Abstract
This study examines psychological determinants and effects of participating in genetic testing among persons diagnosed with or at risk for developing primary pulmonary arterial hypertension. Longitudinal data were drawn from orally administered surveys with 70 affected or at-risk individuals concerning their thoughts, feelings, and decision making about testing for mutations in BMPR2. Distress was measured by use of the Impact of Events Scale. Variations in tolerance for ambiguity were also examined. Although uptake of testing was low, as is common for incompletely penetrant mutations that lack clear therapeutic interventions, we found that those who participated in testing evidenced greater reduction in distress compared to those who had not participated in testing, irrespective of test result. No differences in tolerance for ambiguity by testing status were found. Participation in genetic testing, irrespective of test results, may be particularly beneficial to individuals who may have genetic mutations and who are experiencing high levels of distress.
Introduction
Participation in predisposition genetic testing to assess disease risk is increasingly common. Nonetheless, at-risk patients vary widely in the decision to undergo testing once a test becomes available. This is true even in cases where patients expressed high levels of interest in testing prior to the discovery of the genes involved (Codori et al., 1999). Not surprisingly, the most potent predictor of uptake is the availability of effective and acceptable preventive and therapeutic interventions (Barnoy, 2007). Other variably predictive indicators of uptake (Ropka et al., 2006) include levels of distress (Keller et al., 2004; Dorval et al., 2008), higher perceived disease risk (Shiloh and Ilan, 2005), greater worry about disease (Foster et al., 2004), anticipated ability to handle adverse results (Thompson et al., 2002; Hadley et al., 2003), test readiness (Taylor, 2005), desire for emotional relief (Smith et al., 2004), and lower levels of depression (Codori et al., 1999; Lerman et al., 1999). Often those who undergo predictive genetic testing believe that their risk for developing a disorder is high, even in cases where risk is, in fact, low. In many instances, participation in genetic testing is a method for coping with concerns about disease risk, irrespective of actual level of risk (Metcalfe et al., 2007).
Despite these findings, outcome research on the effects of genetic testing has focused most heavily on reactions to test results and less on reactions to test participation. To date, most research on the effects of predictive genetic testing utilizes an “informative model,” assessing knowledge of and reactions to accurate information, and focusing on processes of cognitive decision making and psychological adaptation prior to and after results (Lerman and Croyle, 1996). In contrast, “transactional models” of stress and coping include an emphasis on behavioral adaptation to aversive states of mind or circumstance (Gooding et al., 2006) and receive less attention. This study seeks to expand the framework for understanding coping responses to genetic testing by focusing on determinants and effects of participation in testing as a form of behavioral coping with distress, irrespective of degree of risk or test results.
Our study further seeks to broaden the array of diseases examined in the literature. Most studies of predisposition testing have concentrated heavily on two broad groups of disorders, cancer and neurological disorders. Because of the particular social meaning of these two groups of disorders, research on predictors of uptake may not be completely generalizable to the full gamut of disorders for which predisposition genetic testing is becoming available. The risk of cancer, for example, evokes complex responses in the United States, ranging from fear to battle cries and solidarity. By contrast, neurological disorders are particularly stigmatizing. Recognizing the need to expand the types of disorders addressed, investigators have begun to explore why people chose to accept or decline predisposition genetic tests for other types of disorders, such as alpha-1-antitrypsin deficiency (Dickson et al., 2008), hemochromatosis (Patch et al., 2005), and inflammatory bowel disease (Lewis et al., 2009). Our focus on pulmonary arterial hypertension (PAH), a disease which has received scant public attention, is an addition to this emergent body of research.
