
| PLA | L BT | 37.9 | 49.1 | 189.6 | 171.9 | |
| 3 | PLA | ALA | 44.44 | 32.98 | 640 | 34.66 |
| PLA | L BT | 54 | 28.29 | 500 | 60.5 | |
| 4 | PLA | ALA | 40.95 | 67.5 | 501.7 | 97.5 |
| PLA | L BT | 50.4 | 31.19 | 462.18 | 144.9 | |
| 5 | LPSTG | L BT | 106.6 | 100 | 365.5 | 138.95 |
| LPSTG | R BT | 111.96 | 200 | 320.61 | 313.43 | |
| 6 | LPSTG | L pIFG | 79.9 | 373.3 | 283 | 401 |
| LPSTG | L BT | 153.5 | 360 | 495 | 460.2 | |
| LPSTG | R BT | 118.3 | 162 | 478 | 189.39 | |
| 7 | L BT | LPSTG | 56 | 35 | Not well defined | |
| R BT | LPSTG | 34.37 | 36 | 81.25 | 162 | |
| 8 | LPSTG | L pIFG | Not well defined | Not well defined | ||
| LPSTG | L BT | |||||
| LPSTG | R BT | |||||
Note: L, left; R, right.
Upon stimulation of the PLAs, the morphology of the CCEPs recorded over the ALAs was very similar among subjects 2–4, as was that of the CCEPs recorded over the left BT areas. No significant difference was found between the voltages of the initial components of the ALA CCEPs and those of the left BT CCEPs (P = 0.202).
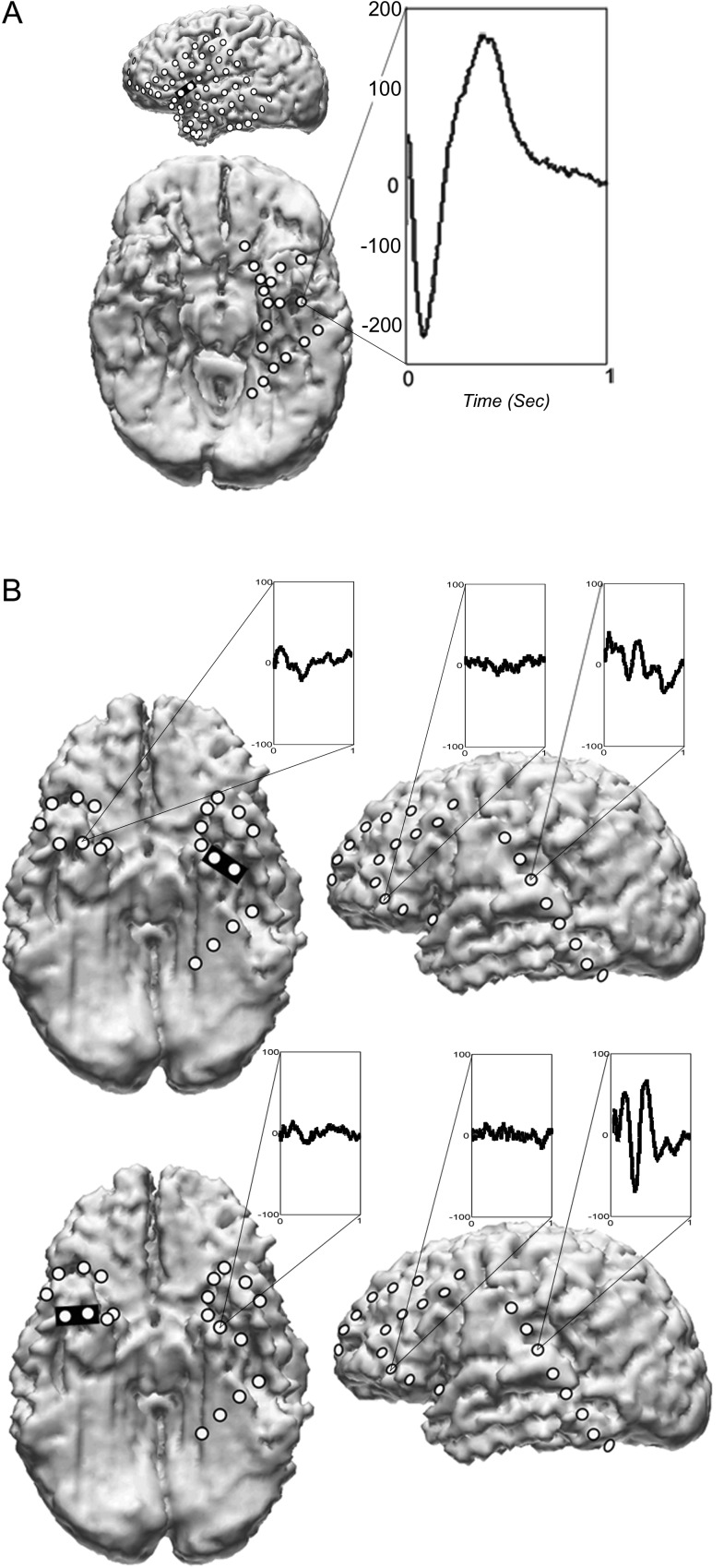
In patient 1, although the ALA and PLA were mapped by electrical stimulation in the left hemisphere, IAP revealed mixed dominance and fMRI showed greater activation in the right hemisphere for both expressive and receptive language tasks. Upon PLA stimulation in that patient, neither ALA nor BT CCEPs were seen. Of note, however, stimulation of the ALA in that patient elicited CCEPs in the left BT cortex (Fig. 3A and Table 2).
Figure 3.
(A) CCEP recorded in the BT area upon stimulation of the ALA in patient 1. (B) CCEPs recorded in the LPSTG in patient 7 upon stimulation of the left (upper part) and the right BT area (lower part). The stimulated electrode pairs are marked by black rectangles. Lower voltage and less-defined contralateral BT and frontal responses are shown as controls.
