Summary
Septo-optic dysplasia (SOD) is a congenital brain anomaly that results in pituitary, optic nerve, and midline forebrain defects. The etiology of SOD is poorly understood, with the majority of cases being sporadic. In rare instances, SOD is caused by mutations in Sox2, Sox3 or Hesx1, but how this manifests in disease is not entirely certain. We demonstrate here that mouse embryos lacking Sonic hedgehog (Shh) in the prospective hypothalamus exhibit key features of SOD, including pituitary hypoplasia and absence of the optic disc. The hypothalamic source of Shh is required to maintain gene expression boundaries along the anteroposterior and mediolateral neural axes that are important for proper pituitary and eye development, respectively. We further reveal that Sox2 and Sox3 are dose dependent regulators of Shh transcription, which directly bind and activate a long-range Shh forebrain enhancer. These data indicate that reduced levels of Shh expression in the hypothalamus cause SOD.
Keywords: Septo-optic dysplasia, Sonic hedgehog, SoxB1, hypothalamus, pituitary, optic disc
Introduction
Shh is a secreted protein that imparts patterns of growth and identity to neuronal progenitors throughout ventral regions of the developing central nervous system (CNS) (Dessaud et al., 2008). For Shh to fulfill these functions, it must be expressed in a temporally and spatially defined manner. Despite a detailed understanding of the signal transduction pathway functioning downstream of Shh, we still know relatively little of the genes operating upstream in the pathway that regulate Shh transcription in key signaling centers mediating CNS development (Dessaud et al., 2008).
During early stages of brain development, Shh is expressed in the prechordal plate, an axial mesendodermal tissue that transiently underlies the anterior CNS (Echelard et al., 1993). Shh signaling from the prechordal plate is necessary for the separation of the cerebral hemispheres, eye fields and other craniofacial structures (Chiang et al., 1996). In humans, SHH haploinsufficiency is the predominant cause of holoprosencephaly (HPE), a structural brain malformation syndrome, indicating that the level of SHH expression in the prechordal plate is important for proper forebrain and craniofacial development (Roessler et al., 1996).
Prechordal plate derived signals also regionalize the ventral forebrain (Shimamura et al., 1997). In chick, the combination of Shh and Bmp7 secreted from the prechordal plate activate markers of hypothalamic identity within the overlying ventral diencephalon (Dale et al., 1997; Manning et al., 2006). Once induced, the hypothalamus becomes a secondary site of Shh expression (Echelard et al., 1993; Dale et al., 1997). This early event in brain regionalization is critical for subsequent functions of the hypothalamus and pituitary, which are often compromised in HPE cases (Kauvar and Muenke, 2010). However, due to the severity of the brain defects in Shh−/− mouse embryos, it has been difficult to fully discern the function of Shh in the hypothalamus from its earlier role in the prechordal plate (Chiang et al., 1996).
Using a conditional gene targeting approach, we now demonstrate that mice lacking Shh in the hypothalamus exhibit a combination of hypothalamic, pituitary and eye defects that are more consistent with a diagnosis of SOD, than they are with HPE. Given the phenotypic similarities between hypothalamic Shh mutants and mouse models of SOD (Kelberman and Dattani, 2008), we postulated that a reduction in Shh signaling from the hypothalamus might underlie the pathogenesis of this disorder. Indeed, Shh expression was downregulated in the hypothalamus of Sox2 and Sox3 mutants, two mouse models of SOD (Kelberman et al., 2006; Rizzoti et al., 2004). Furthermore, we show that Sox2 and Sox3 are direct regulators of a long-range Shh forebrain enhancer. Our data suggest that SOD and HPE are genetically distinct brain anomalies with partially overlapping phenotypes that can be distinguished by the timing and location of Shh downregulation.
Results
The hypothalamic source of Shh is required for proper pituitary morphology
Shh expression in the ventral diencephalon of E10.5 mouse embryos is detected in two bilateral stripes on either side of the ventral midline from the mammillary region posteriorly, to the level of the optic vesicles anteriorly, at which point Shh converges at the midline and extends into the preoptic area (POA) at the base of the telencephalon (Fig. S1a). To address the function of the hypothalamic source of Shh, we used an SBE2-cre mouse line to conditionally inactivate a floxed allele of Shh. SBE2, an upstream regulatory element from Shh, was particularly advantageous for this purpose as it only activates cre transcription within Shh expressing cells of the hypothalamus (Jeong et al., 2006). Attenuation of Shh expression was first apparent in both anterior and posterior regions of the prospective hypothalamus at E9.0 (16 somite stage) in SBE2-cre; Shhloxp/− embryos (herein referred to as ShhΔhyp, for deletion of Shh in the hypothalamus) (Fig. S1b). Complete abrogation of Shh from the SBE2 domain of ShhΔhyp embryos was observed at E9.5 (Fig. S1c). At E12.5, Shh continued to be absent from the ventral hypothalamus of ShhΔhyp embryos, but not other forebrain regions (Fig. 1a,d). Shh signaling activity, as measured by the expression of Gli1, was greatly reduced in Shh responsive cells within the hypothalamus of ShhΔhyp embryos (Fig. 1b,e). This is consistent with recent results demonstrating a requirement for Shh in the formation of ventral hypothalamic nuclei (Szabó et al., 2009; Shimogori et al., 2010). The loss of neuroendocrine and centrally projecting neurons in ShhΔhyp embryos results, in part, from altered dorsoventral patterning in the diencephalon and will be described elsewhere. Instead, our analysis of ShhΔhyp embryos focused on a unique aspect of Shh function, the regulation of gene expression boundaries along the anteroposterior axis of the hypothalamus.
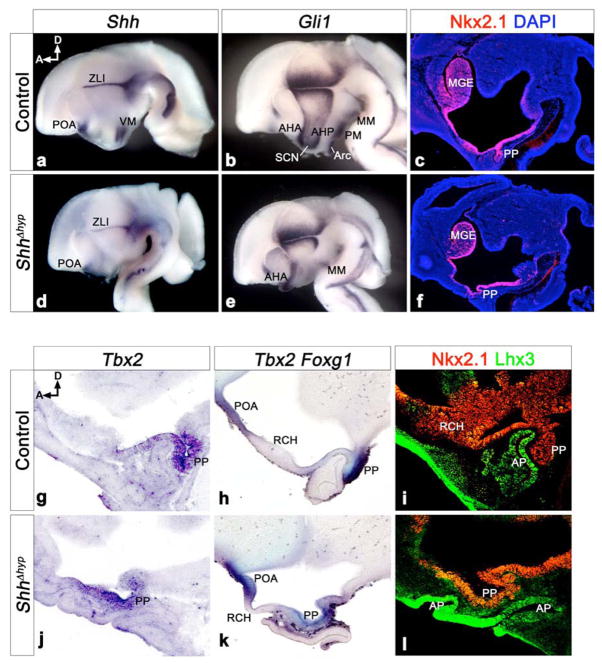
Figure 1. The hypothalamic source of Shh is required for proper pituitary morphology at E12.5.
(a,b,d,e) Whole mount in situ hybridization for Shh and Gli1 on bisected brains from control (SBE2cre; Shh+/loxp) and ShhΔhyp (SBE2cre; Shh−/loxp) embryos. In the hypothalamus of control embryos, Shh is expressed in progenitors of the ventromedial nucleus (VM). Shh is missing from the VM domain of ShhΔhyp embryos, but other forebrain regions, including the zona limitans intrathalamica (ZLI) and preoptic area (POA) maintain Shh expression. Gli1 marks Shh-responsive cells in ventral and anterior hypothalamic nuclei, including the mammillary (MM), premammillary (PM), arcuate (Arc), suprachiasmatic (SCN), anterior hypothalamus posterior region (AHP), and anterior hypothalamus anterior region (AHA). With the exception of the AHA and MM, all other hypothalamic regions show reduced Gli1 expression in ShhΔhyp embryos. (c,f) Nkx2.1 immunostaining on midsagittal sections through control and ShhΔhyp embryos counterstained with DAPI (blue). Nkx2.1 staining is present in the ventral hypothalamus and medial ganglionic eminence (MGE) of ShhΔhyp embryos. However, the posterior pituitary (PP) is dysmorphic and ectopically positioned compared to control embryos. (g,j) In situ hybridization for Tbx2 on midsagittal sections confirms the ectopic PP in ShhΔhyp versus control embryos. (h,k) Double labeling of the POA and PP with Foxg1 and Tbx2, respectively, shows that the intervening region corresponding to the retrochiasmatic hypothalamus (RCH) is reduced in ShhΔhyp embryos. (i,l) Nkx2.1 and Lhx3 immunostaining reveals that the anterior pituitary (AP) is duplicated and shifted anteriorly in ShhΔhyp mutants. D, dorsal; A, anterior.