To gain perspective on how people use predisposition genetic testing, we began studying PAH more than a decade ago. This disorder is insidious, affects primarily younger women, and is poorly amenable to treatment (Badesch et al., 2009). Characterized by increased pressure in the pulmonary arteries, PAH causes shortness of breath, fatigue, chest pain, enlargement of the heart, and eventual heart failure. Although advances in treatment are prolonging life, most of the affected patients die within several years of diagnosis unless they receive a heart–lung transplant. In some cases, this disease is attributable to mutations in BMPR2 (Austin et al., 2009). The mutations in this gene are only approximately 20% penetrant. As a result, PAH can “run in” families, and unaffected carriers can pass the mutation on to their affected children. Because mutations are incompletely penetrant, they are also found in some apparently “sporadic” cases, indicating that their family members are also at risk. Before the role of BMPR2 was defined, the majority of patients with PAH and their at-risk relatives expressed a desire to be tested (Lientz and Clayton, 2000). When testing became clinically available and was offered to patients and at-risk relatives, however, uptake was very low (Hannig et al., 2008; Jones et al., 2008), as is common for predisposition tests for disorders for which there is no curative therapy. Using previously defined survey measures that are administered as part of telephone interviews, we sought to understand why some patients and at-risk individuals chose to pursue or to reject testing as well as the psychological impact of their decisions. We hypothesized that test users would have been more distressed and more intolerant of ambiguity prior to testing and that participation in testing would be helpful to them.
Distress and intolerance for ambiguity are two commonly assessed constructs in the genetic testing literature. As standard measures, we use the impact of events [Impact of Events Scale) IES] (Horowitz et al., 1979) and intolerance for ambiguity scales [Tolerance for Ambiguity Scale (TFA)] (Geller et al., 1993). Distress has been found to increase likelihood of testing (Keller et al., 2004) and to vary, depending upon test results (Lerman and Croyle, 1996). To date, no studies have explored changes to IES prior to and following participation in testing, irrespective of test results. In addition, intolerance for ambiguity is believed to have unique relevance to the field of genetic testing, given the probabilistic nature of test results for incompletely penetrant disorders, like PAH. Previous research suggests that those with a greater tolerance for ambiguity may have a stronger interest in discussing (Geller et al., 1993) and participating (Baty et al., 2006) in testing. We report here the results of these surveys as well as responses to other questions about testing.
Patients and Methods
Setting and participant enrollment
The Institutional Review Board of Vanderbilt University, Nashville, Tennessee, approved this study. Respondents were recruited in one of four ways: in person at the Pulmonary Hypertension clinic at Vanderbilt University; through written correspondence with persons on the Vanderbilt PAH registry of patients known to have familial PAH and their family members; through written correspondence with members of one Southeastern PAH support group; or in person at the 2006 Pulmonary Hypertension conference held in Minneapolis, Minnesota. Upon receipt of signed consent, participants were contacted via phone for interview. Interviews were closed ended and usually lasted 30–45 min, both at time 1 and at follow-up approximately 18 months later. All respondents were offered $25 for their participation and provided copies of written informed consent and contact information to address follow-up questions or concerns.
In order to include only those individuals who had or were at risk for pulmonary hypertension related to mutations in BMPR2, participants were screened to exclude other potential causes of PAH, including scleroderma, scleroderma-related disease, congenital heart disease, liver disease, and human immunodeficiency virus (HIV), as well as previous use of the diet drug Fen-phen. From January 2006 to May 2007, 119 adults diagnosed with or at risk for developing PAH completed the first interview. Approximately 18 months later, from August 2007 to September 2008, attempts were made to conduct a follow-up interview. Eighty-three of the original participants (70%) were reached for follow-up interviews, which included a series of questions regarding their perceptions of genetic testing, genetic testing status, PAH diagnosis, subjective disease-related distress, as assessed by the IES (Horowitz et al., 1979), and other demographic information. Prior to follow-up, all the respondents had been informed about the availability of clinical genetic testing for mutations in BMPR2 through study consent forms as well as from clinicians (91%) or through PHA support groups and newsletters (9%).
Scaled measures
This study used the IES (Horowitz et al., 1979) to assess respondent level of subjective distress about PAH at Survey 1 and at follow-up. The IES is a 15-item self-report questionnaire that has been widely used to assess levels of distress (Weiss, 2004), in response to a range of events including exposure to traumatic incidents, prolonged environmental stressors, poor health status, and disease risk. More specifically, IES has been used and validated among persons at risk for respiratory (Chan and Chan, 2004) and pulmonary (Baumert et al., 2004) conditions. Eight IES items assess avoidance, while seven address intrusion. The frequency of each is rated on a 4-point scale, which is rated as 0 (not at all), 1 (rarely), 3 (sometimes), or 5 (often). The IES has demonstrated high internal consistency with alpha coefficients of 0.82 for avoidance and 0.78 for intrusion subscales. Split-half reliability of the total scale was 0.86 (Horowitz et al., 1979). Alpha coefficients in this study sample are presented in the “Results” section of this article.
