Summary
Pleural tuberculosis (TB) is a common presentation of M. tuberculosis (MTB) infection, and despite spontaneous resolution remains a strong risk factor for reactivation pulmonary TB in a majority of individuals. This study was undertaken to further understand the characteristics of immune cells at sites of pleural TB. A significant shift toward memory CD4+ T cells with an effector phenotype and away from naïve CD4+ T cells in pleural fluid as compared to blood mononuclear cells was found. These data suggest that effector T cells are capable of migrating to sites of active TB infection and/or the differentiation to effector phenotype T cells in situ is highly amplified. Using multiparameter flow cytometry analysis, a significant portion of MTB-specific CD4+ T cells in the pleural space were polyfunctional demonstrating two, three or four simultaneous functions including IFN-gamma, IL-2, TNF-alpha, and or MIP1-alpha production. A greater proportion of these polyfunctional cells were of effector memory rather than central memory phenotype. The role of these polyfunctional MTB-specific CD4+ T cells at sites of pleural TB requires further study.
Keywords: Tuberculosis, pleuritis, CD4+ T cells, immunity, polyfunctional
Introduction
Pleural TB remains a common form of disease both in HIV-infected and uninfected subjects in developing countries (1–3). In two studies of military personnel prior to the HIV era, spontaneous resolution of pleural TB is common but is associated with future development of pulmonary TB in 43–65% of cases (4, 5). Precisely how MTB defies control despite induction of robust TH1 type T cell immune response is unknown. Whether this relates to the inflammatory milieu or types of immune cells recruited at sites of MTB infection is unclear. At the site of active MTB infection, as opposed to other forms of TB, pleural mononuclear cells (PFMC) are readily accessible providing an opportunity to study aspects of TB pathogenesis on cells from the actual site of TB disease.
The immunology of pleural TB has been studied for some time. In an earlier study Barnes et al showed that CD4+ T cell subsets defined at a level of naïve and memory T cells were concentrated at the pleural site and memory cells produce IFN-gamma (6). Since then multiple subsets of CD4+ T cells have been defined by immunophenotyping and functionality (7). Naïve T-cells originate from the thymus and upon antigenic stimulation give rise to memory T cells. Central memory T cells localize to sites of immune activation, such as lymph nodes, and upon co-stimulation and antigen specific activation further differentiate to effector and effector memory T cells.
There have been limited data regarding detailed analysis of CD4+ T cell phenotypes in sites of active TB in humans. Wilkinson et al looked specifically at pleural fluid samples from subjects with TB pleurisy using single parameter IFN-gamma ELISPOT methods and found the greatest proportion of IFN-gamma producing cells were CCR7− effector cells (8). In murine pulmonary tuberculosis models the composition of the CD4+ T cells is predominantly terminally differentiated effector CD4+ T cells (9, 10). In the first part of the present study we determine that there is a shift toward effector phenotype memory CD4+ T cells at the site of pleural TB compared to circulating blood in the same individuals.
More recently the field of viral and HIV immunology has developed sophisticated multicolor flow cytometry methods to determine the polyfunctionality of individual antigen-specific subsets of T cells (11). These methods recently are being employed to understand human immunity to TB (reviewed (12)). The previous studies on TB using multi-parameter polyfunctional flow technology in HIV-uninfected individuals study either blood from active TB patients or blood and bronchoalveolar lavage (BAL) fluid from latently infected individuals (13–16). These previous studies do not obtain clinical material from subjects with active TB from the site of disease. In the current study, we determined the functional profile using multiparameter flow cytometry of the subsets of MTB-specific CD4+ T cells in pleural fluid from HIV-uninfected individuals with active TB pleurisy.
Methods
Study subjects and cell processing
Study subjects were recruited at two sites in Africa under IRB approved protocols. Samples were obtained from 19 patients at Moulay Youssef Hospital in Rabat, Morocco with suspected TB pleuritis. Pleural fluid and blood were obtained simultaneously and peripheral blood mononuclear cells (PBMC) generated using standard methods with Ficoll-Hypaque (Eurobio) and density gradient centrifugation. Cell-free plasma and pleural fluid were stored at −70° C for assessment of IFN-gamma by ELISA (eBiosciences).
Pleural fluid samples were obtained on subjects suspected of having TB pleurisy from 7 Ugandan subjects that were identified at the National Tuberculosis Treatment Center, Mulago Hospital, and Kampala, Uganda. Pleural fluid was spun and pleural fluid mononuclear cells (PFMC) were cryopreserved and stored in liquid nitrogen until shipment using a cryoshipper for analysis at Case Western Reserve University.
Flow cytometry
Moroccan samples: CD4-FITC, CD45RO-PE, CCR7-PECy7 (eBioscience and BD Pharmingen) were used. After staining with antibodies, cells were washed, fixed with 1% paraformaldehyde, and 10,000 events were acquired by a 3-color FACSCalibur flow cytometer (BD) analyzed using CellQuest software.
