Abstract
The prevalence of Fragile X Syndrome (FXS) is 1 in 4000 in males and 1 in 2500 in males and females, respectively, in the general population. Several screening studies aimed at determining the prevalence of FXS have been conducted in individuals with intellectual disabilities (IDs) with a prevalence varying from 1.15% to 6.3% across different ethnic groups. A previous study in Indonesia showed an FXS prevalence of 1.9% among the ID population. A rapid, effective, and inexpensive method for FMR1 screening, using dried blood spots capable of detecting an expanded FMR1 allele in both males and females, was recently reported. We used this approach to screen 176 blood spots, collected from Central Java, Indonesia, for the presence of expanded FMR1 gene alleles. Samples were collected from high-risk populations: 112 individuals with ID, 32 obtained from individuals with diagnosis of autism spectrum disorders, and 32 individuals with a known family history of FXS. Fourteen subjects carrying an FMR1 expanded allele were identified including 7 premutations (55–200 CGG repeats) and 7 full mutations (>200 repeats). Of the seven subjects identified with a full mutation, one subject was from a non-fragile X family, and six from were families with a history of FXS.
Introduction
Fragile X syndrome (FXS), with a prevalence of 1:2500 to 1:4000 in the general population (Turner et al., 1996; Crawford et al., 2002; Hagerman, 2008), is the most common genetic cause of intellectual disability (ID) and the leading genetic cause of autism. Although prevalence estimates vary across studies, there is agreement that the risk of autism spectrum disorders (ASD) is higher in FXS than in many other neuro-developmental disorders. From recent studies, ∼2% to 6% of children with ASD have FXS (Li et al., 1993; Wassink et al., 2001; Estecio et al., 2002; Hagerman, 2002; Reddy, 2005), and ∼30% of children with FXS have ASD (see Appendix in Rogers et al., 2001; Kaufmann et al., 2004); Pervasive Developmental Disorder-Not Otherwise Specified is seen in an additional 30% (Harris et al., 2008). In fact, FXS is characterized by a broad spectrum of behavioral and emotional impairment, psychological problems, and learning disabilities in those without mental retardation or ID. In addition, specific physical features such as long face, macrognathia, prominent ears, hyperextensible joints, flat feet, and macroorchidism in puberty are observed (Hagerman et al., 1991; Lachiewicz and Dawson, 1994; Giangreco et al., 1996). The milder phenotypes are not accounted for in the current prevalence figures (Tassone et al., 1999; Hagerman, 2006). In addition, significant phenotypic involvement has emerged in some individuals with the premutation (55–200 CGG repeats), including fragile X-associated premature ovarian insufficiency (FXPOI) in females, and fragile X-associated tremor/ataxia syndrome (FXTAS) in both male and female aging carriers (Hagerman and Hagerman, 2004; Hagerman et al., 2004; Sullivan et al., 2005). Prevalence for premutation alleles was ∼1 in 110–250 for females and 1 in 250–800 for males (Rousseau et al., 1995; Toledano-Alhadef et al., 2001; Dombrowski et al., 2002; Fernandez-Carvajal et al., 2009).
