Abstract
Few signaling molecules have the potential to influence the developing mammal as the nucleoside adenosine. Adenosine levels increase rapidly with tissue hypoxia and inflammation. Adenosine antagonists include the methlyxanthines caffeine and theophylline. The receptors that transduce adenosine action are the A1, A2a, A2b, and A3 adenosine receptors (ARs). We examined how adenosine acts via A1ARs to influence embryo development.
Transgenic mice were studied along with embryo cultures. Embryos lacking A1ARs were markedly growth retarded following intrauterine hypoxia exposure. Studies of mice selectively lacking A1AR in the heart identify the heart as a key site of adenosines embryo protective effects. Studies of isolated embryos showed that adenosine plays a key role in modulating embryo cardiac function, especially in the setting of hypoxia. When pregnant mice were treated during embryogenesis with the adenosine antagonist caffeine, adult mice had abnormal heart function.
Adenosine acts via A1ARs to play an essential role in protecting the embryo against intra uterine stress, and adenosine antagonists, including caffeine, may be an unwelcome exposure for the embryo.
Keywords: embryo, fetus, adenosine, caffeine, heart, A1 adenosine receptors
ADENOSINE PHYSIOLOGY
Adenosine
Adenosine consists of an adenine group attached to a ribose moiety. Adenosine is present in all cells and is a component of nucleic acids and energy-carrying molecules. 1, 2 Adenosine can be directly released from the cell or generated extracellularly. 3
Within the cell, adenosine is produced from the hydrolysis of S-adenylylhomocysteine, ATP, ADP or cAMP (Fig. 1). 4 Carrier mediated processes transport intracellular adenosine to the extracellular space via bidirectional transporters (1, 5) Intracellular adenosine disposal involves adenosine kinase (AK) that converts adenosine to AMP. 5 Adenosine is also converted to inosine by adenosine deaminase (ADA). 6
Figure 1. A1ARs protects against growth retardation in embryos.
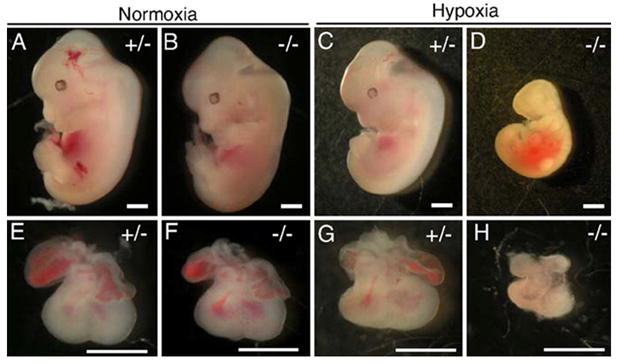
Hypoxia induces severe growth retardation in A1AR−/− embryos. Dams were exposed to 10% O2 from E8.5 to E12.5. C–R length was measured for normoxia and hypoxia-treated embryos at E12.5. Under normoxia conditions (A). A1AR+/− embryos were indistinguishable from A1AR−/− (B) embryos. Under hypoxic conditions, A1AR+/− embryos (C) were smaller then the normoxic controls (A), but A1AR−/− embryos (D) were significantly smaller than A1AR−/− or A1AR+/− normoxic embryos. A1AR−/− hypoxic hearts (H) were smaller than A1AR+/− normoxic (E), A1AR−/− normoxic (F), or A1AR+/− hypoxic hearts (G). Scale bars: 1 mm.
Extracellular ATP is an important source of adenosine following conversion of ATP to ADP and AMP. Enzymes that catalyze these reactions include the ectonucleotidases CD28 and CD39 that convert ATP to ADP and AMP, and CD73 that converts AMP to adenosine. 4 Little is known about the developmental expression and regulation of these enzymes. Contributing to elevations of adenosine levels during hypoxia, there is increased CD39 and CD73 activity, reduced cellular uptake of adenosine, and reduced AK activity in hypoxic conditions. 5, 7
Under basal conditions, interstitial adenosine levels are 1–50 nM. 2, 5 Adenosine levels rapidly rise to more than 1 uM with tissue ischemia, hypoxia, and inflammation. 2 Local adenosine levels thus provide a barometer of tissue activity and oxygenation with tissue oxygenation acting to decrease adenosine levels and oxygen deprivation increasing adenosine concentrations.