Ugandan samples: PFMC (1–3×106/tube) were stimulated with MTB (H37Ra, ATCC) at 5:1 MOI or placed in IMDM with 2 % pooled human serum for 2 hrs and then BFA (5 µg/ml, SIGMA) was added. Anti-CD28/49d (1 µg/ml each, eBioscience and Biolegend) was also added to each tube during the stimulation. After overnight stimulation, PFMC were spun down and surface stained with CCR7-PE-CY7 (BD) for 15 min at room temperature, live dead violet (Invitrogen) for 10 min, and then anti-CD14 and CD19-Pacific Blue (Biolegend), CD27-APC/Cy7 (Biolegend), CD3-PerCP (Biolegend), CD4-V500 (BD), CD45RA-PE/TR (Invitrogen) were added. They were washed and fixed with 4 % paraformaldehyde for 15 min, washed in 0.2 % saponin/PBS, and stained in this buffer with anti-IFN-gamma Alexa 700, anti-IL-2-APC, anti-TNF-alpha-PE (all from Biolegend), and anti-MIP-1alpha-FITC (R & D Systems). Cells were then washed and fixed in 2% paraformaldehyde. Between 500,000 and 1,000,000 total events were collected from each sample on an LSR-II flow cytometer (BD). Analysis was performed using Boolean analysis on Flow Jo (Tree Star) to assess polyfunctionality of the cells.
Results
Description of cohorts of subjects
From both African cohorts, patients suspected of pleural TB with moderate to large pleural effusions by chest X-ray were identified. Subjects underwent pleural space sampling prior to treatment as part of their diagnostic evaluation. The mean age of the Moroccan subjects was 36 years old (range 16–65) with 7 men and 12 women studied. All Moroccan subjects included in this study had positive biopsy results for granulomatous inflammation along with the clinical signs and symptoms of TB pleurisy to establish their diagnosis. The mean age of the Ugandan subjects was 25 years old (range 19–37) with 2 men and 5 women studied. In Uganda pleural biopsies were not uniformly performed, however, 5/7 had either a positive culture for MTB or PCR for MTB genes (17, 18). The other two subjects had signs and symptoms of pleural TB. One subject had responded to MTB treatment, whereas the second was lost to follow-up. The clinical probability that this subject who was lost to followup had TB pleuritis is very high due his clinical presentation in Uganda where MTB is highly endemic. Based on this their data are included in the dataset. All subjects from Morocco and Uganda were HIV-negative by ELISA.
CD4+ T cell Phenotypes in blood vs. pleural fluid
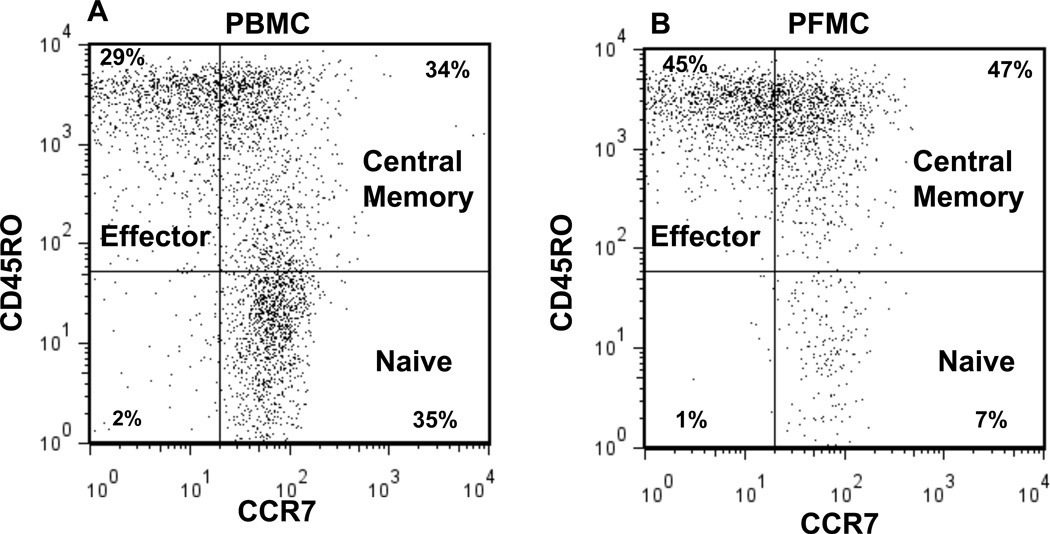
Cellular composition of PBMC and PFMC was assessed by 3-color flow cytometry that was available at the clinical site in Morocco. The gating strategy is demonstrated in Fig. 1. Cells were first gated by forward and side scatter for live cells in the lymphocyte-sized gate. Then CD4+ T cells were gated for analysis of their phenotype. Naïve cells were CD45RO−/CCR7+, central memory cells were CD45RO+/CCR7+, and effector phenotype cells were CD45RO+/CCR7−. Fig. 2 demonstrates the naïve, central memory, and effector CD4+ T cell phenotype composition of PBMC and PFMC in 19 subjects with pleural TB.