Stimulation of Posterior Superior Temporal Gyrus in Patients without Language Mapping
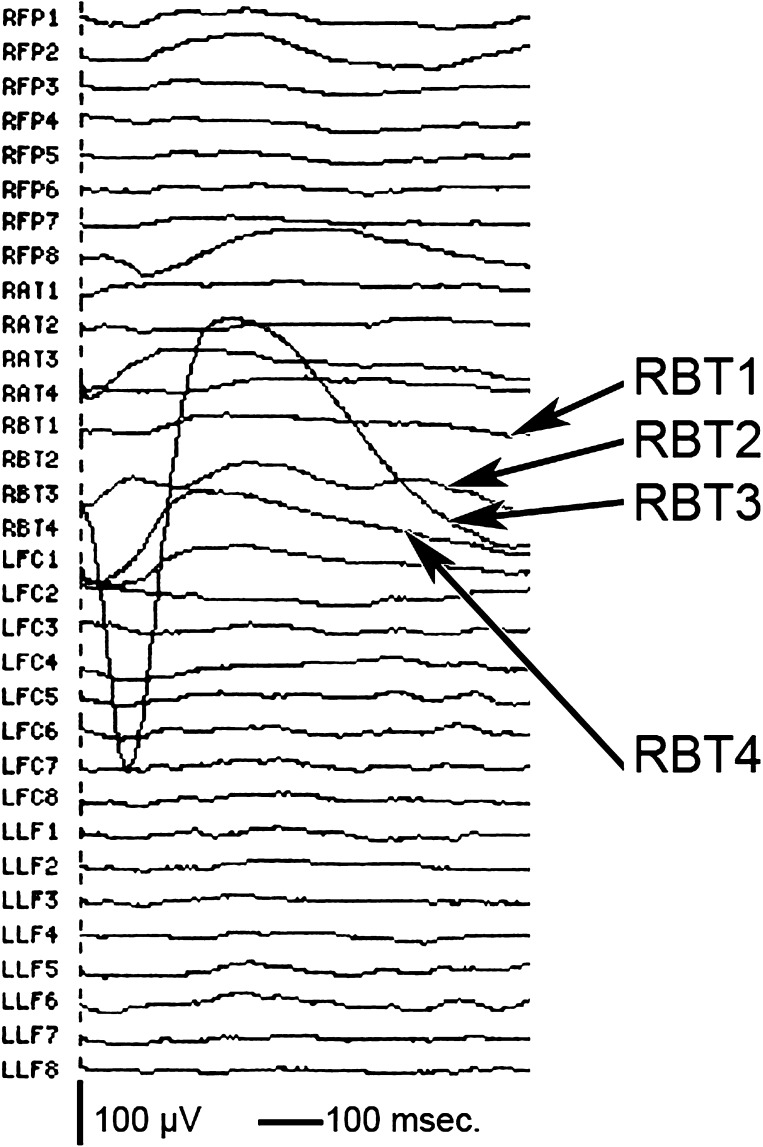
Single pulse stimulation of the LPSTG elicited CCEPs in both BT cortices in patients 5, 6, and 8 and CCEPs in the left pIFG in patients 6 and 8 (Fig. 1). In patients 5 and 6, no clear initial negative peak was seen, and the first component was positive with latencies comparable to those of the positive trough of the CCEPs seen in patients 2–4. The morphologies of the CCEPs in right and left BT areas were remarkably similar to one another in the same subject and between patients 5 and 6. CCEP morphology was different in patient 8 (polyphasic) compared with all other patients (Fig. 1). Interestingly, the stimulation site in patient 8 was posterior to the sites stimulated in all other patients. The CCEPs recorded over both BT areas were focal over one electrode, with a precipitous drop of CCEP within 1 cm (Fig. 4). Thus, not only did the stimulation evoke a stronger response in BT areas than in other regions but it was very focal within the BT itself.
Figure 4.
CCEPs in one subject averaged over multiple strips, including right basal temporal (RBT) upon stimulation of the LPSTG. The maximum recorded response is over RBT3 which is only 1 cm from RBT2 and 1 cm from RBT4 where much smaller responses were recorded.
In patient 7, no CCEPs were seen upon stimulating the part of LPSTG sampled by subdural electrodes. However, single pulse stimulation of either BT area elicited CCEPs in the LPSTG in that patient (Fig. 3B). These CCEPs appeared to have polyphasic morphology. Single pulse stimulation of BT areas was not possible in the other patients because of pain during either clinical stimulation with pulse train or CCEP recordings with single pulses.