The Nkx2.1 homeoprotein is expressed in the ventral hypothalamus in response to Shh signaling from the prechordal plate (Dale et al., 1997) (Fig. 1c). Nkx2.1 staining was maintained in ShhΔhyp embryos (Fig. 1f). This result, along with the observation that the cerebral hemispheres and eyes were appropriately bifurcated in ShhΔhyp embryos, suggests that Shh signaling activity from the prechordal plate was not compromised in these mutants (data not shown). However, we did observe an irregularity in the development of the Nkx2.1+ infundibulum in ShhΔhyp embryos. The infundibulum evaginates from the ventral diencephalon to form the posterior lobe of the pituitary (Zhu et al., 2007). However, in ShhΔhyp embryos, the infundibulum was highly dysmorphic, failed to protrude correctly from the diencephalon, and was shifted anteriorly within the brain (Fig. 1c,f). Examination of Tbx2 confirmed the ectopic position of the infundibulum in ShhΔhyp embryos (Fig. 1g,j). The retrochiasmatic area of the hypothalamus (RCH) lies between the Tbx2+ infundibulum - posteriorly, and the Foxg1+ POA - anteriorly (Fig. 1h). The distance between Tbx2 and Foxg1 was greatly reduced in ShhΔhyp mutants, compared to control littermates, and the RCH took on a thinner cellular morphology, more characteristic of the infundibulum (Fig. 1h,k). Thus, anterior hypothalamic development, including the positioning of the posterior pituitary, was greatly compromised in ShhΔhyp embryos.
Anterior pituitary formation was also affected in ShhΔhyp mutants. Rathke’s pouch, the primordium of the anterior and intermediate lobes of the pituitary, arises from a region of oral ectoderm that invaginates in response to inductive cues from the infundibulum (Zhu et al., 2007). Lhx3 staining, which marks Rathke’s pouch, revealed a duplicated invagination that failed to pinch off from the oral ectoderm and, like the infundibulum, was shifted anteriorly in the brain of ShhΔhyp embryos (Fig. 1i,l and Fig. S3a).
Shh positions the infundibulum by opposing posterior hypothalamic signals
To understand the molecular basis of the pituitary phenotype in ShhΔhyp embryos, we interrogated genes expressed along the anteroposterior axis of the hypothalamus at earlier stages of development (Fig. 2a). Previous studies implicated members of the Bmp and Fgf families in the induction and growth of Rathke’s pouch (Ericson et al., 1998; Treier et al., 1998; Takuma et al., 1998). Bmp4 and Fgf10 are expressed in the ventral midline of the posterior hypothalamus, including the infundibulum (Fig. 2a,b,d). This contrasts with the expression of Shh, Six6 and Tcf4, which mark the ventral midline of the anterior hypothalamus, rostral to the infundibulum (Fig. 2a,f,h,j). In ShhΔhyp mutants, the anterior limit of Bmp4 and Fgf10 expression extended into the RCH territory at the expense of Shh and Six6 (Fig. 2b–i). Evidence in support of this conclusion was gained by our observation that the distance between the anterior extent of Fgf10 expression and the optic recess, a fixed morphological landmark, was dramatically reduced in ShhΔhyp mutants compared to control littermates (Fig. 2d,e). The area of expanded Fgf10 expression coincided roughly with where Shh and Six6 were downregulated (Fig. 2d–i). The anterior expansion of Bmp4 was observed as early as E9.5, suggesting that it occurred in direct response to the loss of Shh. Not all genes expressed in the anterior hypothalamus were downregulated in ShhΔhyp embryos, as evidenced by the persistent expression of Tcf4 in the RCH region (Fig. 2j,k). Importantly, this finding argues that the altered gene expression profile in the hypothalamus of ShhΔhyp mutants does not result from the ablation of anterior hypothalamic tissue, but instead is likely due to the failure to maintain proper gene expression boundaries at, or near, the infundibulum.
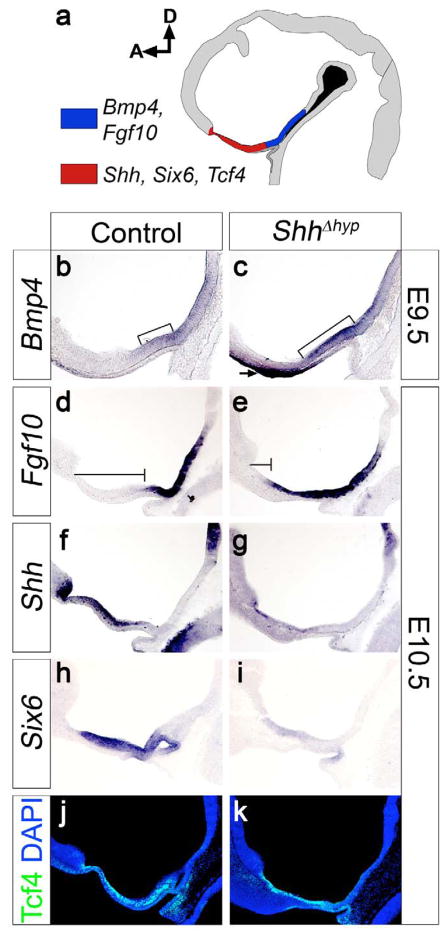
Figure 2. Shh is required for proper anteroposterior patterning in the hypothalamus.
(a) Diagram of a midsagittal section through a control E10.5 brain delineating the approximate expression domains of anteroposterior patterning markers in the hypothalamus. Bmp4 and Fgf10 are co-expressed in the posterior ventral midline (blue), while Shh, Six6, and Tcf4 mark the anterior ventral midline (red). (b,c) At E9.5, Bmp4 expression is rostrally expanded (brackets) in ShhΔhyp embryos. Bmp4 is also ectopically expressed in the oral ectoderm of mutants (arrow). (d,e) Fgf10 expression is expanded rostrally in ShhΔhyp versus control embryos. Notice that the vertical line extending from the optic recess to the rostral extent of the Fgf10 expression domain (horizontal line) is truncated in ShhΔhyp compared to control embryos. (f,g) Shh transcription is repressed in the hypothalamus of ShhΔhyp embryos. (h, i) Six6 expression is downregulated in the hypothalamus of ShhΔhyp embryos. (j, k) Tcf4 expression is unaffected in the hypothalamus of ShhΔhyp embryos. D, dorsal; A, anterior.
We observed a significant reduction in the number of Ki67+ proliferating cells in both anterior (RCH) and posterior (infundibulum) regions of the hypothalamus when comparing ShhΔhyp embryos to control littermates at E10.5 (Fig. S2a–j). While this result is consistent with a mitogenic role for Shh in the hypothalamus, it further suggests that the anteroposterior patterning defect observed in ShhΔhyp embryos is unlikely to be explained by a selective growth advantage of one hypothalamic territory over another, since both regions were equally affected. Nevertheless, the overall reduction in proliferation within the ventral hypothalamus of ShhΔhyp embryos may explain why the infundibulum failed to develop in these mutants, possibly by compromising the forces needed to push the two sides of the evaginating ventral diencephalon together. Alternatively, the altered pattern of gene expression in the ventral diencephalon of ShhΔhyp embryos may have disrupted reciprocal signals from rathke’s pouch that regulate infundibular morphogenesis (Takuma et al., 1998).