Fig. 1. CD4+ T cell subsets in patient with TB pleurisy.
PBMC and PFMC from Moroccan cohort were gated on the live lymphocyte size and then CD4. The percent CD4+ cells in the three subsets of naïve (CD45RO− CCR7+), central memory (CD45RO+ CCR7+), and effector phenotype (CD45RO+ CCR7−) for A) PBMC and B) PFMC are shown. Gates for CCR7 were set by the isotype staining (data not shown) for each individual donor.
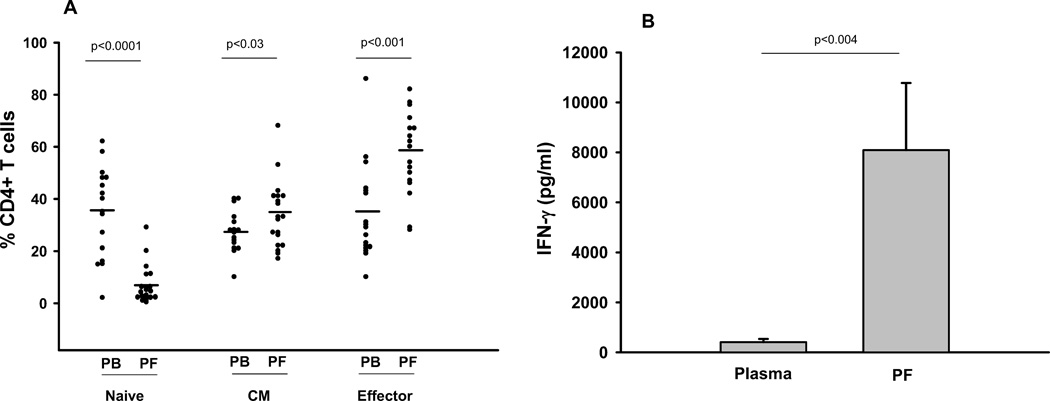
Fig. 2. Cellular analysis of pleural fluid and blood from Moroccan cohort.
A. Percent CD4+ cells in the three subsets of naïve, central memory, and effector phenotype for PBMC and PFMC are depicted. The line represents the median of 19 subjects. B. IFN-gamma in plasma and pleural fluid is determined by ELISA. Means from 9 subjects are shown and error bars represent standard deviations. Wilcoxon Matched-Pairs Signed-Ranks Test was used to calculate statistics in both panels.
There was a significant difference by Wilcoxon Matched-Pairs Signed-Ranks Test between the composition of PBMC and PFMC in naïve, central memory and effector subsets as indicated in Fig. 2A. The most substantial differences were toward a predominance of effector phenotype and lack of naïve CD4 T cells in PFMC compared to PBMC. There was a much smaller difference between the percentage of central memory cells present in PBMC and PFMC.
There were substantial levels of IFN-gamma in pleural fluid over serum in 9 of 19 subjects where paired samples were available (Fig. 2B). This is consistent with the findings of others that the pleural fluid in TB pleurisy is rich in type II IFNs such as IFN-gamma (6, 19). There was no correlation by Spearman of the pleural fluid IFN-gamma levels and any of the T cell subset frequencies (data not shown).
Polyfunctionality of PFMC
After we observed increased numbers of effector group cells in the PFMC from Moroccan subjects, we asked what the phenotype and functionality of the MTB-specific CD4+ T cells from the pleural space of subjects with TB pleurisy was. This analysis was performed on PFMC samples from the Ugandan clinical site that were cryopreserved and shipped to Case Western Reserve where they underwent 10-color flow cytometry analysis.