Single pulse stimulation was safe. Previous studies reported no adverse effects of such stimulation (Valentin et al. 2002; Matsumoto et al. 2004). Except for the discomfort associated with stimulation of the BT cortex, secondary to facial nerve stimulation, resulting in twitching in all but one of our subjects, no side effects such as after discharges or seizures were noted. Surgical resection was done in 6 of the 8 patients. The resected regions and language outcomes are summarized in Table 1.
Discussion
Functional specialization of the cerebral cortex does not shed light on all neural substrates responsible for cortical function. In particular, language processing requires orchestrated interactions among different neural populations. This has led to a shift of focus from functional specialization to functional integration (Lee et al. 2003). Disclosing connectivity among brain areas that subserve language can help shed light on mechanisms of lexical and semantic processing, identify areas that have reorganized outside of their typical anatomical locations, and possibly predict postoperative language outcome after resective surgery. Understanding the functional connections of BT cortex with other language areas is particularly important because standard en bloc anterior temporal lobectomies in patients with epilepsy typically include the BT area, with consequent deficits in confrontation naming (Krauss et al. 1996) or verbal memory deficits (Hermann et al. 1992; Stroup et al. 2003; Baxendale et al. 2006). In this study, we applied single pulse electrical stimulation in patients with implanted intracranial electrodes and found evidence of connectivity between posterior perisylvian cortex and both BT cortices.