Possible IES scores range from 0 to 35 on Intrusion, 0 to 40 on Avoidance, and 0 to 75 on Total Distress. Although IES cannot be used to diagnose posttraumatic stress disorder (PTSD), since the scale does not address all PTSD symptoms, a suggested cutoff for identifying moderate-to-severe PTSD on the IES Total Distress Scale is > 26. During the initial interview, respondents were also asked questions from the TFA (Geller et al., 1993). Both the IES and TFA scales have been independently validated.
Statistical analysis
All analyses were performed using Statistical Package for the Social Sciences (SPSS), version 18.0. Descriptive percentage and mean summary statistics were conducted on demographic and other descriptive data. Comparisons of persons who reported having been tested for mutations in BMPR2 and those not tested were conducted by performing mean comparison t-tests across demographic and other previously identified measures. Repeated measures Analysis of Variance (ANOVA) was conducted to assess the effects of distress (Total, Intrusive, and Avoidant) by testing status over time.
Results
Demographics
One hundred and nineteen respondents participated in an initial interview from January 2006 to 2007. Seventy respondents were interviewed 18 months later (59%) and were included in this analysis. Descriptive statistics for the respondents are shown in Table 1.
Table 1.
Descriptive Statistics
| |
First interview |
Follow-up interview |
|---|---|---|
| |
n=119 |
n=70 |
| N (%) | N (%) | |
| Recruitment site | ||
| Vanderbilt clinic | 33 (29) | 20 (29) |
| Vanderbilt registry | 44 (33) | 19 (27) |
| Southeastern PAH support group | 9 (8) | 6 (9) |
| Pulmonary hypertension conference 2006 | 32 (27) | 25 (36) |
| Self reported diagnostic status | ||
| Sporadic | 66 (55) | 42 (60) |
| FPAH | 22 (18) | 16 (23) |
| At risk | 31 (26) | 12 (17)a |
| Demographics | ||
| Female | 95 (80) | 56 (80) |
| Average Age | 50 | 50 |
| White | 104 (87) | 62 (89) |
| Married | 90 (76) | 54 (77) |
| Any children | 98 (82) | 57 (81) |
| Some college | 79 (66) | 53 (77)a |
| Income | ||
| <35k | 34/114 (30) | 14 (21) |
| 35–50k | 22/114 (19) | 11 (16) |
| >50k | 58/114 (51) | 42 (63)a |
| Insured | 115 (97) | 68 (97) |
Differences are statistically significant at p<0.05.
PAH, pulmonary arterial hypertension; FPAH, familial PAH.
Response rates for the first interview varied across recruitment sites, including a rate of 71% for the Vanderbilt clinic, 28% for the Vanderbilt PAH registry, and 7% from the Southeastern PAH support group mailing. Four persons who participated in the first interview were known to be deceased at follow-up (3% of Interview 1 respondents). Four persons who gave incomplete responses to items on the IES were excluded from the analysis. No statistical differences in attrition rates by recruitment site were found between the first interview sample and the 59% (70/119) of respondents included in this analysis. For the second interview, 29% (20) of respondents were recruited at the Vanderbilt clinic, 27% (19) were recruited through the Vanderbilt PAH registry, 9% (6) were recruited from the Southeastern PAH support group, and 36% (25) were recruited at the Pulmonary Hypertension Association meeting. At the time of the first interview, 74% (88/119) of respondents reported that they had been diagnosed with PAH. Fifty-five percent had no known family history of the disease (PAH–sporadic), 18% (n=22) had PAH and a known family history of the disease (familial PAH [FPAH]–diagnosed), and 26% (n=31) had a family history of PAH and were considered potentially “at risk” but did not have a PAH diagnosis (FPAH–at risk). Eighty percent of those interviewed at follow-up reported that they had been diagnosed with PAH (56/70). Sixty percent (n=42) were PAH–sporadic and 23% (n=16) were FPAH–diagnosed, one of whom at initial interview had previously been at risk. Seventeen percent (12/70) of those participating in follow-up interview were in the “at-risk” category.