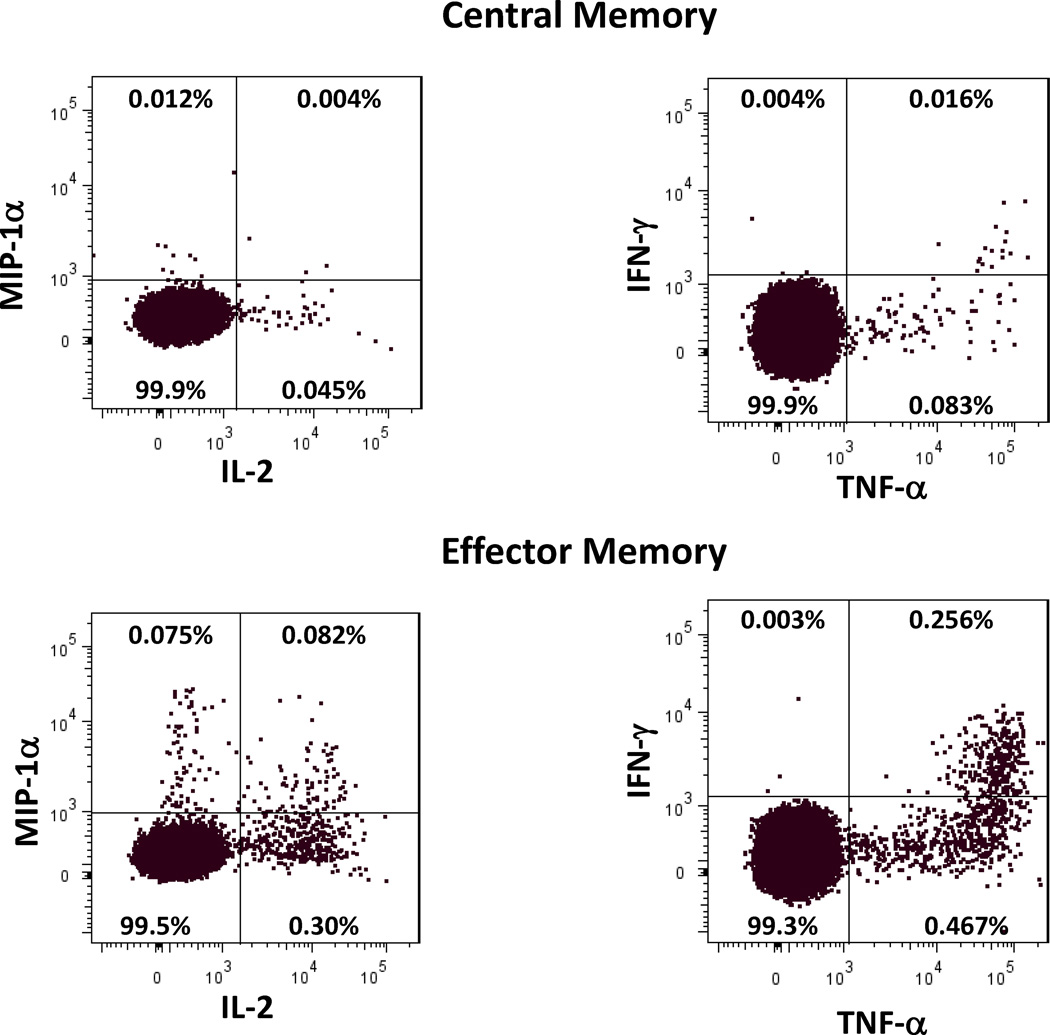
We developed a polychromatic flow cytometry panel that simultaneously determines CD4+ T cell memory phenotype and 4 unique effector functions. We have chosen to study the proinflammatory cytokines, TNF-alpha, IFN-gamma, and IL-2, and the β-chemokine MIP-1 alpha. PFMC were incubated with media or MTB overnight and analyzed by intracytoplasmic flow cytometry. The gating strategy is shown in Fig. 3. In the Ugandan PFMC analysis, staining with CD45RA and CCR7 allowed us to differentiate specifically effector and effector memory cells. Similar to the PFMC analysis of the Moroccan subjects, the Ugandan PFMC had preponderance of effector type cells (Fig. 4A). Gates for MTB-specific production of cytokines and MIP1-alpha from of each CD4+ T cell subsets were determined based on spontaneous levels on unstimulated cells. Fig. 5 demonstrates representative dot plots to demonstrate the magnitude cytokine secretion observed after overnight MTB stimulation.
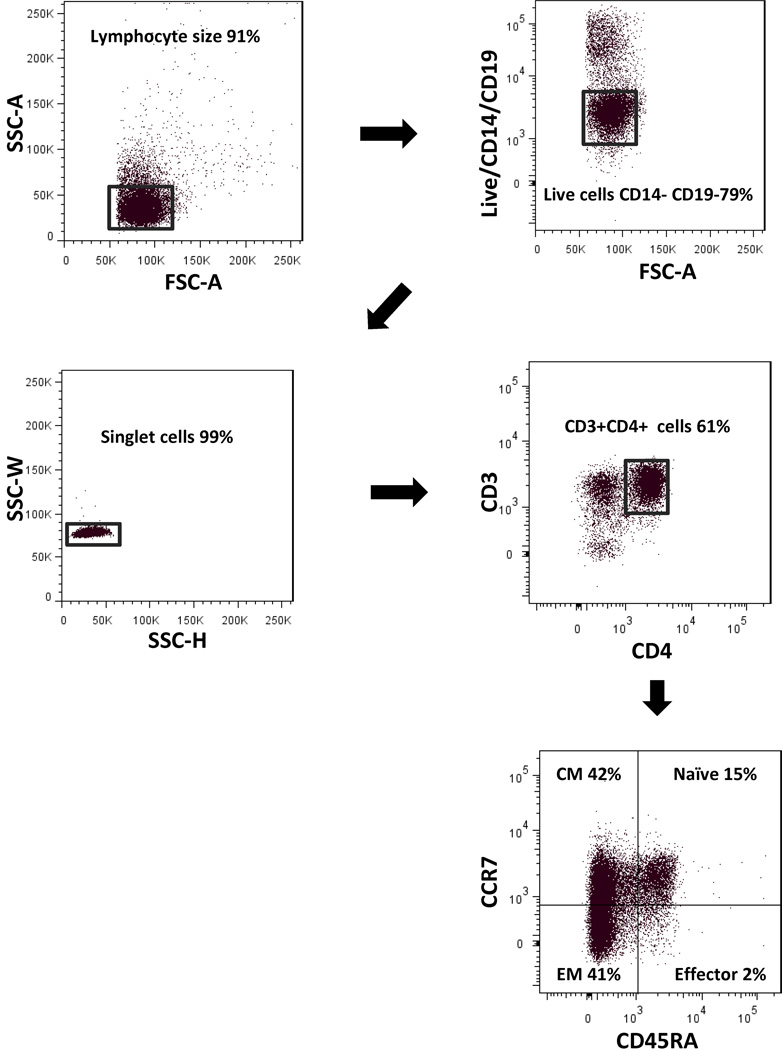
Fig. 3. Gating strategy for flow.