The first population study of FXS performed in Indonesia, based on the screening of 176 subjects (92 males and 84 females), indicates a prevalence of 1.9% (Faradz et al., 1999). Special education needs schools, developmental disorders clinics, or health care centers are the most appropriate places for FXS screening in developing countries. In the past several years, the interest of FXS studies has turned treatment studies, both pharmacological and nonpharmacological intervention, and has emphasized the importance of early intervention for minimizing behavioral impairment and optimizing cognitive functioning. FXS, caused by immature branching of dendrites, results in lack of fragile X mental retardation protein (FMRP), an RNA binding protein important for synaptic plasticity (Zalfa et al., 2003). The awareness of fragile X-associated disorders, such as FXTAS and FXPOI, recently became more pronounced and reinforced the statement that FXS is a genetic disorder with broad clinical family involvement (Chonchaiya et al., 2009). Expanded clinical involvement, especially medical co-morbidity and neurobehavioral disorders that include thyroid dysfunction, high blood pressure, peripheral neuropathy, fibromyalgia, depression, and anxiety, has been reported as common among premutation females (Johnston et al., 2001; Hessl et al., 2005; Coffey et al., 2008; Bourgeois et al., 2009, 2010; Roberts et al., 2009; Hunter et al., 2010; Lachiewicz et al., 2010). Finally, lowered FMRP expression and/or elevated FMR1 mRNA, observed in premutation carriers, combined with environmental factors may lead to ASD and/or symptoms of attention deficit hyperactivity disorders (ADHD), social deficits, and learning disabilities in young premutation individuals (Tassone et al., 2000; Aziz et al., 2003; Goodlin-Jones et al., 2004; Farzin et al., 2006; Hagerman, Hoem et al., 2010).
Thus, to identify individuals with FXS and FMR1-associated disorders and to provide early intervention services for children, an FMR1-sensitive and -specific screening method for both males and females is needed in countries with a large population, such as Indonesia. Tassone et al. (2008) introduced a rapid, effective, and inexpensive method for screening both males and females for FMR1 allele sizes throughout the premutation and full-mutation range and is possible by using dried blood spots (Fernandez-Carvajal et al., 2009; Yuhas et al., 2009); thus, this method is suitable for large population screenings. Here, we present a high-risk screening in Central Java, Indonesia, including 176 subjects (92 males and 84 females) with ID and/or known family history of FXS.
Materials and Methods
Participants
Blood spots were collected from 92 males and 84 females (n=176), including 112 individuals with IDs, 32 with a diagnosis of ASD, and 32 individuals who were the relative of someone with a previous diagnosis of FXS. Samples were collected from Central Java, Indonesia. One hundred twelve subjects with ID were screened from special need schools, 26 of whom were from schools for children with autism, and 6 subjects were mothers of children with autism. This study was approved by the Institutional Review Board of the University of California Davis and the Medical Ethical Committee of the Faculty of Medicine, Diponegoro University.
Screening methodology
Blood spots were collected from each subject on FTA cards (QIAcard FTA; Qiagen, Valencia, CA). Polymerase chain reaction (PCR) analysis was performed either directly on a punch from the dry blood spot, or from isolated DNA. Samples were prepared for PCR in two ways: washing the blood spots and DNA isolation. Washing a 2 mm blood spot disk was performed by using FTA purification reagents (Qiagen) using manufacturer's instructions. Briefly, three 5-min incubations with 200 μL of purification reagents were followed by two 5-min incubations with 200 μL of Tris-sodium EDTA buffer. Spots had been dried at room temperature for 1 h before they were directly introduced into the PCR mix (Tassone et al., 2008). For DNA isolation, two disks of 3 mm in diameter were punched from the blood spot, heated with digest solution at 57°C, and processed in a Qiaxtractor (Qiagen) according to the manufacturer's protocol. The PCR amplification was performed by using primer c and f conditions as previously described (Fu et al., 1991; Fernandez-Carvajal et al., 2009). The PCR products were visualized by using the Qiaxcel genetic analyzer (Qiagen) or by capillary electrophoresis (CE) (ABI 3100; Applied Biosystems, Carlsbad, CA). Results from the Qiaxcel genetic analyzer were analyzed as described in Fernandez-Carvajal et al. (2009). The CE results were analyzed by using the ABI Peak Scanner software (Applied Biosystems). DNA samples from men that did not yield a band after the first round of PCR, or DNA samples from females that yielded only one normal band with primers c and f, were subjected to a secondary, chimeric-CGG-primer-based PCR screening, as previously described (Tassone et al., 2008; Chen et al., 2010). The PCR amplicons were subjected to visualization on an agarose gel (Tassone et al., 2008) or by (CE) (Chen et al., 2010).