Proportions of sporadic and FPAH diagnosed were statistically similar to the first interview, as were respondents' gender, age, race, marital status, and parental status. Most of the respondents were female (which is not surprising since this disease disproportionately affects women), White, married, and had children, and were, on average, 50–51 years of age. Persons who were FPAH–at risk were less likely to participate in follow-up interview. Persons with more education and higher income were somewhat more likely to participate in follow-up interview.
Of those who had been diagnosed with PAH at the time of the follow-up interview, the mean time since diagnosis was 6.5 years (a median of 6 years), with a range of 6 months to 22 years since initial diagnosis. The 28 respondents (40%) who reported having other family members previously diagnosed with PAH (FPAH–total) had a range of 1–14 affected family members, with an average of 4 blood relatives (not including the respondent) previously diagnosed with the disease.
Testing for mutations in BMPR2
In the follow-up interview, 9% of respondents (6/70) reported that they had had the clinical test for mutations in BMPR2 since the first interview. Length of time between testing and follow-up interview ranged from several weeks to more than a year. Four of those tested had been recruited through the Vanderbilt clinic, one through the Vanderbilt PAH registry, and one through the Southeastern PAH support group. Given the small number of test accepters, there were no statistically significant differences by recruitment site or in the rate at which PAH-sporadic, FPAH, or FPAH–at-risk persons pursued testing. Similarly, there were no statistically significant differences in the demographic characteristics between those who had been tested and those who had not. By the follow-up interview, four individuals who been tested had received their test results and two had not.
Distress and participation in testing
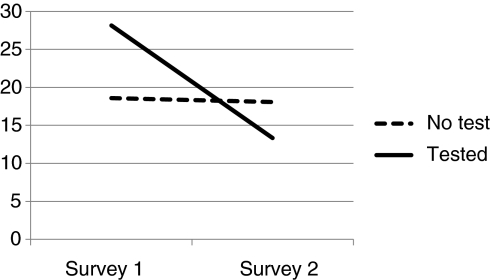
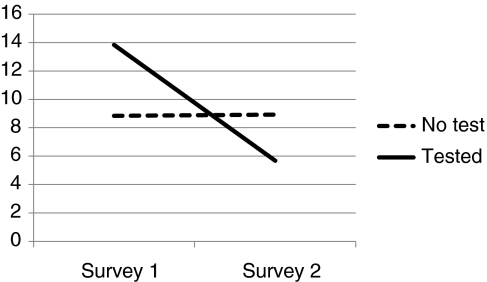
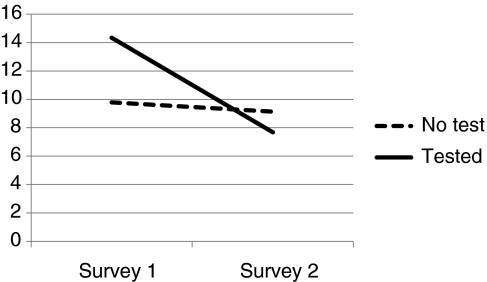
Figures 1–3 depict comparison of testing status by level of distress. IES Total and IES Intrusion and Avoidant subscales are used. Chronbach alpha for Survey 1 and Survey 2 include IES Intrusion (0.88; 0.86), IES Avoidance (0.76; 0.77), and IES Total Distress (0.90; 0.89).
FIG. 1.
Total IES.
FIG. 3.
IES Avoidance.
FIG. 2.
IES Intrusion.
When compared with nontesters, those who had chosen to have clinical testing for mutations in BMPR2 were more distressed at baseline, often exceeding levels diagnostic of PTSD, and they experienced a significant reduction in overall level of distress, including distress associated with intrusive thoughts about PAH and avoidance. Scores overall and in the nontested sample were positively skewed. This is consistent with other studies utilizing IES measures. To correct for skewness, square root transformations were conducted on each of the values when performing parametric statistics (Dougall et al., 1999). Overall F values for repeated measure ANOVA are reported in Table 2. Differences in IES by testing status are reported by Cohen's D. Effect sizes for the tested group are large (1.8 or above) and quite small for those who did not undergo genetic testing. Covariates' age, gender, education, and race (White/non-White) were not significant, which did not impact significance of testing status, and were not included in Table 2 analysis. By contrast, those who were tested did not differ from those who were not tested in terms of their tolerance for ambiguity (No Test=21.17, Tested=22.84) (Geller et al., 1993) (Table 3).