Adenosine Receptors
There are two major classes of purine receptors - P1 and P2 8, 9. ATP and ADP bind to P2 purine receptors that include P2Y purine metabotropic receptors that couple with G-proteins. 9 P2 receptors also include the P2X receptors that are ion channels. 9
Adenosine receptors (ARs) are P1 purine receptors. 8, 10, 11 A1 and A3ARs inhibit adenylyl cyclase and A2a and A2bARs stimulate adenylyl cyclase. 8, 10, 11 Similar to other G protein-coupled receptors (GPCRs), adenosine receptors contain seven putative transmembrane (TM) spanning domains. 8, 10, 11 Adenosine receptors were initially cloned as orphan receptors. 12 The identities of the genes encoding the A2a, A1, A2b, and A3 adenosine receptors were subsequently established in sequential order. 13-18
Each adenosine receptor subtype has a different pattern of tissue expression and ligand binding properties. In cell-based systems, A1ARs have the highest affinity for adenosine (Ki 10 nM). 8, 10, 11 The Ki values for adenosine for the A2a, A2b and A3 adenosine receptors are 200, 2000, and 10,000 nM, respectively, for the human receptors. 8, 10, 11 A3ARs are also activated by the adenosine metabolite inosine (Ki 2300 nM). 8, 10, 11
During development, A1ARs play an important role in transducing adenosine physiological effects. A1ARs are 326 amino acids in length with seven transmembrane-spanning domains. 17 A1ARs activate Gi and Go, inhibit cAMP accumulation, activate phospholipase C, and open ion channels. 10
Adenosine and adenosine receptors influence a number of cellular processes, as well. For example, adenosine receptors activate transcription factors, e.g. NF-κB that in turn activates pro-inflammatory molecules. 19 Adenosine also plays a role in regulating cellular event by influencing the expression of the transcription factor hypoxia-inducible factor (HIF-1). 19
A1AR-selective compounds are available and include the agonist N6-cyclopentyladenosine (CPA). 20 Specific A1AR antagonists include 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). 20 Methlyxanthines, including caffeine and aminophylline, are nonselective adenosine antagonists that block A1ARs and other adenosine receptors. 20
Adenosine receptor expression in mature mammals
Highest levels of A1AR gene expression are detected in adult brain, fat, and testis. 17 Less prominent A1AR expression is seen in the heart and kidneys. 17 A2aAR gene expression is seen in brain, heart, and lung. 16 A2bAR mRNA expression is highest in colon and bladder. 21 A2bARs expression is also high in retina. 22 A3AR gene expression is found in testis, heart, and retina. 18 Whereas levels of gene and binding site expression are proportional for A1 and A2aARs, gene expression is much greater than binding site expression for A3ARs. 23
In the brain, A2aARs are expressed in several brain regions, and heavy expression is seen in the striatum on cells expressing D2 dopamine receptors, an observation that dates back two decades. 16 A2bAR expression is localized to the pars tuberalis region of the hypophysis. 15, 21 Functional studies have suggested the presence of A3ARs in the central nervous system. 24 A1ARs are among the most widespread GPCRs in the brain. In comparison with the relatively discrete expression of other receptor subtypes, A1AR expression is at high level throughout the brain. 17, 25
In the heart, A1AR expression is present in atria and ventricles, and atrial A1AR expression is greater than that seen in the ventricles. 26 A2aARs are present in coronary vessels in endothelial cells, smooth muscle cells of blood vessels and on myocytes. 27 A3ARs are present in myocardial tissue, although at low levels. 18 A2bARs are present on endothelial cells, smooth muscles cells and fibroblasts. 28 Adenosine receptors are thus localized at sites to modulate cardiovascular system function.