Mouse models of SOD, including Hesx1−/−, Sox2+/− and Sox3−/− mutants, display hypothalamo-pituitary patterning defects of similar character to Shhhyp embryos (Kelberman et al., 2006; Rizzoti et al., 2004; Dattani et al., 1998; Dasen et al., 2001). The anterior expansion of Fgf and Bmp signals is a common occurrence in these mutants and is postulated to enhance the recruitment of oral ectoderm to Rathke’s pouch resulting in the formation of multiple and ectopic anterior pituitaries. Consequently, SOD patients often present with deficiencies in one or more pituitary hormones (Kelberman and Dattani, 2008). Likewise, ShhΔhyp mutants displayed significant reductions in the number of somatotropes, corticotropes, and thyrotropes in the anterior pituitary at E18.5 (Fig. S3b,c). The reduced number of pituitary cell types in ShhΔhyp mutants may be a primary consequence of the loss of Shh signaling from the diencephalon and/or a secondary consequence of the patterning alterations in the hypothalamus. While our experiments cannot distinguish between these two possibilities, other studies have implicated Shh signaling in the proliferation of anterior pituitary progenitors (Treier et al., 2001; Wang et al., 2010).
Optic disc formation is dependent on Shh signaling from the hypothalamus
SOD patients also exhibit varying degrees of blindness due to optic nerve hypoplasia (Kelberman and Dattani, 2008). To determine if ShhΔhyp embryos were afflicted with a similar eye defect, we stained retinal ganglion cell (RGC) axons for neurofilament. Unlike control embryos, which showed a tight bundle of RGC axons exiting the eye at E14.5, the majority of RGC axons remained trapped in the eye of ShhΔhyp embryos, resulting in a severely hypoplastic optic nerve (Fig. 3a,b). RGC axons exit the eye through the optic disc, which forms at the juncture of the optic stalk and cup (Otteson et al., 1998). Pax2+, Netrin1+ optic disc cells were not detected in the eyes of ShhΔhyp mutants, although expression of these markers was detected in the optic stalk (Fig. 3c–f). The absence of optic disc cells was not due to altered Shh signaling in the eye, as previously shown (Dakubo et al., 2003), since the expression of Shh and Gli1, in RGCs and retinal progenitor cells, respectively, was unaffected in ShhΔhyp embryos (Fig. 3g–j).
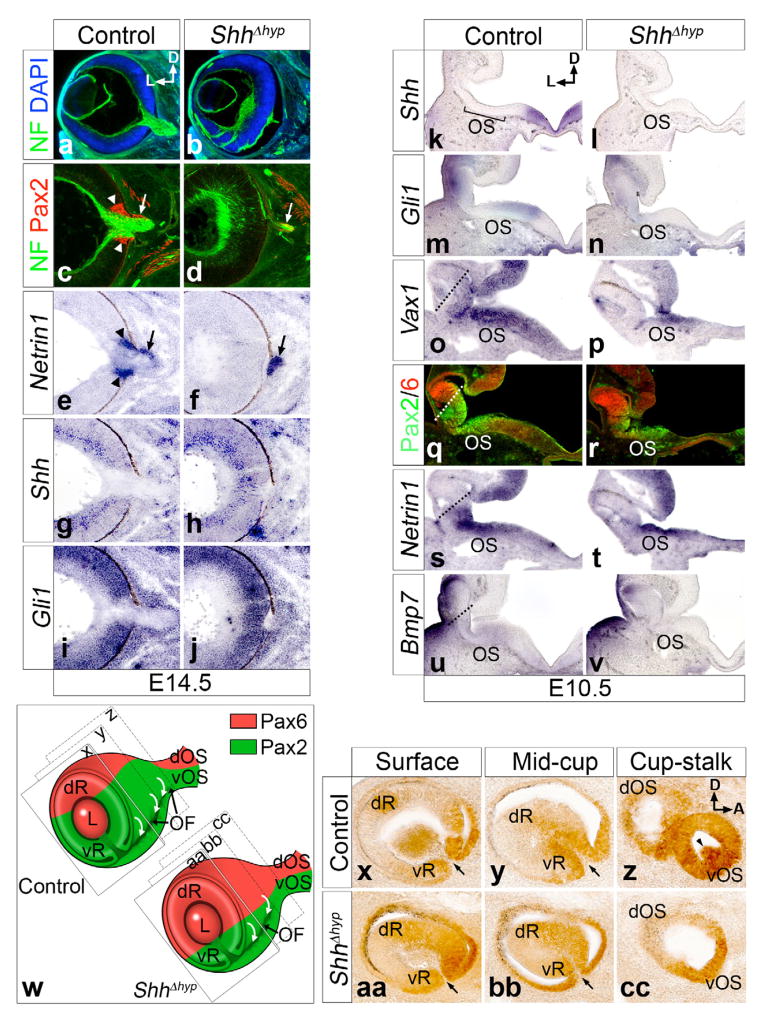
Figure 3. The optic disc fails to form in ShhΔhyp embryos.
(a–j) Coronal sections through the eyes of control and ShhΔhyp embryos at E14.5. (a,b) RGC axons labeled with neurofilament (NF) bundle and exit the eye at the optic disc in control, but not ShhΔhyp embryos. (c–f) Pax2 and Netrin1 mark cells of the optic disc (arrowheads) and optic stalk (arrows). The optic disc is absent in ShhΔhyp mutants. (g–j) Shh and Gli1 expression is unaltered in the eyes of ShhΔhyp versus control embryos. (k–v) Coronal sections through the hypothalamus of E10.5 embryos at the level of the eye. (k–n) The expression of Shh in the ventral midline of the hypothalamus and Gli1, in the optic stalk (OS) is lost in ShhΔhyp mutants. (o–t) The expression of Vax1, Pax2, and Netrin 1 is reduced in the ventral retina (below dotted line) of ShhΔhyp mutants, but is preserved in the optic stalk. Pax6 staining in the dorsal retina (above dotted line) expanded ventrally in ShhΔhyp embryos. (u,v) The weak Bmp7 expression in the ventral midline of the hypothalamus, optic stalk, and ventral retina in control embryos is absent in ShhΔhyp mutants. (w) Diagram of the eye and optic stalk in control and mutant, tilted to expose the ventral optic fissure (OF). The fissure and invagination of cells (curved arrows) does not extend to the stalk in mutants. The level of section in panels x-cc is shown. Labels: dR, dorsal retina; L, lens; vR, ventral retina; dOS, dorsal optic stalk; vOS, ventral optic stalk. (x–cc) Sections cut parallel to the eye surface at E10.5. Pax2+ cells invaginate at the lips of the optic fissure (arrows) in control and ShhΔhyp at surface and mid-cup levels. However, Pax2+ optic disc precursors at the interface between the eye cup and stalk (arrowhead) fail to invaginate in ShhΔhyp. D, dorsal; L, lateral; A, anterior.
To elucidate the molecular mechanism responsible for the optic disc defect in ShhΔhyp embryos, we assayed the expression of genes with known roles in eye development. At E10.5, Shh and Gli1 were missing from the ventral midline of the diencephalon and optic stalk, respectively, of ShhΔhyp embryos (Fig. 3k–n). Other markers, including Vax1, Pax2 and Netrin1 maintained their expression in the optic stalk, but were reduced from the ventral portion of the retina (Fig. 3o–t). Consequently, the dorsal retinal marker, Pax6, expanded ventrally in ShhΔhyp embryos. These data indicate that the role of the hypothalamic source of Shh in eye development is mostly limited to patterning the retina, whereas the earlier source of Shh in the prechordal plate is required for the separation of the eye fields and formation of medial structures, such as the optic stalk (Chiang et al., 1996).