Table 2.
IES and Repeated Measures ANOVA
| n | Distress mean (SD) Survey 1 | Distress mean (SD) Survey 2 | F value | Siga | Cohen's D | |
|---|---|---|---|---|---|---|
| Intrusion | ||||||
| No test | 64 | 9.78 (9.2) | 9.12 (9.3) | 2.12 | 0.150 | 0.16 |
| Test | 6 | 14.33 (10.8) | 7.67 (5.5) | 1.83 | ||
| Avoidance | ||||||
| No test | 64 | 8.84 (8.3) | 8.91 (8.9) | 6.34 | 0.014 | 0.02 |
| Test | 6 | 13.83 (9.9) | 5.67 (6.1) | 2.28 | ||
| Total distress | ||||||
| No test | 64 | 18.62 (16.2) | 18.08 (16.5) | 4.54 | 0.037 | 0.07 |
| Test | 6 | 28.17 (20) | 13.33 (7.7) | 2.4 | ||
Computed using square root transformations of values.
IES, impact of events scale; ANOVA; SD, standard deviation.
Table 3.
Demographics by Testing Status
| |
Not tested |
Tested |
|---|---|---|
| |
(n=64) |
(n=6) |
| N (%) | N (%) | |
| Female | 52 (83) | 4 (67) |
| Average age | 50 | 50 |
| White | 56 (88) | 6 (100) |
| Married | 50 (78) | 4 (67) |
| Any children | 52 (81) | 5 (83) |
| Some college | 48 (75) | 5 (83) |
| Income | ||
| <50k | 23 (36) | 2 (33) |
| >50k | 38 (59) | 4 (67) |
| Diagnosed PAH | 52 (77) | 6 (100) |
| Familial | 14 (21) | 2 (33) |
| Sporadic | 38 (59) | 4 (67) |
| At risk | 12 (19) | 0 (0) |
All (100%, 6/6) of those tested cited children or desire to help other family members as a motivator for testing. Of those who were not tested, 53% (34/64) nonetheless reported that they still believed they would probably or definitely participate in testing, with half of those reporting (16/34) that there would be “no reason not to be tested.” Forty-four percent (28/64) identified children or desire to help family members as a potential motivator. Those who identified a barrier to participating in testing cited the cost of the test and lack of curative therapy. Those who felt they would definitely never participate in testing mentioned cost of the test, a decision to never have children, and lack of curative therapy as the most important factors for not participating in testing.
Discussion
This study includes 70 persons at risk for mutations in BMPR2. The individuals were predominantly White, middle class, female, married, and with children. In this study, we sought to define the characteristics of individuals who chose to have predisposition genetic testing for low-penetrance autosomal dominant mutations that cause PAH. As reported previously, the rate of test uptake among those we interviewed, which included individuals from at-risk families as well as sporadic cases, was quite low (Jones et al., 2008).
Given the low level of uptake, it is striking that test acceptors evidenced dramatic changes in levels of distress, often meeting levels associated with PTSD prior to testing, and evidencing dramatically lower levels of distress following testing. Similar pretest patterns of distress have been reported by other investigators. Women who pursued testing for mutations in BRCA1/2, for example, often had higher levels of distress than those who did not (Lerman et al., 1997; Dorval et al., 2008). Similarly, those who accept genetic counseling for cancer predisposing mutations were more often distressed than those who refused counseling (Halbert et al., 2004; Keller et al., 2004). Following testing, others have found that the majority of test recipients do not experience significant levels of distress (Schwartz et al., 2002; Claes et al., 2005; Gritz et al., 2005; Bleiker et al., 2007; Hiraki et al., 2009). Our findings provide support for the contention of Gooding et al. (2006) that participation in genetic testing may serve as a mechanism to alleviate distress for some at-risk individuals even when there are no clear preventive actions available. Test acceptors and decliners did not differ, however, in their tolerance for test ambiguity even though desire to reduce ambiguity is often cited as a major reason for pursuing testing (Baty et al., 2006).