To minimize background staining and ensure that we were only detecting MTB-specific CD4+ T cells, a dump gate to exclude dead cells, CD14+ monocytes, and CD19+ B cells was used. Singlet cells were then gated on CD3+ CD4+ cells and analyzed for CCR7 and CD45RA expression to determine T cell subsets. CD4+ T cell subsets were defined as naïve CCR7+ CD45RA+, central memory CCR7+ CD45RA−, effector memory CCR7− CD45RA−, and effector as CCR7− CD45RA+.
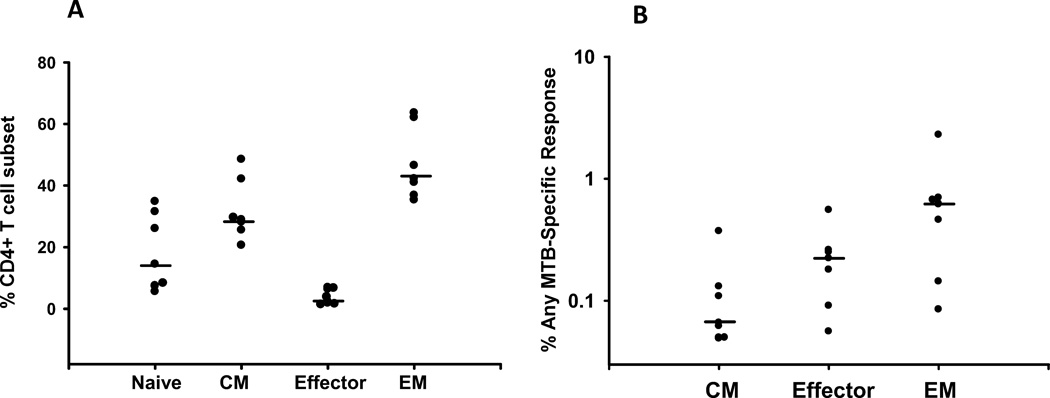
Fig. 4. Frequency of MTB-specific CD4+T cells in T cell subsets from Ugandan PFMC.
A. Percent CD4+ T cells in the subsets of naïve, central memory, effector, and effector memory phenotype of the Ugandan PFMC are depicted. The line represents the median of 7 subjects. B. PFMC from 7 subjects were incubated overnight with media or MTB and stained with markers for T cells subsets, TNF, IFN, IL-2, and MIP. Percent of each CD4+ T cell subset responding with any MTB-induced response minus the media only background is shown. A Boolean matrix was used to assure that a given positive event was not double counted. Sensitivity of <0.01% generally corresponded to <10 flow events less and was considered a lower limit of detection. Naïve cells were excluded from analysis as there were <10 flow events positive in all but one individual. The line represents the median of the 7 subjects.
Fig. 5. Cytokine expression patterns of PFMC.
Cytokine and MIP-1 alpha secretion of central and effector memory CD4+ T cells after MTB stimulation are shown. Percentages are of the MTB induced presentation within the T cell subsets. >89,000 cells were analyzed in each T cell subset. The background percentage in the unstimulated groups of central memory cells was <0.035% for TNF and <0.001 for MIP, IL-2, and IFN and for effector memory cells <0.018% for TNF and IL-2 and <0.007% for MIP and IFN.
Each CD4+ T cell subset underwent Boolean analysis to define one, two, three, and four function cells. The overall percentage of memory CD4+ T cell subsets demonstrating production of any of the three cytokines or MIP1-alpha after MTB stimulation is shown in Fig. 4B. MTB-specific production of each individual cytokine was determined using histogram analysis of unstimulated in comparison to MTB-stimulated cells. Central memory had less MTB-specific CD4+ T cells while effector memory cells have the greatest proportion of MTB-specific CD4+ T cells. Fritsch et al have shown that CCR7− CD27− memory CD4+ T cells are the more differentiated subpopulation (20). The IFN-gamma producing MTB-specific effector memory population in the PFMC had a higher proportion of CCR7− CD27− cells (median 84% S.D. 17.5%) than the overall population of effector memory cells (median 39% S.D. 9.9) suggesting that the MTB-specific cells overall were a more differentiated subpopulation of these cells.