Subtle reductions in the ventral retina domain marked by Pax2 are known to affect the morphogenesis of the optic disc (Dakubo et al., 2003). Pax2+ cells invaginate in the ventral retina and optic stalk to generate the optic fissure (curved white arrows in Fig. 3w). The optic disc forms from the population of Pax2+ cells that invaginate at the juncture between the optic cup and optic stalk. We examined Pax2+ cells on sections cut parallel to the surface of the eye in control and ShhΔhyp embryos (Fig. 3x–cc). While Pax2+ cells successfully invaginated to form the optic fissure at lateral levels of ShhΔhyp embryos (black arrows in Fig. 3aa, bb) they failed to invaginate at the medial extent of the optic cup (note the missing arrowhead in Fig. 3cc). A similar phenotype was observed in Bmp7−/− mutants, which lack the optic disc as well as the optic fissure and show a downregulation in Pax2 expression (Morcillo et al., 2006). Interestingly, the faint domains of Bmp7 in the ventral retina, stalk and midline of the hypothalamus were absent in ShhΔhyp embryos (Fig. 3u–v). Therefore, the optic disc defect likely stems from the failure of the hypothalamic source of Shh to properly maintain Bmp7 and/or Pax2 expression in the ventral region of the eye.
Dose dependent regulation of Shh in the hypothalamus by SoxB1 family members
We next investigated how Shh, Sox2, Sox3 and Hesx1 might be integrated in a signaling network required for hypothalamo-pituitary development by assessing Shh expression in several mouse models of SOD. A previous study revealed that the hypothalamic expression of Shh was unaltered in Hesx1−/− mutants, ruling out a role for this homedomain transcription factor in regulating Shh (Dattani et al., 1998). We therefore focused our analysis on single and compound mutants in Sox2 and Sox3, two high-mobility group (HMG) containing DNA binding proteins that along with Sox1 comprise the SoxB1 subfamily (Pevny and Placzek, 2005). Sox2 and Sox3 are expressed throughout the ventricular zone of the CNS where they function to maintain neuronal progenitor identity (Pevny and Placzek, 2005).
Sox2 and Sox3 are coexpressed with Shh from the onset of its transcription in the ventral diencephalon and continue to share overlapping expression in the RCH at E10.5 (Fig. 4a–d and data not shown).
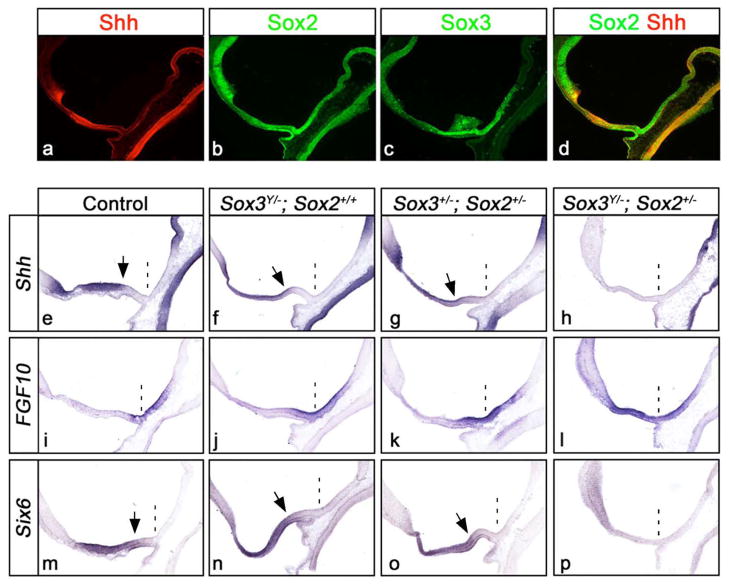
Figure 4. SoxB1 proteins are dose dependent regulators of Shh expression in the anterior hypothalamus.
(a–d) Coexpression of Shh, Sox2 and Sox3 on midsagittal sections through the hypothalamus of wild type embryos at E10.5. (e–p) In situ hybridization for Shh (e–h), Fgf10 (i–l) and Six6 (m–p) on midsagittal sections through the hypothalamus of control and SoxB1 mutant embryos at E10.5. The position of the infundibulum is marked with a dashed line, and the arrows point to the posterior boundaries of Shh and Six6 expression. A subtle reduction in the intensity and posterior demarcation of Shh and Six6 expression was detected in Sox3Y/− and Sox3+/−; Sox2+/− mutants, concomitant with an anterior expansion in Fg10 expression. Sox3Y/−; Sox2+/− mutants showed a profound loss of Shh and Six6 expression in the anterior hypothalamus and corresponding gain in Fgf10.
The intensity of Shh staining in the anterior hypothalamus of male hemizygous Sox3Y/− mutants (Sox3 is X-linked) was subtly, albeit consistently, downregulated, especially at its posterior limit adjacent to the infundibulum (Fig. 4e,f). This area of reduced Shh transcription was coincident with an expansion in the anterior boundary of Fgf10 expression (Fig. 4i,j). A similar reduction in the anterior hypothalamic domain of Six6 was observed (Fig. 4m,n). Taken together with the results from our analysis of ShhΔhyp mutants, these data suggest that the reduced expression of Shh in the hypothalamus of Sox3Y/− embryos may contribute to the pituitary hypoplasia described in these animals (Rizzoti et al., 2004).
SOD is typically less severe in Sox3Y/− and Sox2+/− mice, compared to ShhΔhyp mutants (Rizzoti et al., 2004; Kelberman et al., 2006). To determine whether this is due to functional redundancy between SoxB1 family members, we examined the expression of hypothalamic markers in various compound mutants. A very similar pattern of misexpression was observed for Shh, Fgf10 and Six6 in Sox3+/−;Sox2+/− compared to Sox3Y/− mutants, suggesting that the overall dose rather than the specific function of Sox2 and Sox3 is important for hypothalamic development (compare Fig. 4g,k,o to f,j,n). Remarkably, in embryos lacking Sox3 and heterozygous for Sox2 (Sox3Y/−; Sox2+/−), the ventral midline of the anterior hypothalamus was completely devoid of Shh and Six6 expression and instead was replaced by an expanded domain of Fgf10 (Fig. 4h,l,p). The hypothalamic patterning defect in Sox3Y/−; Sox2+/− embryos is highly reminiscent of ShhΔhyp mutants and is consistent with the notion that SoxB1 family members function in a dose dependent manner to regulate the expression of Shh in the ventral midline of the anterior hypothalamus. Interestingly, Sox2 also regulates the postnatal expression of Shh in neuronal stem cell populations in the hippocampus and lateral ventricle (Favaro et al., 2009).
Sox2 and Sox3 are direct regulators of Shh transcription
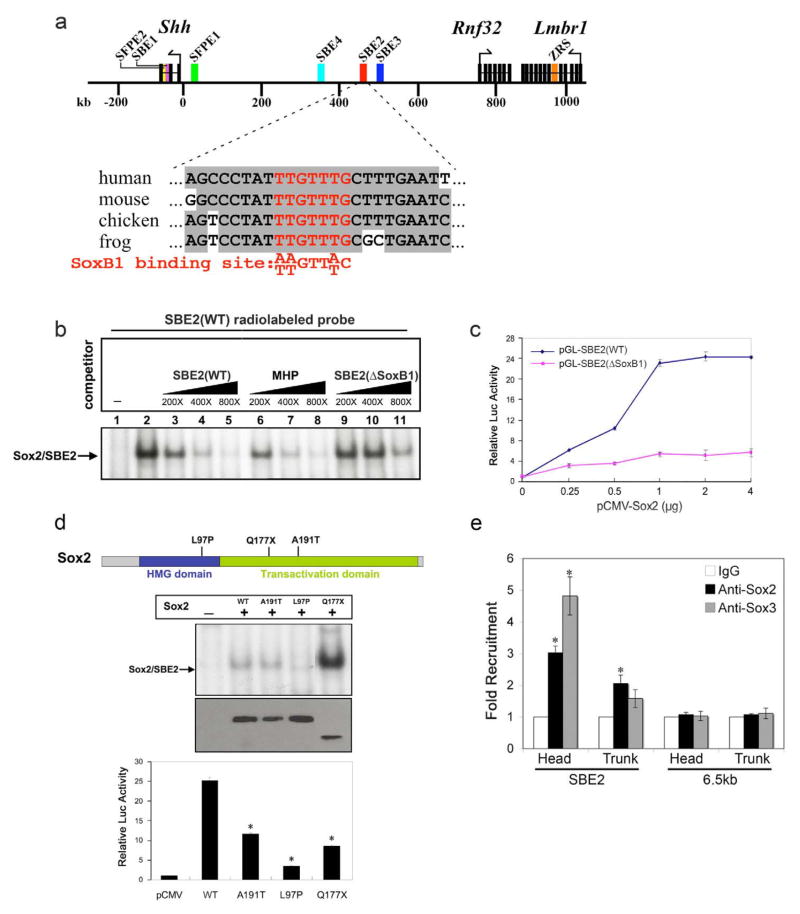
The loss of Shh expression in compound SoxB1 mutant embryos was limited to the hypothalamus, implying a high degree of specificity to the SoxB1 dependent regulation of Shh transcription (Fig. 4e,h). One mechanism by which this might occur is through the direct binding of SoxB1 factors to SBE2. To explore this possibility, we scanned the 750 bp SBE2 sequence for SoxB1 binding sites using the rVista tool and identified a single site closely matching the consensus sequence that was invariant across multiple phyla (Loots et al., 2002) (Fig. 5a).