We did not observe differences in levels of distress between mutation carriers and noncarriers, which may be attributable to the small number of test acceptors. If more people pursue testing, then it will be appropriate to reassess the level of pre- and posttest distress. Two unique mechanisms effect post test distress. The first is participation in testing (“participation effects”) as our study finds. The second is knowledge of test results (“information effects”) as some investigators report, including Broadstock et al. (2000) and others who found that mutation carriers experience greater distress than nonmutation carriers (Broadstock et al., 2000; Tercyak et al., 2001; van Roosmalen et al., 2004; Claes et al., 2005; Hiraki et al., 2009). Others researches have observed that poor mental health status (Licklederer et al., 2008) and a history of depression prior to testing (Gargiulo et al., 2009) increase the likelihood of increased distress and negative effects following testing. The effects of these, and other factors that are likely to influence participation in genetic testing, were not addressed in this analysis and reflect an important limitation of our study.
Two other significant limitations are the small sample size (albeit large for this rare disease) and low rate of test uptake, which made it possible to detect only the largest differences between test users and those who did not undergo testing. Covariates' age, race, educational status, gender, and other factors might well be influential predictors, not revealed in this small sample. This limitation may be unavoidable in studies that attempt to examine the impact of predisposition genetic testing for rare, difficult-to-treat disorders with low penetrance, since the population affected is notably small and uptake is frequently low. For example, although the majority of the respondents in this study said that they planned to be tested, the level of uptake we observed is consistent with that of Hannig et al. (2008) who specifically offered testing to 23 families with known mutations in BMPR2. Moreover, unaffected respondents with a family history of PAH were less likely to participate in the second interview. This is unfortunate since this group could have different motivations and fears regarding genetic testing than people who are already affected, who frequently pursue testing to obtain information for family members (Jones et al., 2008). Despite these limitations, the study adds to the literature in a number of ways. It is one of the first longitudinal studies of individuals who may have inherited a predisposing mutation linked to a single-gene disorder other than cancer or neurological diseases. The low level of uptake may well be characteristic of tests for partially penetrant mutations, especially those associated with poorly treatable diseases. Clinicians need to be aware of the correlation of high levels of disease-related distress with test acceptance observed in this study and others. Participation in genetic testing, irrespective of test results, may be particularly beneficial to individuals who may have genetic mutations and who are experiencing high levels of distress.
Future studies should examine both the most immediate and long-term effects of testing to assess more fully whether or not reduction in distress is wholly attributable to participation in testing, coincident with a natural propensity for PTSD symptoms to level off and/or to participants' broader psychological coping approach in dealing with distress.
Acknowledgments
This research was supported by 5 P01 HL 072058–02 NIH/NHLBI, “Genetic and Environmental Pathogenesis of PPH.” We would like to thank Mary J. Rosenthal, Christi Ulmer, Robert Saunders, Emily Tanner-Smith, Joanne Sandberg, Lisa Wheeler, Jim Loyd, Ivan Robbins, the Vanderbilt PH clinic staff, Sally Maddox, and especially the participants of our study.
Disclosure Statement
No competing financial interests exist.