Developmental expression of A1ARs
Whereas it is likely that several of the different adenosine receptor subtypes play important and possibly protective roles during development, we know more about the role of A1ARs in this regard, which is the major focus of this report. A1AR expression is present in the brain when neural tissue first appears, and A1ARs are one of the earliest expressed GPCRs in the fetal heart. 26
During early embryogenesis, when the primitive cardiac cylinder appears, A1AR gene expression is seen over the developing myocardium. Labeling of the nodal region, that controls embryo situs, is seen. 26 Later in embryogenesis, A1AR expression is seen in the heart, brain, spinal cord, and kidney. 26 Within the heart, A1AR binding site expression is more prominent over the atria than the ventricles. 26
Reporter assay studies reveal that 500 base pairs of the proximal A1AR promoter contains essential elements for A1AR gene expression. 29 Within the proximal A1AR promoter, putative binding sites for cardiac transcription factors GATA4 and Nkx2.5 were identified. 29 Embryonic A1AR expression thus involves activation of the A1AR promoter by GATA-4 and Nkx2.5.
ADENOSINE INLFUENCES ON THE EMBRYO
Influences on Embryogenesis
In different species, adenosine has been shown to exert potent effects on the developing cardiovascular system. In chicken embryos, A1ARs are expressed in the heart during early embryogenesis. 30 Treatment of Hamburger and Hamilton stage 4 embryos with the A1AR agonist N6-cyclopentyladenosine caused cardiac bifida and looping defects in 55% of embryos. Hamburger and Hamilton stage 4 embryos exposed to hypoxia followed by recovery in room air until stage 11, exhibited cardiac bifida and looping defects 30. Hypoxia-induced abnormalities were reduced when A1AR signaling was inhibited by the A1AR antagonist 1,3 dipropyl-8-cyclopentylxanthine or by siRNA-targeting A1ARs. Thus in chickens, adenosine appears to adversely influence embryogenesis 30, which differs from that seen in mammals.
Because adenosine and A1ARs mediate adverse effects of hypoxia on the developing postnatal mammalian brain and lung 31, we anticipated that blockade of adenosine action would protect embryos from hypoxia. 31 To our surprise we observed that adenosine exerts dramatic protective effects during mammalian embryogenesis. 32, 33
Timed pregnant dams from A1AR +/- x A1AR -/- matings were exposed to hypoxia or room air during early embryogenesis. 33 Under normoxic conditions, embryos lacking A1ARs develop normally. However, embryos lacking A1ARs were markedly growth retarded in hypoxia 33 (Fig. 1). These data show that adenosine acting via A1ARs play an important role in protecting the embryo from hypoxia.
Observing A1AR embryo protective roles, the molecular pathways that may mediate these effects were examined. We found differences in networks of molecular responses to hypoxia suggesting the adenosine alters hypoxia-inducible factor 1 (HIF1-α) signaling. 33 We also found that the amount of stabilized HIF-1α protein was markedly reduced in A1AR-/- embryos exposed to hypoxia. 33
After embryonic day (E) 10 in mice, the embryo is dependent on the fetal heart for adequate nutrient delivery. 5 Thus, to test if adenosine confers embryo protective effects by acting at the heart, mice that lack A1ARs only in the heart were developed. 32 Remarkably, we observed that embryos lacking cardiac A1ARs had reduced survival in hypoxia, and those that survived were growth retarded.
These observations show that adenosine plays a key role in protecting the embryo against intrauterine stress, and adenosine exerts protective effects through A1ARs expressed in the heart. It is likely that adenosine action on embryo cardiac function plays a major role in embryonic responses to intrauterine stress.
The role of other adenosine receptor subtypes
In addition to A1ARs, adenosine exerts effects during development via other adenosine receptor subtypes, in mammalian and non-mammalian species 34. In developing lambs, A2aARs in the brain regulate ventilatory responses to hypoxia. 35 A2aARs also are involved in O2 sensing in fetal carotid bodies and brains. 35 In mice maternal treatment with A2aARs antagonists influence embryonic hemodynamic function and growth, and A2aAR gene expression is detected at E11.5. 36 In mice, overexpression of the A3AR gene in development, induces embryo death, suggesting that proper levels of A3AR expression is needed for normal embryo development 37.