Figure 5. Sox2 binds directly to SBE2 and is necessary for its activation.
(a) Schematic map showing the distribution of genes/exons (black rectangles) and regulatory elements (colored rectangles) within the 1Mb genomic region upstream of Shh on mouse chromosome 5. The SoxB1 binding site (red) in SBE2 is highly conserved across species. (b) EMSAs performed with Cos-1 cell extracts transfected with pcDNA3-Flag (lane 1) or pcDNA3-Flag-Sox2 (lanes 2–11) expression vectors, incubated with a radiolabeled SBE2 wild type (WT) probe. The arrow points to the specific protein/DNA complex. Increasing concentrations of unlabeled wild type probes overlapping the SoxB1 site in SBE2 (lanes 3–5), or MHP (lanes 6–8) effectively competed for Sox2 binding, whereas the mutated SBE2 (ΔSoxB1) probe did not (lanes 9–11). (c) Sox2 regulates SBE2 activity in vitro. Cos-1 cells were co-transfected with either WT (blue) or ΔSoxB1 (magenta) versions of SBE2-luciferase constructs and increasing amounts of pcDNA3-Flag-Sox2 plasmids. Sox2 activated SBE2-luc (WT) in a dose-dependent manner, but not SBE2-luc (ΔSoxB1). Each point on the curve represents an average of three experiments performed in triplicate (*p<0.02, Student’s t-test). (d) Human mutations in Sox2 affect SBE2 binding and/or activation. Top: Schema of Sox2 protein showing amino acid substitutions arising from point mutations in either the HMG DNA-binding or transactivation domains. Middle: Cos-1 cell lysates transfected with pcDNA3-Flag (lane 1), pcDNA3-Flag-Sox2 (lane 2), or pcDNA3-Flag-muSox2 (lanes 3–5) were analyzed for binding to a radiolabeled SBE2(WT) probe. No complex formation was observed for the L97P mutant (lane 4), whereas, the A191T and Q177X mutants (lanes 3 and 5) showed normal or increased DNA binding, respectively. αFlag immunoblot demonstrates that wild type and mutant Sox2 proteins were expressed at equivalent levels, although the size of Q177X is smaller than WT. Bottom: Mutant Sox2 proteins showed significantly impaired transactivation of the SBE2 (WT) luciferase reporter construct. Each bar represents the average of three experiments performed in triplicate. Asterisk represents a statistically significant difference from wild type (p<0.05). (e) ChIP from embryos using anti-Sox2, Sox3 or IgG antibodies. QPCR results from three independent experiments revealed SBE2 enrichment in Sox2 and Sox3 bound chromatin isolated from forebrain and posterior trunk regions of E10.5 mouse embryos (*p<0.01, Student’s t-test). A control sequence, 6.5 kb downstream of SBE2, was not enriched in Sox2 or Sox3 bound chromatin. Error bars in all graphs represent standard error of the mean.
Since the SoxB1 binding site was not a perfect match with the consensus sequence, we evaluated its potential to be bound by a representative SoxB1 family member in an electrophoretic mobility shift assay (EMSA). A specific protein-DNA complex was observed when Cos-1 cell lysates transfected with Flag-tagged Sox2 were incubated with a radiolabeled probe overlapping the SoxB1 binding site in SBE2 (Fig. 5b, lane 2). Further evaluation of the specificity of the Sox2/SBE2 interaction was addressed in competition assays using unlabeled probes overlapping the SoxB1 motif in SBE2, or a previously characterized SoxB1 site in the mouse Hesx1 promoter (MHP) (Eroshkin et al., 2002). Both of these cold probes displaced Sox2 binding to the radiolabeled SBE2 (WT) probe with equivalent efficiency (Fig. 5b, lanes 3–8). In contrast, an unlabeled mutant SBE2 (ΔSoxB1) probe was unable to compete efficiently for Sox2 binding (Fig. 5b, lanes 9–11).
To determine if SoxB1 factors are sufficient to stimulate transcription of an SBE2 driven luciferase reporter construct (pGL-SBE2 WT), we conducted co-transfection assays. Cos-1 cells transfected with increasing amounts of Sox2 showed a robust dose-dependent gain in luciferase activity, which was highly dependent on the presence of the SoxB1 binding site (Fig. 5c). Similar results were obtained for Sox3 (data not shown). Our finding that SoxB1 factors are potent and direct activators of SBE2-mediated transcription, afforded us with an assay to assess the mechanisms by which SOD causing mutations in these genes might affect Shh transcription. It should be noted that mutations in Sox2 are not strictly associated with SOD, but also cause a range of other developmental disorders including anopthalmia, developmental delay, oesophageal atresia and genital defects (Kelberman and Dattani, 2008). A previously described mutation in the HMG domain of Sox2 (L97P) impeded its binding to SBE2 (Fig. 5d) (Ragge et al., 2005). Two other Sox2 variants carrying point mutations in the transactivation domain showed either no effect on DNA binding (A191T), or enhanced binding to SBE2 (Q177X), the mechanism for which is unclear (Kelberman et al., 2006; Fantes et al., 2003). Interestingly, none of the Sox2 variants activated SBE2 dependent luciferase expression to a level comparable to wild type Sox2 (Fig. 5d). These findings indicate that disease-causing variants in Sox2 failed to either bind and/or activate SBE2.
We next performed chromatin immunoprecipitation (ChIP) experiments to determine if SoxB1 binding to SBE2 occurred in vivo. A significant enrichment of SBE2 was detected in Sox2 and Sox3-bound chromatin isolated from embryonic brain extracts at E10.5 (Fig. 5e). No such enrichment was detected in chromatin pulled down with an antibody against Sox9 (data not shown). Moreover, the presence of a DNA sequence 6.5 kb downstream of SBE2 was not enhanced in Sox2 or Sox3-bound chromatin, suggesting that the recruitment of SoxB1 factors to SBE2 was specific. Unexpectedly, a slight enrichment in SBE2 sequence was also observed in Sox2 bound chromatin isolated from the trunk region of the embryo. The significance of this finding is unclear as SBE2 is not active in posterior regions of the embryo. Nonetheless, these data indicate that Sox2 and Sox3 are recruited to SBE2 in the embryonic forebrain at a stage when Shh is dependent on SoxB1 factors for its expression.