Follow-up FXS molecular diagnosis
Diagnosis of FXS was confirmed on DNA isolated from peripheral blood leucocytes by using standard procedures; PCR and Southern blot analysis were performed as detailed in Tassone et al. (2008).
Results
One hundred seventy-six blood spots (144 from populations with ID/ASD and 32 from known fragile X families) were screened for the presence of an expanded FMR1 allele. One full mutation (one male) (1/144) was identified within the ID population, and six full mutations (six females) and seven premutation individuals (two males, five females) were identified in families with a history of FXS (Fig. 1). Thus, prevalence of FXS full mutation in this high-risk population from Central Java, Indonesia, is 4.0% (1.1% in males and 7.1% in females; 95% confidence interval (2.0%, 8.1%) in agreement with reports from previous studies in other populations) (Sutherland, 1985; Syrrou et al., 1998; de Vries et al., 1999; Faradz et al., 1999; Pouya et al., 2009). However, the FXS full mutation was 0.72% (1/139) in the ID populations alone.
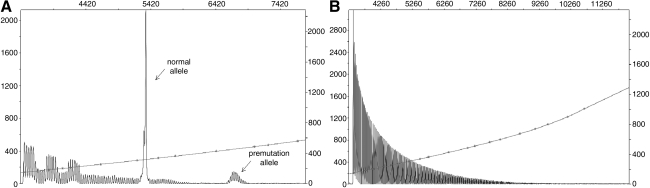
FIG. 1.
(A) Capillary Electrophoregram (CE) from a premutation female showing the normal and premutation alleles (arrows) obtained by CGG repeat-primed PCR. (B) Electrophoregram from a full mutation male. The CGG repeat PCR produces in this case an uninterrupted series of triplet repeat products that indicate the presence of a long, expanded FMR1 allele.
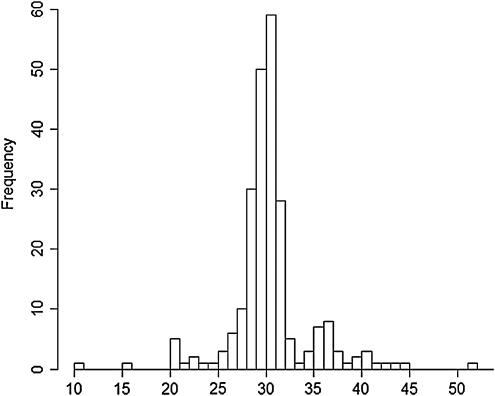
The FMR1 gene allele size distribution in both genders ranged between 10 and 51, with a modal number of 30 CGG repeats (25.0%), followed by 29 CGG repeats (21.2%). Interestingly, 3% and 3.4% of the alleles contained 35 and 36 CGG repeats, respectively, as previously reported in an Asian population (Zhong et al., 1994; Chiang et al., 1999; Faradz et al., 2001). Allele size distribution is shown in Figure 2.
FIG. 2.
Allele size distribution from the 176 subjects (92 men and 84 women) screened. The most common alleles in this population were 30 and 29 CGG repeats. An increased prevalence for 35 and 36 CGG repeats was also observed.
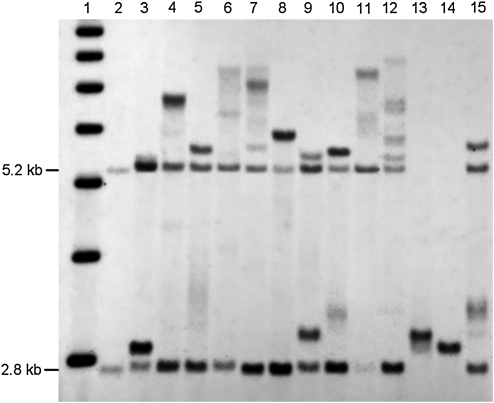
Follow-up studies to confirm FXS diagnosis were performed on those subjects found to be carriers of an FMR1 expanded allele during the blood spot screening process. Additional family members of those who had screened positive were also tested for the FXS mutation. Fragile X diagnosis was confirmed in one full mutation subject among the ID group, in addition to an uncle and a cousin. Among three families with known cases of FXS, 14 expanded alleles were identified. Confirmatory diagnosis was performed by Southern Blot/PCR analysis as depicted in Figure 3. Detailed results of individuals with an expanded FMR1 allele from known fragile X families are presented in Table 1.