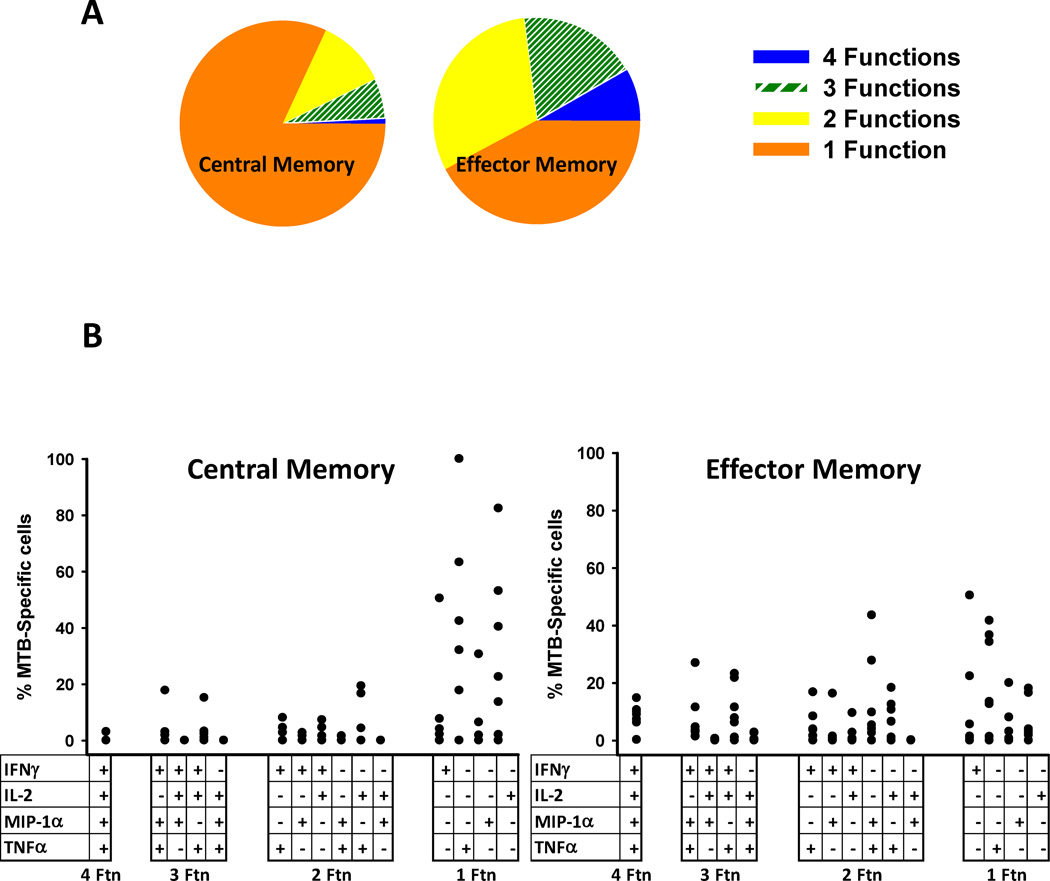
Due to the low percentage of effector cells (Fig. 4A) there were not enough flow events available for meaningful analysis of all of the 15 possible polyfunctional groups and the subsequent analysis will focus on central memory and effector memory cells. Fig. 6A demonstrates that >50% of the MTB-specific effector memory cell have at least 2 functions and >25% have 3 or 4 functions. Polyfunctional breadth is lower in central memory cells. Fig. 6B demonstrates the detailed profiles of both MTB-specific CD4+ T cell subsets. Overall this analysis of individual combinations of cytokine producing cells shows that there is a breadth of patterns of responses present.
Fig. 6. Polyfunctionality of MTB-specific CD4+ T cells in PFMC.
A. Average polyfunctionality of MTB-specific cells in each T cell subset. B. Total frequency of each individual response profile is shown on the y axis from the 7 subjects. Dots in each column represent data from each individual subject.
Discussion
We found the composition of T cell subsets was significantly biased toward effector and effector memory cells in pleural fluid compared to blood in subjects with active TB. Although several prior studies have noted increased percentages of memory cells as a whole in pleural fluid (6, 21–24) there has never been a report in human studies that further discriminated these memory populations into effector and central memory cells. Mouse models of tuberculosis infection have shown that terminally differentiated effectors cells accumulate in the lungs (9, 10). These are consistent with our model of active pleural TB in humans in that the MTB-specific effector cells have a marker prolife of increased differentiation (CD27 dim).
There are several possible mechanisms for the shift away from naïve cell in PFMC from TB patients. These include primarily differentiation of naive or central memory cells in situ or recruitment of effector cells to the site. We favour the later mechanism for several reasons. In the current studies the precursor frequency of MTB specific CD4+ T cells was not very high generally less than 1%. That would suggest that most of the effector memory cells that were present were recruited into rather than differentiated at the pleural site. Pokkali et al report a rich expression of CXC and CC chemokines in pleural fluid of subjects with TB pleurisy (19). These chemokines include a number that are known to recruit memory lymphocytes such as MCP-1 and IFN-gamma induced IP-10 and MIG (25, 26). These data indicate a strong basis for recruitment of memory T cells to pleural sites of MTB infection, rather than a predominance of local differentiation of naive cells at the pleural site.