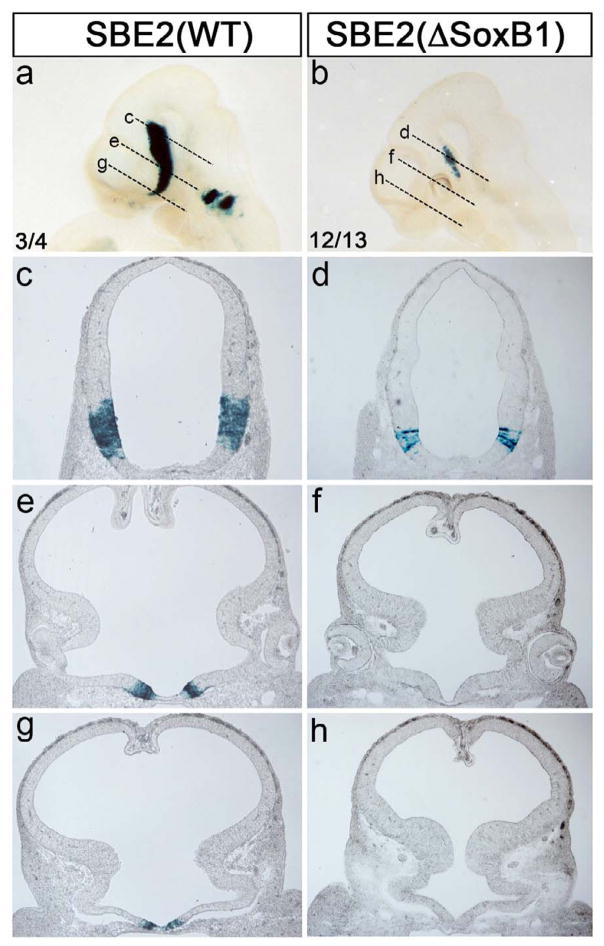
To address the in vivo significance of the SoxB1 binding site, we performed transgenic reporter assays with constructs containing either wild type (WT) or mutant (ΔSoxB1) versions of SBE2 lacking the SoxB1 motif. Embryos carrying the SBE2 (WT) lacZ construct showed a pattern of X-gal staining in the ventral diencephalon that is both typical for this enhancer and the endogenous expression of Shh (n = 3/4; Fig. 6a,c,e,g) (Jeong et al., 2006). In contrast, embryos carrying the mutated SBE2 (ΔSoxB1) lacZ construct displayed a truncated pattern of X-gal staining that consistently failed to extend into the anterior hypothalamic domain, including the area showing reduced Shh expression in SoxB1 mutant embryos (n = 12/13; Fig. 5b,d,f,h). Thus, the putative SoxB1 binding site in SBE2 is essential for transcriptional activity in the hypothalamus.
Figure 6. SBE2 activity in the hypothalamus is dependent on a highly conserved SoxB1-binding site.
X-gal staining of representative transgenic embryos carrying either wild type (WT; a,c,e,g) or SoxB1 deleted (ΔSoxB1; b,d,f,h) SBE2-lacZ reporter constructs at E10.5. The dashed lines in (a,b) show the plane of section in panels (c–h). The number of embryos showing representative reporter activity over the total number of transgenic embryos is indicated for each construct (a,b).
Discussion
Our results identify unique roles for the hypothalamic source of Shh in regulating eye and pituitary morphogenesis. The phenotypes shared between ShhΔhyp embryos and other mouse models of SOD suggest that reduced Shh signaling from the hypothalamus underlies the pathogenesis of this disorder. In agreement with this hypothesis, we show that the hypothalamic expression of Shh is directly dependent on Sox2 and Sox3, two genes that are mutated in humans and mice with SOD. Thus, SOD represents a congenital brain anomaly with a later manifestation compared to HPE, due to differences in the timing and location of Shh signal attenuation.
The unique functions of Shh in the prechordal plate versus the hypothalamus distinguish HPE from SOD. This is particularly apparent by the distinct sets of genetic variants responsible for each of these heterogeneous disorders (Kelberman et al., 2008; Geng and Oliver, 2009). The prechordal plate has long been regarded as an important signaling center for forebrain development. Surgical removal of the prechordal plate in a variety of experimental organisms, results in alobar HPE, a severe midline brain defect that presents with complete fusions of the cerebral hemispheres, eyes, nasal placodes and other craniofacial structures (Li et al., 1997; Pera and Kessel, 1997; Aoto et al., 2009). A similar HPE phenotype is observed in humans and mice lacking Shh, or other genes required for prechordal plate specification (Geng and Oliver, 2009). Shh is only expressed in the prechordal plate for a relatively brief period, lasting approximately 24 hours from E7.75 to E8.75 (Echelard et al., 1993). During this time, Shh signals to the overlying neuroepithelium to fulfill three crucial patterning events: 1) bifurcation of the telencephalic vesicles; 2) induction of the hypothalamus; and 3) formation of the bilateral optic stalks (Chiang et al., 1996). Moreover, Shh is required to maintain the viability of prechordal plate cells for their subsequent development into ventral cranial mesoderm and foregut endoderm (Aoto et al., 2009).
While Shh−/− embryos reveal the importance of Shh signaling from the prechordal plate, the HPE phenotype is further enhanced by the loss of Shh from secondary and tertiary signaling centers, including the ventral hypothalamus, ventral telencephalon (medial ganglionic eminence), oral ectoderm and pharyngeal endoderm, all of which make important contributions to brain and craniofacial development (Shimogori et al., 2010; Helms et al., 2005; Xu et al., 2010; Flandin et al., 2011).
As mentioned, Shh expression in the hypothalamus is dependent on Shh signaling from the prechordal plate (Dale et al., 1997; Manning et al., 2006). Members of the Gli family of zinc finger containing transcriptional regulators mediate this homeogenetic (like-by-like) signaling cascade. Gli2−/−; Gli3−/− embryos show a pronounced downregulation of Shh expression in the hypothalamic region of the neural tube (Motoyama et al., 2003). This Gli-dependent activation of Shh transcription in the ventral hypothalamus is not direct since Gli binding sites were not identified in SBE2 (Jeong et al., 2006). Instead, it is the combined action of Nkx2.1, Six3, Sox2 and Sox3 that is required for the initiation of Shh transcription in the hypothalamus (Sussel et al., 1999; Geng et al., 2008; Jeong et al., 2008). The precise mechanism by which these transcription factors function, either independently or in a complex, to regulate Shh expression is unclear. Nevertheless, our current and previous studies show that SoxB1 and Six3 factors are direct regulators of SBE2 activity (Jeong et al., 2008).
Bmp signaling from the prechordal plate in chick embryos further elaborates on the identity of ventral hypothalamic cells by activating Tbx2, which in turn, represses Shh expression in the ventral midline of the tuberomamillary region (Dale et al., 1997; Manning et al., 2006). This explains why Shh expression is split into bilateral stripes running through the posterior portion of the hypothalamus. Continuous Bmp signaling is also likely responsible for establishing the ventral midline domains of Bmp4, Fgf8 and Fgf10 in the posterior hypothalamus (Manning et al., 2006). Hence, an expression boundary is created at the point where Bmp/Fgf in the ventral midline of the posterior hypothalamus abuts the expression of Shh in the ventral midline of the anterior hypothalamus. The positioning of this boundary is essential for proper pituitary morphogenesis as it delineates the anterior limit of a set of reciprocal signaling interactions between the infundibulum and oral ectoderm, from where the posterior and anterior lobes of the pituitary derive, respectively (Zhu et al., 2007).
The boundary between Shh and Bmp/Fgf is shifted anteriorly in several mouse models of SOD. In ShhΔhyp and SoxB1 deficient embryos, the rostral expansion of Bmp4 and Fgf10 is due to the loss of Shh on the anterior side of the infundibulum. Interestingly, a similar function was previously ascribed to Hedgehog (Hh) in zebrafish. However, in this scenario, Hh signaling was required to prevent the entire anterior hypothalamic domain, rather than just the ventral midline, from adopting a posterior hypothalamic fate (Mathieu et al., 2002). Nevertheless, the general principle that Shh regulates anteroposterior patterning within the hypothalamus is likely conserved across phyla.
Tcf4−/−, Noggin−/− and Wnt5a−/− mouse mutants also show alterations in the positioning of the infundibulum, with associated pituitary dysmorphology (Davis and Camper, 2007; Brinkmeier et al., 2007; Potok et al., 2008). Consequently, these genes are good candidates for mutation in humans with SOD. In the case of Noggin, a secreted Bmp4 inhibitor, its absence results in a rostral expansion in the Bmp4 signaling domain and concomitant reduction in Shh expression in the anterior hypothalamus (Davis and Camper, 2007). Therefore, the antagonism between Shh and Bmp signaling that plays an essential role in regulating dorsoventral identity in the neural tube may also be instrumental in regulating anteroposterior patterning in the hypothalamus.