FIG. 3.
Diagnosis of FXS was confirmed by Southern Blot analysis in 13 subjects identified through the blood spot screening. DNA was digested with Eco RI and Nru I, and stB 12.3 probes were used (Tassone et al. 2008). The 1 kb ladder markers is show in lane1. Lane 2: normal female control showing a normal unmethylated band (2.8Kb) and normal methylated band (5.2Kb). Lanes 3, 5, 9, 10, and 15: premutation females. Lanes 4, 6, 7, 8, 11, and 12: full mutation females. Lanes 13 and 14: premutation male.
Table 1.
Result from Blood Spot Screening Among Individuals with Known Family History of Fragile X Syndrome
| Family | Σ Screened (M, F) | Σ Premutation (M, F) | Σ Full mutation (M, F) | Σ Normal (M, F) |
|---|---|---|---|---|
| A | 22 (6, 16) | 3 (1, 2) | 5 (0, 5) | 14 (5, 9) |
| B | 4 (0, 4) | 1 (0, 1) | 2 (0, 2) | 1 (0, 1) |
| C* | 6 (4,2) | 2 (1, 1) | 1 (1, 0) | 3 (2, 1) |
| Total | 32 (10, 22) | 6 (2, 4) | 8 (1, 7) | 18 (7, 11) |
C* New FXS case from ID and or autism population.
M, male; F, female; FXS, fragile X syndrome; ID, intellectual disability.
Discussion
A number of studies have been carried out in populations of individuals with IDs, with a prevalence varying from ∼1% to 6.3% (Sutherland, 1985; Syrrou et al., 1998; de Vries et al., 1999; Faradz et al., 1999; Pouya et al., 2009). A previous study in Indonesia showed an FXS prevalence of 1.9% among the ID population (Faradz et al., 1999).
We present the first blood spot screening for FXS in a high-risk population in Indonesia by using a new approach that is capable of screening for FMR1 mutations throughout premutation and the full mutation range in both males and females. This screening approach is affordable, effective, and less time consuming compared with the standard diagnostic methods for FXS, and, therefore, feasible for large population screening. Results of this screening found 1 full mutation allele out of 139 subjects (0.72%) with ID, which is lower than the prevalence previously described in another study (1.9%) (Faradz et al., 1999) among 254 children with ID, likely due to their smaller sample size.
The most common alleles contain 30 and 29 CGG repeats, which is concordant with our previous studies (Faradz et al., 2001) among a Chinese population (Zhong et al., 1994) and among a non-Caucasian population (Kunst et al., 1996; Faradz et al., 2001). An allele frequency of 28 CGG repeats was also common in the Asian population. In 1999, Chiang et al. reported 45% of individuals carrying an allele of 28 CGG repeats, among a representative Chinese population (Chiang et al., 1999). Interestingly, a minor peak was found in 3% and 3.4% of alleles with higher CGG-repeat numbers (35 and 36). A previous study done in Indonesia reported a frequency of 8.7% for alleles with 36 CGG repeats, whereas higher frequencies were reported for alleles with 34, 35, and 36 CGG repeats in an Asian population (Zhong et al., 1994; Chiang et al., 1999; Faradz et al., 2001).