For important methodologic and conceptual reasons, a distinction needs to be made between anatomic, functional, and effective connectivity (Fingelkurts and Kahkonen 2005). Anatomic connectivity may be demonstrated by tract tracing in postmortem studies or by diffusion tensor imaging (DTI) in vivo. Tract tracing is excellent for studying neural pathways postmortem but is rarely done in the human language system, and DTI reveals similarly oriented myelinated axonal processes but not necessarily effective connections between brain areas (Catani et al. 2002). Functional connectivity is inferred when spatially disparate neurophysiological events appear to be temporally related, whereas effective connectivity refers to the influence of one neural system over another (Friston et al. 1993; Lee et al. 2003). A main way of inferring effective connectivity is via perturbation studies. This consists of applying an activating or deactivating stimulus that is precisely defined in time and space and assessing its effects on other brain regions. CCEPs rely on measuring cortical responses to an electrophysiological perturbation and thus can also be considered a measure of “effective connectivity.” CCEP recordings offer a spatial resolution of typically 1 cm interelectrode distance and a temporal resolution of ECoG-recorded responses on the order of tens to hundreds of milliseconds. These features offer an excellent opportunity to measure the temporal sequence of separate neurophysiological events, thereby allowing inferences about the likelihood of causal interactions between different cortical sites. Although these inferences are necessarily limited by the nonphysiological characteristics of the perturbing stimulus, they reflect presence of connections between different cortical regions. Interestingly, striking similarity has been observed in cat visual cortex between responses to physiologic stimuli and responses to direct electrical stimulation (Lee et al. 2003).
One study used CCEPs to study connectivity among language areas and found connectivity between PLA and ipsilateral BT area in one patient (Matsumoto et al. 2004). The stimulated electrodes in the PLA were different from those used to elicit CCEPs in the ALA, and the CCEPs recorded in that study were of low voltage and less well defined than those recorded elsewhere. To the best of our knowledge, our study is the first to report connectivity between the LPSTG, a putative language area, and both BT cortices, in addition to confirming connectivity between the PLA and BT cortex in the dominant hemisphere.
Effective Connectivity between PLA and BTLA
The CCEPs we observed in BT cortex with stimulation of PLA suggest effective connectivity between these cortical regions. Previous authors have hypothesized that BT area plays a role in linking visual semantic representations in occipital–temporal cortex with phonological representations in posterior superior temporal gyrus (Lüders et al. 1991; Usui et al. 2003, 2005). In a visual semantic decision task during which subjects were asked to judge whether pairs of words were related or unrelated, both the left fusiform gyrus and the superior temporal cortex showed fMRI activation for object names and their associated actions (Tyler et al. 2003). Additionally, a decrease in confrontation naming has been reported in some patients after resections in dominant BT area (Krauss et al. 1996), and impaired semantic processing (Gainotti et al. 1995; Schmolck et al. 2002) and word-finding difficulties (Riva 1998) have been observed with natural lesions of the fusiform gyrus. Although the aforementioned studies have suggested that dominant BT area plays a role in linking visual semantic knowledge with phonological knowledge, it has not been clear exactly how such a link would occur. The BT area includes the anterior fusiform gyrus and is located at the end of the ventral visual object processing stream, that is, the “what” pathway. As such, its role in visual semantic knowledge representations is quite plausible, but phonological representations are much more likely stored in auditory association cortex in and around the LPSTG. A putative cross-modal link between these higher order visual and auditory areas would presumably require substantial connectivity between BT area and PLA. The CCEPs observed in the present study in 3 patients with left language dominance offer a direct evidence for this. In the patient with mixed language dominance, there was no evidence of connectivity between the PLA and BT cortex. It is possible that higher order auditory processing occurs in the right hemisphere in that patient, although we cannot generate meaningful conclusions because adequate sampling of the right hemisphere by intracranial electrodes was not done.
Connectivity between Right BT and Left Perisylvian Areas
If the function of BT cortex includes linking visual semantic knowledge with phonological representation as discussed above, then this function is likely to be bilaterally distributed. In this study, we stimulated the LPSTG and found bilateral BT responses in 3 out of 4 patients with bilateral implanted strip electrodes. In the fourth patient, simulation of either BT area elicited a clear LPSTG CCEP. These results constitute an evidence for effective 2-way connectivity between LPSTG and both anterior BT areas. Numerous other studies using different methodologies have demonstrated varying degrees of language processing in the nondominant BT cortex.