Mouse embryos carrying mutations in Vax1, a homeodomain containing transcriptional regulator, also show phenotypes that overlap with SOD, including eye and pituitary defects (Bertuzzi et al., 1999; Bharti et al., 2011). Moreover, Shh from the prechordal plate regulates the expression of Vax1 and Vax2 in both the developing eye and ventral diencephalon (Take-uchi et al. 2003; Kim and Lemke 2006). However, despite the seemingly linear relationship between prechordal plate Shh and Vax1 expression in early brain patterning, differences in the molecular mechanisms underlying the Vax1−/− and ShhΔhyp mutant phenotypes point to a divergence in their regulatory relationship and function at later stages. For instance, the ectopic pituitary that forms in Vax1−/− embryos appears to result from the failure to directly repress Fgf10 transcription in only a relatively small patch - compared to ShhΔhyp embryos- of the most anterior region of the diencephalon, with no obvious consequence to the neuroepithelial expression of Bmp4, Six6 or Shh (Bharti et al., 2011). Likewise, Vax1 continues to be expressed in the anterior hypothalamus of ShhΔhyp mutants (data not shown). Taken together, these results suggest that Vax1 and Shh repress Fgf10 in the anterior hypothalamus through distinct mechanisms.
The reduction of Shh expression in the ventral midline of the anterior hypothalamus can explain each of the primary defects observed in mouse models of SOD. Portions of the hypothalamus, pituitary and eye are dependent on the ventral midline source of Shh in the anterior hypothalamus for specific aspects of their development. While ShhΔhyp embryos may reflect an extreme example of SOD that is incompatible with life, subtle reductions in Shh expression, such as those observed in Sox2 and Sox3 mutants may be more typical of human SOD cases that survive postnatally.
It is intriguing to speculate that the phenotypic variability that is commonly associated with SOD in humans may be due to subtle differences in the timing, extent and duration of Shh downregulation, resulting from either genetic or environmental means. Support for this supposition is drawn from studies assessing the developmental consequences of prenatal exposure to teratogenic agents (Lipinski et al., 2010). For instance, fetal alcohol syndrome shares a similar spectrum of defects with HPE and SOD, depending on the gestational period of ethanol exposure (Lipinski et al., 2010; Coulter et al., 1993; Hellström 1999). Shh appears to be an important target of ethanol judging from the loss of its expression in the prechordal plate, and subsequent HPE phenotype, in mouse embryos subjected to ethanol during gastrulation (Aoto et al., 2008). It will be important to determine whether embryos exposed to ethanol later in development recapitulate aspects of SOD by interfering with Shh signaling activity in the hypothalamus.
Experimental Procedures
Electromobility Shift Assays (EMSA)
Cos-1 cells were transfected with pcDNA3-Flag, pcDNA3-Flag-Sox2 or pcDNA3-Flag-muSox2 using FuGENE 6 (Roche Applied Science) according to the manufacturer’s instructions. 48 hours after transfection, whole cell lysates were prepared in a RIPA buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, and 0.5% sodium deoxycholate and protease inhibitor cocktail (Roche Applied Science). EMSA was performed as described (Jeong et al., 2008). The sequence of the sense strand of oligonucleotide probes is as follows: SBE2(WT):
5′- ACTAGTTAGCCCTATTTGTTTGCTTTGAATTAC -3′,
SBE2(ΔSoxB1): 5′- ACTAGTTAGCCCTATAGCTCAGCTTTGAATTAC -3′,
MHP: 5′- CGCTTAAGGAGTTAATTGTTTATTTGTTTGCAAG -3′.
Transient transfection and dual reporter assays
Cos-1 cells were cultured under standard conditions in DMEM (GIBCO BRL) supplemented with 10% fetal bovine serum, and transfected at 50–70% confluency using FuGENE 6 (Roche Applied Science). 1 μg of an SBE2-luciferase reporter construct pGL4.23[luc2/minP] (Promega) was mixed with 0.25–4 μg of pcDNA3-Flag-Sox2 expression plasmid or empty vector for compensation, and 5 ng of pRL-TK vector (Promega) as an internal control and applied to cells grown in a 3-cm dish. Cells were harvested 48 hours after transfection and assayed for firefly and renilla-luciferase activities (Dual Luciferase assay system, Promega). Enhancer activity was expressed as fold induction relative to that of cells transfected with the empty pcDNA3 vector. At least three independent experiments were performed for each construct in triplicate.
Chromatin Immunoprecipitation
Approximately, 10–15 embryos at E10.5 were harvested in DMEM (with 10% fetal bovine serum), pooled into head and trunk fractions, cut into small pieces and fixed with 1% PFA for 15 minutes with shaking. Crosslinking was blocked by treating samples with 100 mM glycine for 15 min. Tissues were homogenized with a B dounce pestle in cell lysis buffer (5 mM Pipes, pH8, 85 mM KCl, 0.5% NP-40) with protease inhibitor cocktail, then nuclei were spun down and resuspended in nuclei lysis buffer (50 mM Tris-HCl, pH8.1, 10 mM EDTA, 1% SDS). Chromatin was sonicated and diluted in buffer (0.5% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH8.1, 150 mM NaCl). After preclearing with protein A and G agarose beads (Upstate), the chromatin was incubated overnight with 4μg of Anti-Sox2 (Abcam), Anti-Sox3 (R&D Systems), or Anti-IgG (Santa cruz) antibodies, followed by 2 hours of incubation with protein A and G agarose beads which were washed overnight in dilution buffer. Beads were washed twice with low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH8.1, 150 mM NaCl), twice with high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH8.1, 500 mM NaCl), twice again with low salt buffer, once with Li buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH8.1), and finally once with TE. After elution with 100 mM NaHCO3 and 1% SDS and decrosslinking, DNA was purified with phenol/chloroform/isoamylalcohol and subjected to quantitative PCR as described (Jeong et al., 2008) with the following primer sets:
SBE2: (F) 5′-CATATTCTAACCAGTTGGCCCTA-3′,
(R) 5′-TTGTCCACATGATCCATTTCA-3′.
6.5 kb 3′ of SBE2: (F) 5′-CACATCAGCATCCTAGCCTAC-3′,
(R) 5′-GGTACATTTCTTGTAGCTTCG-3′.
Plasmid Construction
SBE2 reporter constructs were generated by cloning a 750 bp SBE2 sequence into a vector containing the Shh minimal promoter, lacZ gene and SV40 poly(A) signal. A construct containing a deletion of the SoxB1-binding site (TTGTTTG) was generated by ligating two PCR products immediately flanking this site that were amplified with the following primer sets:
E355 (5′-GCAGCTGGATCCACAAAGCGACTTAGAGAATCTT-3′) and
E360 (5′- GCAGCTTCTAGATAGGGCCACTGGTTAGAATATG-3′);
E361 (5′-GCAGCTTCTAGAGCTTTGAATCACTCGTTTCAATA-3′) and
E343 (5′-GCAGCTGCGGCCGCACAGTCATTAGAGGCAAACAG-3′).
The cloning of full-length wild-type or mutant versions of human Sox2 into the pCMV/SV-Flag expression vector was described previously (Kelberman et al., 2006).
Mouse lines
Transient transgenic embryos were generated by pronuclear injection of SBE2 reporter constructs into fertilized eggs derived from the (BL6×SJL) F1 mouse strain (Jackson Labs, Bar Harbor ME). Sox2; Sox3 compound mutant embryos were produced by crossing Sox2+/− males with Sox3+/− females (Rizzoti et al., 2007). The SBE2-cre line was generated by pronuclear injection of a construct containing cre cloned downstream of SBE2 and a minimal Shh promoter. Stable lines were screened by in situ hybridization for cre expression in the SBE2 domain within the ventral diencephalon. The Shh+/− and Shhfl/fl (Shhtm2Amc) mouse strains were procured from the Jackson labs (Bar Harbor, ME).