A recent report showed evidence of broad clinical involvement in FMR1 gene mutations reported among premutation and gray zone alleles (Hall et al., 2006; Loesch et al., 2009) including Idiopathic Parkinson's diseases, ASD and ADHD, FXPOI, and FXTAS (Tassone et al., 2000; Jacquemont et al., 2003; Goodlin-Jones et al., 2004; Jacquemont et al., 2004; Sullivan et al., 2005; Toft et al., 2005; Kraff et al., 2007; Cilia et al., 2009). Thus, a robust screening method is needed to accommodate future directions of early intervention and anticipation. We have performed our novel blood spot screening method on a high-risk population in Indonesia to demonstrate the validity of this screening tool for the diagnosis of FXS, which is especially valuable in locations where genetic testing is virtually inaccessible.
Acknowledgments
This work was supported by the National Institutes of Health (R01 HD02274; FT). The authors thank all the participants and their families for their contributions. They also thank Lidia Tilehun for her technical assistance. The first author is the addressee of an Excellent Scholarship Program of The Bureau of Planning and International Cooperation, Ministry of National Education, Government of Indonesia.
This work is dedicated to the memory of Matteo.
Disclosure Statement
No competing financial interests exist.
References
- Aziz M. Stathopulu E, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet. 2003;121B:119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- Bourgeois J. Seritan A, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2010;72:175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JA. Coffey SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70:852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Hadd A, et al. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn. 2010;12:589–600. doi: 10.2353/jmoldx.2010.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S-C. Lee Y-M, et al. Allele distribution at the FMR1 locus in the general Chinese population. Clin Genet. 1999;55:353–356. doi: 10.1034/j.1399-0004.1999.550509.x. [DOI] [PubMed] [Google Scholar]
- Chonchaiya W. Utari A, et al. Broad clinical involvement in a family affected by the fragile X premutation. J Dev Behav Pediatr. 2009;30:544–551. doi: 10.1097/DBP.0b013e3181c35f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R. Kraff J, et al. Screening for the presence of FMR1 premutation alleles in women with parkinsonism. Arch Neurol. 2009;66:244–249. doi: 10.1001/archneurol.2008.548. [DOI] [PubMed] [Google Scholar]
- Coffey SM. Cook K, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC. Meadows KL, et al. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- de Vries BBA. Mohkamsing S, et al. Screening for the among the mentally retarded: a clinical study. J Med Genet. 1999;36:467–470. [PMC free article] [PubMed] [Google Scholar]
- Dombrowski C. Levesque S, et al. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002;11:371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- Estecio M. Fett-Conte AC, et al. Molecular and cytogenetic analyses on Brazilian youths with pervasive developmental disorders. J Autism Dev Disord. 2002;32:35–41. doi: 10.1023/a:1017952123258. [DOI] [PubMed] [Google Scholar]
- Faradz SM. Leggo J, et al. Distribution of FMR1 and FMR2 alleles in Javanese individuals with developmental disability and confirmation of a specific AGG-interruption pattern in Asian populations. Ann Hum Genet. 2001;65(Pt 2):127–135. doi: 10.1017/S0003480001008521. [DOI] [PubMed] [Google Scholar]
- Faradz SMH. Buckley M, et al. Molecular screening for fragile X syndrome among Indonesian children with developmental disability. Am J Med Genet A. 1999;83:350–351. [PubMed] [Google Scholar]
- Farzin F. Perry H, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(2 Suppl):S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Fernandez-Carvajal I. Walichiewicz P, et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009;11:324–329. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y-H. Kuhl DPA, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Giangreco CA. Steele MW, et al. A simplified six-item checklist for screening for fragile X syndrome in the pediatric population. J Pediatr. 1996;129:611–614. doi: 10.1016/s0022-3476(96)70130-0. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones B. Tassone F, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25:392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ. Hagerman RJ. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) Ment Retard Dev Disabil Res Rev. 2004;10:25–30. doi: 10.1002/mrdd.20005. [DOI] [PubMed] [Google Scholar]