An event-related potential study (Nobre et al. 1994) showed that both anterior fusiform gyri were involved in semantic processing in a manner sensitive to the nature of the written stimulus: words with semantic content elicited responses, whereas nonword letter strings did not. Additionally, a positron emission tomography study (Sharp et al. 2004) concluded that both BT areas were involved in access to stores of semantic knowledge. In that study, the accuracy of semantic decision predicted activation within the right BT area. Our findings support the view that both BT areas function within a distributed network in language processing.
The functions of the right anterior BT area include higher order visual processing, such as face recognition (Levy et al. 1972; Uhl et al. 1990). Thus, connectivity between the right BT area and Wernike’s area may participate in visual naming and visual–auditory matching by linking higher order visual material with verbal material. Indeed, a postmortem study of a patient who had suffered from a right BT infarction used the Nauta method and found monosynaptic connections between the right BT area and perisylvian language areas, particularly the PLA (Di Virgilio and Clarke 1997). The Nauta method for anterogradely degenerating axons would yield meaningful results in determining corticocortical connectivity if original lesions are circumscribed and limited to the cortex. In that study, however, the lesion was not limited to the fusiform gyrus but also comprised the hippocampus and parahippocampus, as well as the pulvinar and other thalamic nuclei. Thus, our data appear to provide more direct evidence that the BT area is connected with contralateral perisylvian regions. Connectivity between extrastriate BT cortex and language areas, as suggested by our data, may support parallel processing of language functions (Petersen et al. 1988), with auditory and visual perception of words initially involving modality-specific codes and later sharing common semantic and articulatory codes.
Surgical Outcome
Of the 6 patients who underwent epilepsy surgery, the left BT area was included in the resection in 4 patients and the right BT area in one patient, while another patient underwent a frontal topectomy. Right BT CCEPs were recorded upon LPSTG stimulation in the patient who underwent right temporal resection, but surgery did not result in any language deficits. Transient language deficits (Table 1) were seen in 2 of 4 patients who underwent left temporal lobectomy, and these were 2 of the 3 who had left BT CCEPs upon LPSTG stimulation, the third patient’s response being polyphasic. Whether these results suggest that left BT CCEPs with certain characteristics predict language deficits remains to be established in a larger sample and with more extensive implantation of electrodes in BT regions.
Limitations
The spatial distribution of the observed CCEPs depended on the extent of electrode coverage determined solely by clinical considerations. For example, we did not see CCEPs in the BT areas in one patient with bilateral strip electrodes, possibly due to insufficient sampling of the fusiform gyri by electrodes. Moreover, BT CCEPs were of higher voltage on the right in one patient but on the left in another. Although this could have been due to actual differences between the 2 sides, possibly in turn due to signal amplification through multisynaptic transmission, we cannot rule out the effects of spatial undersampling. Thus, the actual maximum response over BT areas may be different from the CCEPs we recorded with a limited number of BT electrodes. In addition, we did not map language function in the BT cortex due to discomfort, and our conclusions about bilateral BT areas being part of the language system would have been stronger if mapping was done over these regions. Finally, the subjects of this study were all patients with epilepsy in whom reorganization of language cortex was a possibility. Thus, conclusions about structure–function relationships with respect to language processing are necessarily tentative.
Funding
National Institute of Neurological Disorders and Stroke (R01-NS40596to N.E.C.) and National Epifellows Foundation to M.Z.K.
Acknowledgments
The authors would like to thank Roy Shipley and Robert Webber, PhD for technical assistance. The results were presented in part at the American Academy of Neurology meeting in San Diego, April 2006. Conflict of Interest : None declared.