Whole mount β-galactosidase staining and whole mount in situ hybridization
The activity of β-galactosidase was detected using X-gal (Sigma) as substrate. Whole-mount RNA in situ hybridization was performed using digoxygenin-UTP-labeled riboprobes according to a previously described protocol (Jeong et al., 2006). Stained embryos were dehydrated in methanol, cleared in benzyl alcohol:benzyl benzoate (1:1), and photographed. Representative embryos were rehydrated, sunk in 30% sucrose overnight, embedded in O.C.T (optimal cutting temperature embedding medium), quick frozen on dry ice and cryosectioned at 20 μm.
Immunohistochemistry and section in situ hybridization
Embryos were collected at specified developmental stages (plug day = 0.5) and fixed in 4% paraformaldehyde at 4°C for either 90 min, for immunohistochemistry, or overnight, for in situ hybridization. Embryos were washed extensively in PBS, sunk in 30% sucrose overnight at 4°C, embedded in OCT, quick frozen on dry ice and cryosectioned at 20 μm. Primary antibodies used for immunohistochemistry and dilutions are as follows: Shh (5E1; 1:100); Lhx3 (1:100); Pax6 (1:100); Neurofilament (1:250) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City Iowa); Nkx2.1 (1:1000; Dr. Carole Mendelson, UT Southwestern Medical School), Pax2 (1:250; Invitrogen), Tcf4 (1:200; Exalpha Biologicals, Inc.), Sox2 (1:250; Millipore), Sox3 (1:100; R&D Systems). Detection of primary antibodies was achieved using Cy3-(Jackson ImmunoResearch Laboratories) or Alexa 488- (Molecular Probes) conjugated secondary antibodies. Vector Labs’ Vectastain Elite ABC Kit (Rabbit IgG) and ImmPACT DAB Peroxidase Substrate were used to detect Pax2 staining on sagittal sections through the eye. Section in situ hybridization was performed as described (Nissim et al., 2007). At least 3–5 control and mutant embryos were evaluated for each antibody or in situ probe.
Supplementary Material
Acknowledgments
We thank Dr. Jean Richa and his staff at the University of Pennsylvania Transgenic and Mouse Chimeric Facility for their assistance in transgenic mouse production, as well as the staff in the Biological Services Section at NIMR. We are grateful to Drs. Dan Kelberman and Mehul Dattani (University College London, London, UK) for kindly providing the human Sox2 expression constructs. We also thank Drs. Steve Liebhaber and Kenny Campbell for constructive comments on the manuscript. This work was supported by NIH grant R01 NS039421 from NINDS (DJE), March of Dimes grant #1-FY08-421 (DJE), and the MRC (U117512772) (KR and RLB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res A Clin Mol Teratol. 2008;82:224–31. doi: 10.1002/bdra.20447. [DOI] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, Shioda S, Yamada G, Motoyama J. Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev Biol. 2009;327:106–20. doi: 10.1016/j.ydbio.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Bharti K, Gasper M, Bertuzzi S, Arnheiter H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development. 2011;138:873–8. doi: 10.1242/dev.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi S, Hindges R, Mui SH, O’Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105. doi: 10.1101/gad.13.23.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol. 1993;50:771–5. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Dakubo GD, Wang YP, Mazerolle C, Campsall K, McMahon AP, Wallace VA. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development. 2003;130:2967–80. doi: 10.1242/dev.00515. [DOI] [PubMed] [Google Scholar]
- Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, Dodd J, Placzek M. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal plate. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Barbera JP, Herman TS, Connell SO, Olson L, Ju B, Tollkuhn J, Baek SH, Rose DW, Rosenfeld MG. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev. 2001;15:3193–207. doi: 10.1101/gad.932601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattani MT, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–33. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–60. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules is implicated in the regulation of CNS and limb polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Eroshkin F, Kazanskaya O, Martynova N, Zaraisky A. Characterization of cis-regulatory elements of the homeobox gene Xanf-1. Gene. 2002;285:279–86. doi: 10.1016/s0378-1119(02)00393-1. [DOI] [PubMed] [Google Scholar]
- Fantes J, et al. Mutations in Sox2 cause anopthalmia. Nat Genet. 2003;33:461–3. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–56. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JL. Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors. Neuron. 2011;70:939–50. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–47. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Oliver G. Pathogenesis of holoprosencephaly. J Clin Invest. 2009;119:1403–13. doi: 10.1172/JCI38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A. Optic nerve morphology may reveal adverse events during prenatal and perinatal life--digital image analysis. Surv Ophthalmol. 1999;44(Suppl 1):S63–73. doi: 10.1016/s0039-6257(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Helms JA, Cordero D, Tapadia MD. New insights into craniofacial morphogenesis. Development. 2005;132:851–61. doi: 10.1242/dev.01705. [DOI] [PubMed] [Google Scholar]
- Jeong Y, El-Jaick K, Roessler E, Muenke M, Epstein DJ. A functional screen for Sonic hedgehog regulatory elements across a 1 Mb interval identifies long range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- Jeong Y, et al. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet. 2008;40:1348–53. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauvar EF, Muenke M. Holoprosencephaly: recommendations for diagnosis and management. Curr Opin Pediatr. 2010;22:687–95. doi: 10.1097/MOP.0b013e32833f56d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–55. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D, Dattani MT. Septo-optic dysplasia - novel insights into the aetiology. Horm Res. 2008;69:257–265. doi: 10.1159/000114856. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lemke G. Hedgehog-regulated localization of Vax2 controls eye development. Genes Dev. 2006;20:2833–2847. doi: 10.1101/gad.1462706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tierney C, Wen L, Wu JY, Rao Y. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development. 1997;124:603–15. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski RJ, Godin EA, O’leary-Moore SK, Parnell SE, Sulik KK. Genesis of teratogen-induced holoprosencephaly in mice. Am J Med Genet C Semin Med Genet. 2010;154C:29–42. doi: 10.1002/ajmg.c.30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res. 2002;12:832–9. doi: 10.1101/gr.225502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell. 2006;11:873–85. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Barth A, Rosa FM, Wilson SW, Peyriéras N. Distinct and cooperative roles for Nodal and Hedgehog signals during hypothalamic development. Development. 2002;129:3055–65. doi: 10.1242/dev.129.13.3055. [DOI] [PubMed] [Google Scholar]
- Morcillo J, Martínez-Morales JR, Trousse F, Fermin Y, Sowden JC, Bovolenta P. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006;133:3179–90. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Milenkovic L, Iwama M, Shikata Y, Scott MP, Hui CC. Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification. Dev Biol. 2003;259:150–61. doi: 10.1016/s0012-1606(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Nissim S, Allard P, Bandyopadhyay A, Harfe BD, Tabin CJ. Characterization of a novel ectodermal signaling center regulating Tbx2 and Shh in the vertebrate limb. Dev Biol. 2007;304:9–21. doi: 10.1016/j.ydbio.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, Shelden E, Jones JM, Kameoka J, Hitchcock PF. Pax2 expression and retinal morphogenesis in the normal and Krd mouse. Dev Biol. 1998;193:209–24. doi: 10.1006/dbio.1997.8794. [DOI] [PubMed] [Google Scholar]
- Pera EM, Kessel M. Patterning of the chick forebrain anlage by the prechordal plate. Development. 1997;124:4153–62. doi: 10.1242/dev.124.20.4153. [DOI] [PubMed] [Google Scholar]
- Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237:1006–20. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge NK, et al. SOX2 anophthalmia syndrome. Am J Med Genet A. 2005;135:1–7. doi: 10.1002/ajmg.a.30642. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–55. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. SOX3 activity during pharyngeal segmentation is required for craniofacial morphogenesis. Development. 2007;134:3437–48. doi: 10.1242/dev.007906. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–18. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Shimogori T, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–75. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–70. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Szabó NE, Zhao T, Cankaya M, Theil T, Zhou X, Alvarez-Bolado G. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci. 2009;29:6989–7002. doi: 10.1523/JNEUROSCI.1089-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-uchi M, Clarkem JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–86. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2010;348:199–209. doi: 10.1016/j.ydbio.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–40. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87:933–63. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.