Effects of Caffeine on the Embryo
Because caffeine is widely consumed, potential effects of caffeine on the developing fetus have been examined in animals and humans. 38 A cup of coffee, tea, or cola contains 100-300 mg of caffeine and it is estimated that more than 60% of pregnant women consume caffeine containing beverages. 38 Following administration to pregnant rodents, embryo and fetal caffeine levels are 90% of maternal levels. 38, 39 Fetal caffeine clearance is much longer than that observed in the dam, with a half-life of 12-24 hrs. 38, 39 Maternal caffeine intake also induces down-regulation of A1ARs in mothers and fetuses suggesting that caffeine will also be able modify receptor action. 40
In rats, teratogenic effects of caffeine on the fetal heart are observed at doses in excess of 50 mg/kg.41 The most common cardiovascular malformations are ventricular defects. 42 Cardiac morphogenesis has been found to be impaired in embryos from mothers treated with both ethanol and caffeine 43, showing that caffeine can amplify effects of other toxins.
In contrast to animal studies, major teratogenic effects of caffeine have not been found in humans. 41 Few studies, though, have evaluated effects of caffeine consumption during early embryogenesis. 38, 41 Recent studies reveal that coffee consumption is associated with an increased risk of cardiovascular malformations. 38, 44 Caffeine consumption during pregnancy is also associated with an increased risk of miscarriage in a dose-dependent manner, an effect most pronounced in early pregnancy. 45, 46
Clinical studies suggest that caffeine may influence fetal growth. The risk of having small for gestational age infants is doubled if mothers have high caffeine intake. 47 Women who reduce their caffeine intake from greater than 300 mg/day to less than that amount early in pregnancy, have lower risks of delivering infants with low birth weight than women who do not. 47, 48
Considering the above, we tested if caffeine exerts effects on the embryo similar to that seen when A1ARs are deleted. 49 Pregnant mice in room air or hypoxia were treated with a single dose of caffeine at E8.5 resulting in circulating concentrations in the dam equivalent to those seen with two cups of coffee. 39 The time of exposure was equivalent to 20-30 days of human gestation, a time when many women are not aware that they are pregnant.
Caffeine was associated with reduced fetal viability. 49 When embryo size was assessed, the caffeine treated embryos were smaller than vehicle-treated embryos. 49 When cardiac histology was examined, caffeine resulted in reduced ventricular myocardial area. Caffeine also reduced HIF-1α protein expression in hypoxia. 49
We next assessed if there were long-term effects of prenatal caffeine exposure. Pregnant dams were exposed to hypoxia or room air from E8.5 to 10.5 and treated with caffeine or vehicle. At two months of age, the hypoxia-caffeine exposed male mice were significantly heavier than controls, and body fat content was significantly greater when there was prenatal caffeine exposure. 49. Echocardiography of adult animals revealed decreases in cardiac function in the groups exposed to the single dose of caffeine. 49
At present, the adenosine-mediated effects which are disrupted by caffeine that trigger embryo loss or altered fetal development are not known. During early embryogenesis, cardiac output is very much dependent on fetal heart rate. We observe that caffeine leads to alterations in embryo heart rate. It has also been observed that caffeine alters maternal cardiac output and effects embryo cardiovascular function. 36 Thus, it is likely that alteration in embryo cardiac activity by caffeine leads to altered tissue perfusion, contributing to embryo loss or altered embryo development.
CONCLUSION
An expanding body of data shows that adenosine plays an important role during prenatal development. Reduced A1AR action during embryogenesis leads to embryo loss, acute growth retardation, and hearts with thinner ventricular walls. Caffeine induces defects like that seen in embryos lacking A1ARs exposed to hypoxia. A1ARs are needed for full stabilization of HIF-1α protein in hypoxia. Embryonic caffeine treatment is associated with increased body fat and reduced cardiac function in adulthood. Thus, we have identified unique aspects of A1AR action that protects the embryo against acute hypoxic insults at embryonic stages (Fig. 2). As such, it is possible that the mechanism by which caffeine leads to embryo loss during early gestation is via blockade of A1AR action.
Figure 2. Cartoon depicting contribution of adenosine to protecting the embryo against intrauterine stress and how this is disrupted by caffeine.