- Hagerman R. Hoem G, et al. Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ. Physical and behavioral phenotype. Fragile X syndrome: diagnosis, treatment and research. In: Hagerman RJ, editor; Hagerman PJ, editor. Fragile X syndrome. 3rd. The Johns Hopkins University Press; Baltimore: 2002. pp. 3–109. [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Amiri K, et al. Fragile X checklist. Am J Med Genet. 1991;38:283–287. doi: 10.1002/ajmg.1320380223. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Leavitt BR, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA. Hagerman RJ, et al. Prevalence of FMR1 repeat expansions in movement disorders. A systematic review. Neuroepidemiology. 2006;26:151–155. doi: 10.1159/000091656. [DOI] [PubMed] [Google Scholar]
- Harris SW. Hessl D, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D. Tassone F, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hunter JE. Rohr JK, et al. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77:374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S. Hagerman RJ, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S. Hagerman RJ, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Johnston C. Eliez S, et al. Neurobehavioral phenotype in carriers of the fragile X premutation. Am J Med Genet. 2001;103:314–319. [PubMed] [Google Scholar]
- Kaufmann WE. Cortell R, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kraff J. Tang HT, et al. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol. 2007;64:1002–1006. doi: 10.1001/archneur.64.7.1002. [DOI] [PubMed] [Google Scholar]
- Kunst CB. Zerylnick C, et al. FMR1 in global populations. Am J Hum Genet. 1996;58:513–522. [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz A. Dawson D, et al. Indicators of anxiety and depression in women with the fragile X premutation: assessment of a clinical sample. J Intellect Disabil Res. 2010;54:597–610. doi: 10.1111/j.1365-2788.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Lachiewicz AM. Dawson DV. Do young boys with fragile X syndrome have macroorchidism? Pediatrics. 1994;93(6 Pt 1):992–995. [PubMed] [Google Scholar]
- Li SY. Chen YC, et al. Molecular and cytogenetic analyses of autism in Taiwan. Hum Genet. 1993;92:441–445. doi: 10.1007/BF00216447. [DOI] [PubMed] [Google Scholar]
- Loesch DZ. Khaniani MS, et al. Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism. Clin Genet. 2009;76:471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouya AR. Abedini SS, et al. Fragile X syndrome screening of families with consanguineous and non-consanguineous parents in the Iranian population. Eur J Med Genet. 2009;52:170–173. doi: 10.1016/j.ejmg.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE. Bailey DB, Jr, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. Wehner EA, et al. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rousseau F. Rouillard P, et al. Prevalence of carriers of premutation-size alleles of the FMRI gene—and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57:1006–1018. [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK. Marcus M, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Sutherland GR. Heritable fragile sites on human chromosomes. XII. Population cytogenetics. Ann Hum Genet. 1985;49(Pt 2):153–161. doi: 10.1111/j.1469-1809.1985.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Syrrou M. Georgiou I, et al. FRAXA and FRAXE prevalence in patients with nonspecific mental retardation in the Hellenic population. Genet Epidemiol. 1998;15:103–109. doi: 10.1002/(SICI)1098-2272(1998)15:1<103::AID-GEPI8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tassone F. Hagerman RJ, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- Tassone F. Hagerman RJ, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F. Pan R, et al. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft M. Aasly J, et al. Parkinsonism, FXTAS, and FMR1 premutations. Mov Disord. 2005;20:230–233. doi: 10.1002/mds.20297. [DOI] [PubMed] [Google Scholar]
- Toledano-Alhadef H. Basel-Vanagaite L, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351–360. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. Webb T, et al. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wassink TH. Piven J, et al. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11:57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Yuhas J. Walichiewicz P, et al. High-risk fragile x screening in Guatemala: use of a new blood spot polymerase chain reaction technique. Genet Test Mol Biomarkers. 2009;13:855–859. doi: 10.1089/gtmb.2009.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F. Giorgi M, et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhong N. Liu X, et al. Distribution of FMR-1 and associated microsatellite alleles in a normal Chinese population. Am J Med Genet. 1994;51:417–422. doi: 10.1002/ajmg.1320510423. [DOI] [PubMed] [Google Scholar]