Advances in flow cytometry over the last few years have allowed us to further define both T cell subsets and the functionality of antigen specific cells. A review on use of this technology to study polyfunctional T cells in TB was recently published (12). We will discuss our findings in the context of a number of other studies in the field. Two recent studies of blood from subjects pre- and post- treatment for pulmonary TB demonstrate that polyfunctionality of MTB-specific CD4+ cells is increased during active infection and declines after treatment (13, 16). Our studies of Ugandan PFMC showed that the percentage of highly polyfunctional cells was greater than they reported in PBMC from subjects with active pulmonary TB. This could reflect an expansion of antigen specific T cells at sites of active disease rather than blood. Another explanation could be the higher number of the more polyfunctional effector memory cells that are more abundant in PFMC than in PBMC.
It has recently been shown that even in PPD+ healthy subjects with no evidence of active disease that MTB-responsive CD4+ T cells in the lung are primarily of the effector memory phenotype and are at significantly greater frequency in the lung than in the blood (27). In a South African HIV-negative control population, Kaldorf et al found mycobacteria-specific CD4+ T cells in BAL had significant polyfunctionality although they did not determine the T cell subset types (14). Our data of the pleural space during active MTB disease showed the greatest proportion of MTB-specific cells were effector memory cells and that they also had the greatest polyfunctionality. The clinical significance of this subset compartmentalization and polyfunctional capability is yet to be determined. One outstanding issue is if increased polyfunctionality of T cells is an inevitable consequence of active infection or if these cells are necessary to provide protection and cure of active disease.
The recent review on the studies of polyfunctional T cell and TB has been published by Wilkinson and Wilkinson discusses some of these conundrums in the field (12). There appears to be great potential in use of mutiparameter flow cytometry to evaluate the polyfunctionality of T cells on a single cell basis. A key issue is the need to better understand what the role of polyfunctional T cells have with respect to protection and immunity to TB. They suggest that that the interpretation of polyfunctional T cell responses in MTB infection needs to be evaluated in longitudinal studies to determine the clinical relevance and correlates of the findings. This type of study was not possible in the case of pleural TB as the pleural effusion resolves readily after treatment.
Our data and others emerging now suggest that focusing only on MTB-induced IFN-gamma production may be missing a significant portion of MTB-responsive cells (28). We saw a significant portion of MTB-responsive cells that made more than just IFN-gamma or in fact do not produced IFN-gamma but rather made other responses including IL-2, TNF-alpha or MIP-1 alpha. This could have ramifications for example on the use of IFN-gamma release assays that are now widely used as a TB diagnostic tool. This suggests that future studies that focus on TB responsive cells may need to include other cytokines and chemokines. Also studying a broader array of effector molecules such as perforin and granulysin may be useful in future work using polyfunctional methods of study.
These data support the concept that the pleural space in TB pleurisy is an activated site with expanded memory CD4+ T cells predominantly of the effector and effector memory phenotypes. These are the cells most poised to generate a polyfunctional inflammatory response and also to contain mycobacterial infection. It is well known that a portion of subjects with TB pleural disease are able to resolve their TB pleurisy even without treatment. We postulate that a large influx of effector and effector memory CD4+ T cells into these sites is a key factor for this containment. In spite of this burst of effector type cells at this site, a significant portion of subjects are unable to contain their TB infection and develop clinical signs and symptoms of pulmonary disease. Thus, the burst of effector T cells at this site may be insufficient in most cases to both contain and eradicate TB infection. Future studies to understand the basis of CD4+ T cell immunity that favour control and eradication of MTB infection is essential for the development of a successful vaccine and for the design of therapeutic strategies to prevent disease.
Acknowledgements
Thank you for the assistance of Dr. W. Henry Boom and the Case Western Reserve School of Medicine TB Research Unit. This project was funded by the NIH grants AI-73217, AI-70022, AI-36219, and HL-51636.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
L El Fenniri, Email: elfenniril@yahoo.fr.
Z Toossi, Email: zxt2@case.edu.
H Aung, Email: hxa11@case.edu.
J Bourkkadi, Email: jebourkadi@yahoo.com.
J Benamor, Email: drjoudabenamor@yahoo.fr.
A Laskri, Email: hmedlaskri@live.fr.
N Berrada, Email: berrada-noama@hotmail.fr.
H Mayanja-Kizza, Email: hmk@chs.mak.ac.ug.
MR Betts, Email: betts@mail.med.upenn.edu.
R El Aouad, Email: rajaeelaouad@yahoo.fr.
DH Canaday, Email: dxc44@case.edu.