References
- Austin ED. Loyd JE. Phillips JA., 3rd Genetics of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30:386–398. doi: 10.1055/s-0029-1233308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badesch DB. Champion HC. Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Barnoy S. Genetic testing for late-onset diseases: effect of disease controllability, test predictivity, and gender on the decision to take the test. Genet Test. 2007;11:187–192. doi: 10.1089/gte.2006.0509. [DOI] [PubMed] [Google Scholar]
- Baty BJ. Dudley WN. Musters A. Kinney AY. Uncertainty in BRCA1 cancer susceptibility testing. Am J Med Genet C Semin Med Genet. 2006;142C:241–250. doi: 10.1002/ajmg.c.30112. [DOI] [PubMed] [Google Scholar]
- Baumert J. Simon H. Gundel H, et al. The impact of event scale-revised: evaluation of the subscales and correlations to psychophysiological startle response patterns in survivors of a life-threatening cardiac event. J Affect Disord. 2004;82:29–41. doi: 10.1016/j.jad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Bleiker EM. Menko FH. Kluijt I, et al. Colorectal cancer in the family: psychosocial distress and social issues in the years following genetic counselling. Hered Cancer Clin Pract. 2007;5:59–66. doi: 10.1186/1897-4287-5-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadstock M. Michie S. Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8:731–738. doi: 10.1038/sj.ejhg.5200532. [DOI] [PubMed] [Google Scholar]
- Chan A. Chan YH. Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occup Med. 2004;54:190–196. doi: 10.1093/occmed/kqh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes E. Denayer L. Evers-Kiebooms G, et al. Predictive testing for hereditary nonpolyposis colorectal cancer: subjective perception regarding colorectal and endometrial cancer, distress, and health-related behavior at one year post-test. Genet Test. 2005;9:54–65. doi: 10.1089/gte.2005.9.54. [DOI] [PubMed] [Google Scholar]
- Codori AM. Petersen GM. Miglioretti DL, et al. Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiol Biomarkers Prev. 1999;8:345–351. [PubMed] [Google Scholar]
- Dickson MR. Carter CL. Carpenter MJ, et al. Barriers to genetic testing among persons at risk for alpha-1 antitrypsin deficiency. Genet Test. 2008;12:501–505. doi: 10.1089/gte.2008.0028. [DOI] [PubMed] [Google Scholar]
- Dorval M. Bouchard K. Maunsell E, et al. Health behaviors and psychological distress in women initiating BRCA1/2 genetic testing: comparison with control population. J Genet Couns. 2008;17:314–326. doi: 10.1007/s10897-008-9150-7. [DOI] [PubMed] [Google Scholar]
- Dougall AL. Craig K. Baum A. Assessment of characteristics of intrusive thoughts and their impact on distress among victims of traumatic events. Psychosom Med. 1999;61:38–48. doi: 10.1097/00006842-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Foster C. Evans DG. Eeles R, et al. Non-uptake of predictive genetic testing for BRCA1/2 among relatives of known carriers: attributes, cancer worry, and barriers to testing in a multicenter clinical cohort. Genet Test. 2004;8:23–29. doi: 10.1089/109065704323016003. [DOI] [PubMed] [Google Scholar]
- Gargiulo M. Lejeune S. Tanguy ML, et al. Long-term outcome of presymptomatic testing in Huntington disease. Eur J Hum Genet. 2009;17:165–171. doi: 10.1038/ejhg.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller G. Tambor ES. Chase GA. Holtzman NA. Measuring physicians' tolerance for ambiguity and its relationship to their reported practices regarding genetic testing. Med Care. 1993;31:989–1001. doi: 10.1097/00005650-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Gooding HC. Linnenbringer EL. Burack J, et al. Genetic susceptibility testing for Alzheimer disease: motivation to obtain information and control as precursors to coping with increased risk. Patient Educ Couns. 2006;64:259–267. doi: 10.1016/j.pec.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Gritz ER. Peterson SK. Vernon SW, et al. Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2005;23:1902–1910. doi: 10.1200/JCO.2005.07.102. [DOI] [PubMed] [Google Scholar]
- Hadley DW. Jenkins J. Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163:573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- Halbert CH. Wenzel L. Lerman C, et al. Predictors of participation in psychosocial telephone counseling following genetic testing for BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2004;13:875–881. [PubMed] [Google Scholar]
- Hannig VL. Wheeler L. Phillips JA, III, et al. Interest in clinical genetic testing for familial pulmonary arterial hypertension (FPAH) Paper presented at American Thoracic Society. 2008.