References
- Baxendale S, Thompson P, Harkness W, Duncan J. Predicting memory decline following epilepsy surgery: a multivariate approach. Epilepsia. 2006;47:1887–1894. doi: 10.1111/j.1528-1167.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- Burnstine TH, Lesser RP, Hart J, Jr, Uematsu S, Zinreich SJ, Krauss GL, Fisher RS, Vining EP, Gordon B. Characterization of the basal temporal language area in patients with left temporal lobe epilepsy. Neurology. 40:966–970. doi: 10.1212/wnl.40.6.966. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S. Direct interhemispheric visual input to human speech areas. Hum Brain Mapp. 1997;5:347–354. doi: 10.1002/(SICI)1097-0193(1997)5:5<347::AID-HBM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Kahkonen S. Functional connectivity in the brain-is it an elusive concept? Neurosci Biobehav Rev. 2005;28:827–836. doi: 10.1016/j.neubiorev.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, Conry JA, Pearl PL, Ritter FF, Sato S, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L. Neuroanatomical correlates of category-specific semantic disorders: a critical survey. Memory. 1995;3:247–264. doi: 10.1080/09658219508253153. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Berry AD, 3rd, Dohan FC., Jr Pathological status of the mesial temporal lobe predicts memory outcome from left anterior temporal lobectomy. Neurosurgery. 1992;31:652–656. doi: 10.1227/00006123-199210000-00006. ; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, Lesser RP, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lee L, Harrison LM, Mechelli A. A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage. 2003;19:457–465. doi: 10.1016/s1053-8119(03)00062-4. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Luders H, Klem G, Dinner DS, Morris HH, 3rd, Hahn J. Ipsilateral trigeminal sensory responses to cortical stimulation by subdural electrodes. Neurology. 1985;35:1760–1763. doi: 10.1212/wnl.35.12.1760. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Luders H, Klem G, Dinner DS, Morris HH, Hahn JF, Wyllie E. Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol. 4:27–53. doi: 10.1097/00004691-198701000-00003. [DOI] [PubMed] [Google Scholar]
- Levy J, Trevarthen C, Sperry RW. Reception of bilateral chimeric figures following hemispheric deconnexion. Brain. 1972;95:61–78. doi: 10.1093/brain/95.1.61. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris H, Resor S, Harrison M. Basal temporal language area demonstrated by electrical stimulation. Neurology. 36:505–510. doi: 10.1212/wnl.36.4.505. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, et al. Basal temporal language area. Brain. 1991;114(Pt 2):743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Riva D. Language deficits in a child with omolateral (left) temporo-basal and cerebellar lesions. Neuropsychologia. 1998;36:71–75. doi: 10.1016/s0028-3932(97)00095-x. [DOI] [PubMed] [Google Scholar]
- Schaffler L, Luders HO, Morris HH, 3rd, Wyllie E. Anatomic distribution of cortical language sites in the basal temporal language area in patients with left temporal lobe epilepsy. Epilepsia. 1994;35:525–528. doi: 10.1111/j.1528-1157.1994.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H.M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002;12:520–533. doi: 10.1002/hipo.10039. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJ. Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann Neurol. 2004;56:836–846. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy (ATL) Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss H. Objects and their actions: evidence for a neurally distributed semantic system. Neuroimage. 2003;18:542–557. doi: 10.1016/s1053-8119(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Uhl F, Lang W, Spieth F, Deecke L. Negative cortical potentials when classifying familiar and unfamiliar faces. Cortex. 1990;26:157–161. doi: 10.1016/s0010-9452(13)80082-2. [DOI] [PubMed] [Google Scholar]
- Usui K, Ikeda A, Takayama M, Matsuhashi M, Yamamoto J, Satoh T, Begum T, Mikuni N, Takahashi JB, Miyamoto S, et al. Conversion of semantic information into phonological representation: a function in left posterior basal temporal area. Brain. 126:632–641. doi: 10.1093/brain/awg057. [DOI] [PubMed] [Google Scholar]
- Usui K, Ikeda A, Takayama M, Matsuhashi M, Satow T, Begum T, Kinoshita M, Miyamoto S, Hashimoto S, Nagamine T, et al. Processing of Japanese morphogram and syllabogram in the left basal temporal area: electrical cortical stimulation studies. Brain Res Cogn Brain Res. 24:274–283. doi: 10.1016/j.cogbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Valentin A, Anderson M, Alarcon G, Seoane JJ, Selway R, Binnie CD, Polkey CE. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain. 125:1709–1718. doi: 10.1093/brain/awf187. [DOI] [PubMed] [Google Scholar]