References
- 1.Luzze H, Elliott AM, Joloba ML, Odida M, Oweka-Onyee J, Nakiyingi J, Quigley M, Hirsch C, Mugerwa RD, Okwera A, Johnson JL. Evaluation of suspected tuberculous pleurisy: clinical and diagnostic findings in HIV-1-positive and HIV-negative adults in Uganda. Int J Tuberc Lung Dis. 2001;5:746–753. [PubMed] [Google Scholar]
- 2.Ozvaran MK, Baran R, Tor M, Dilek I, Demiryontar D, Arinc S, Toker N, Chousein EU, Sogukpinar O. Extrapulmonary tuberculosis in non-human immunodeficiency virus-infected adults in an endemic region. Ann Thorac Med. 2007;2:118–121. doi: 10.4103/1817-1737.33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyderman RS, Makunike R, Muza T, Odwee M, Kadzirange G, Manyemba J, Muchedzi C, Ndemera B, Gomo ZA, Gwanzura LK, Mason PR. Pleural tuberculosis in Harare, Zimbabwe: the relationship between human immunodeficiency virus, CD4 lymphocyte count, granuloma formation and disseminated disease. Trop Med Int Health. 1998;3:14–20. doi: 10.1046/j.1365-3156.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 4.Roper WH, Waring JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955;71:616–634. doi: 10.1164/artpd.1955.71.5.616. [DOI] [PubMed] [Google Scholar]
- 5.Patiala J. Initial tuberculous pleuritis in the Finnish armed forces in 1939–1945 with special reference to eventual postpleuritic tuberculosis. Acta Tuberc Scand Suppl. 1954;36:1–57. [PubMed] [Google Scholar]
- 6.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 7.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, Maskell N, Davies R, Pasvol G, Lalvani A. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis. 2005;40:184–187. doi: 10.1086/426139. [DOI] [PubMed] [Google Scholar]
- 9.Kapina MA, Shepelkova GS, Mischenko VV, Sayles P, Bogacheva P, Winslow G, Apt AS, Lyadova IV. CD27low CD4 T lymphocytes that accumulate in the mouse lungs during mycobacterial infection differentiate from CD27high precursors in situ, produce IFN-gamma protect the host against tuberculosis infection. J Immunol. 2007;178:976–985. doi: 10.4049/jimmunol.178.2.976. [DOI] [PubMed] [Google Scholar]
- 10.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 2010;40:2139–2142. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 13.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 14.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young JM, Adetifa IM, Ota MO, Sutherland JS. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One. 2010;5:e11237. doi: 10.1371/journal.pone.0011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Mahan CS, Palaci M, Horter L, Loeffelholz L, Johnson JL, Dietze R, Debanne SM, Joloba ML, Okwera A, Boom WH, Eisenach KD. Sputum Mycobacterium tuberculosis mRNA as a marker of bacteriologic clearance in response to antituberculosis therapy. J Clin Microbiol. 2010;48:46–51. doi: 10.1128/JCM.01526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desjardin LE, Perkins MD, Wolski K, Haun S, Teixeira L, Chen Y, Johnson JL, Ellner JJ, Dietze R, Bates J, Donald CM, Eisenach KD. Measurement of Sputum Mycobacterium tuberculosis Messenger RNA as a Surrogate for Response to Chemotherapy. Am J Respir Crit Care Med. 1999;160:203–210. doi: 10.1164/ajrccm.160.1.9811006. [DOI] [PubMed] [Google Scholar]
- 19.Pokkali S, Das SD, L R. Expression of CXC and CC type of chemokines and its receptors in tuberculous and non-tuberculous effusions. Cytokine. 2008;41:307–314. doi: 10.1016/j.cyto.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 21.Mitra DK, Sharma SK, Dinda AK, Bindra MS, Madan B, Ghosh B. Polarized helper T cells in tubercular pleural effusion: phenotypic identity and selective recruitment. Eur J Immunol. 2005;35:2367–2375. doi: 10.1002/eji.200525977. [DOI] [PubMed] [Google Scholar]
- 22.Aguiar LM, Antonangelo L, Vargas FS, Zerbini MC, Sales MM, Uip DE, Saldiva PH. Malignant and tuberculous pleural effusions: immunophenotypic cellular characterization. Clinics (Sao Paulo) 2008;63:637–644. doi: 10.1590/S1807-59322008000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faith A, Schellenberg DM, Rees AD, Mitchell DM. Antigenic specificity and subset analysis of T cells isolated from the bronchoalveolar lavage and pleural effusion of patients with lung disease. Clin Exp Immunol. 1992;87:272–278. doi: 10.1111/j.1365-2249.1992.tb02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues DS, Medeiros EA, Weckx LY, Bonnez W, Salomao R, Kallas EG. Immunophenotypic characterization of peripheral T lymphocytes in Mycobacterium tuberculosis infection and disease. Clin Exp Immunol. 2002;128:149–154. doi: 10.1046/j.1365-2249.2002.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 27.Walrath J, Zukowski L, Krywiak A, Silver RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am J Respir Cell Mol Biol. 2005;33:48–55. doi: 10.1165/rcmb.2005-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, Hanekom WA. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]