- Hiraki S. Chen CA. Roberts JS, et al. Perceptions of familial risk in those seeking a genetic risk assessment for Alzheimer's disease. J Genet Couns. 2009;18:130–136. doi: 10.1007/s10897-008-9194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M. Wilner N. Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Jones DL. Sandberg JC. Rosenthal MJ, et al. What patients and their relatives think about testing for BMPR2. J Genet Couns. 2008;17:452–458. doi: 10.1007/s10897-008-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M. Jost R. Kadmon M, et al. Acceptance of and attitude toward genetic testing for hereditary nonpolyposis colorectal cancer: a comparison of participants and nonparticipants in genetic counseling. Dis Colon Rectum. 2004;47:153–162. doi: 10.1007/s10350-003-0034-5. [DOI] [PubMed] [Google Scholar]
- Lerman C. Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology. 1996;10:191–195. [PubMed] [Google Scholar]
- Lerman C. Hughes C. Trock BJ, et al. Genetic testing in families with hereditary nonpolyposis colon cancer. Jama. 1999;281:1618–1622. doi: 10.1001/jama.281.17.1618. [DOI] [PubMed] [Google Scholar]
- Lerman C. Schwartz MD. Lin TH, et al. The influence of psychological distress on use of genetic testing for cancer risk. J Consult Clin Psychol. 1997;65:414–420. doi: 10.1037//0022-006x.65.3.414. [DOI] [PubMed] [Google Scholar]
- Lewis JR. Konda V. Rubin DT. Genetic testing for inflammatory bowel disease: focus group analysis of patients and family members. Genet Test Mol Biomarkers. 2009;13:495–503. doi: 10.1089/gtmb.2008.0102. [DOI] [PubMed] [Google Scholar]
- Licklederer C. Wolff G. Barth J. Mental health and quality of life after genetic testing for Huntington disease: a long-term effect study in Germany. Am J Med Genet A. 2008;146A:2078–2085. doi: 10.1002/ajmg.a.32423. [DOI] [PubMed] [Google Scholar]
- Lientz EA. Clayton EW. Psychosocial implications of primary pulmonary hypertension. Am J Hum Genet. 2000;59(suppl 2):209. [Google Scholar]
- Metcalfe A. Werrett J. Burgess L. Clifford C. Psychosocial impact of the lack of information given at referral about familial risk for cancer. Psycho-oncology. 2007;16:458–465. doi: 10.1002/pon.1081. [DOI] [PubMed] [Google Scholar]
- Patch C. Roderick P. Rosenberg W. Comparison of genotypic and phenotypic strategies for population screening in hemochromatosis: assessment of anxiety, depression, and perception of health. Genet Med. 2005;7:550–556. doi: 10.1097/01.gim.0000182466.87113.ce. [DOI] [PubMed] [Google Scholar]
- Ropka ME. Wenzel J. Phillips EK, et al. Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev. 2006;15:840–855. doi: 10.1158/1055-9965.EPI-05-0002. [DOI] [PubMed] [Google Scholar]
- Schwartz MD. Peshkin BN. Hughes C, et al. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- Shiloh S. Ilan S. To test or not to test? Moderators of the relationship between risk perceptions and interest in predictive genetic testing. J Behav Med. 2005;28:467–479. doi: 10.1007/s10865-005-9017-4. [DOI] [PubMed] [Google Scholar]
- Smith CO. Lipe HP. Bird TD. Impact of presymptomatic genetic testing for hereditary ataxia and neuromuscular disorders. Arch Neurol. 2004;61:875–880. doi: 10.1001/archneur.61.6.875. [DOI] [PubMed] [Google Scholar]
- Taylor SD. Predictive genetic test decisions for Huntington's disease: elucidating the test/no-test dichotomy. J Health Psychol. 2005;10:597–612. doi: 10.1177/1359105305053442. [DOI] [PubMed] [Google Scholar]
- Tercyak KP. Lerman C. Peshkin BN, et al. Effects of coping style and BRCA1 and BRCA2 test results on anxiety among women participating in genetic counseling and testing for breast and ovarian cancer risk. Health Psychol. 2001;20:217–222. [PubMed] [Google Scholar]
- Thompson HS. Valdimarsdottir HB. Duteau-Buck C, et al. Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev. 2002;11:1579–1585. [PubMed] [Google Scholar]
- van Roosmalen MS. Stalmeier PF. Verhoef LC, et al. Impact of BRCA1/2 testing and disclosure of a positive test result on women affected and unaffected with breast or ovarian cancer. Am J Med Genet A. 2004;124A:346–355. doi: 10.1002/ajmg.a.20374. [DOI] [PubMed] [Google Scholar]
- Weiss S, editor. Assessing Psychological Trauma and PTSD. Guilford Press; New York: 2004. [Google Scholar]