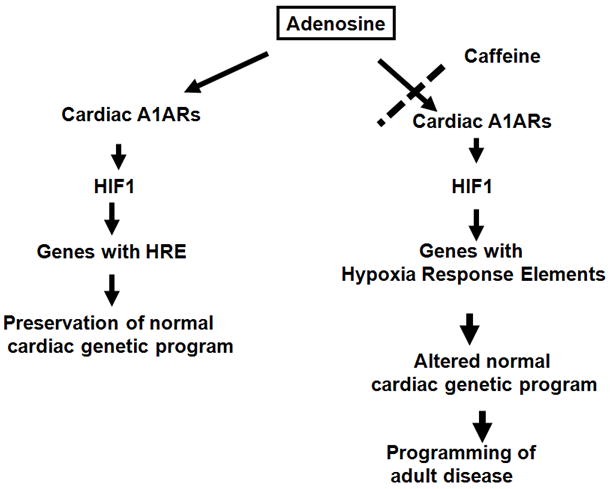
Adenosine plays important modulatory roles in mammalian development, conferring protective or deleterious effects depending on the timing of exposure and site of action. As such, adenosine antagonists, including caffeine, may be an unwelcome exposure for the embryo.
Building on these observations, additional clinical investigation is needed to better address caffeine safety during pregnancy. Studies are also needed to match known prenatal caffeine intake with long-term postnatal outcomes to determine if caffeine contributes to the programming of adult disease. As such, determining if prenatal caffeine exposure exerts epigenetic effects is needed too. Studies that better define the cellular targets of caffeine and adenosine action will also better define fundamental mechanisms that play adaptive roles in responses to hypoxia and other environmental insults.
Acknowledgments
Sources of Funding: Supported by NIH grant 1R01HD056281
Footnotes
Disclosures: There are no conflicts to report.
References
- 1.Jacobson KA. Introduction to adenosine receptors as therapeutic targets. Handb Exp Pharmacol. 2009:1–24. doi: 10.1007/978-3-540-89615-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- 3.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 5.Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- 6.Van Linden A, Eltzschig HK. Role of pulmonary adenosine during hypoxia: Extracellular generation, signaling and metabolism by surface adenosine deaminase/cd26. Expert Opin Biol Ther. 2007;7:1437–1447. doi: 10.1517/14712598.7.9.1437. [DOI] [PubMed] [Google Scholar]
- 7.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5’-nucleotidase (cd73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. Xxv. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 11.Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 12.Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, Simons MJ, Dumont JE, Vassart G. Selective amplification and cloning of four new members of the g protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- 13.Maenhaut C, Van Sande J, Libert F, Abramowicz M, Parmentier M, Vanderhaegen JJ, Dumont JE, Vassart G, Schiffmann S. Rdc8 codes for an adenosine a2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990;173:1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- 14.Libert F, Schiffmann SN, Lefort A, Parmentier M, Gerard C, Dumont JE, Vanderhaeghen JJ, Vassart G. The orphan receptor cdna rdc7 encodes an a1 adenosine receptor. EMBO J. 1991;10:1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivkees SA, Reppert SM. Rfl9 encodes an a2b-adenosine receptor. Mol Endocrinol. 1992;6:1598–1604. doi: 10.1210/mend.6.10.1333049. [DOI] [PubMed] [Google Scholar]
- 16.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat a2 adenosine receptor: Selective co-expression with d2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 17.Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat a1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- 18.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: The a3 adenosine receptor. Proc Natl Acad Sci U S A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltzschig HK. Adenosine: An old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi BH, Bridges AJ, Bruns RF. Structure-activty relationships of adenosine a1 and a2 receptors. In: Williams M, editor. Adenosine and adenosine receptors. Clifton: Humana Press; 1990. pp. 57–106. [Google Scholar]
- 21.Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM. Molecular cloning and expression of the cdna for a novel a2- adenosine receptor subtype. Mol Endocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- 22.Blazynski C. Characterization of adenosine a2 receptors in bovine retinal pigment epithelial membranes. Exp Eye Res. 1993;56:595–599. doi: 10.1006/exer.1993.1073. [DOI] [PubMed] [Google Scholar]
- 23.Rivkees SA, Thevananther S, Hao H. Are a3 adenosine receptors expressed in the brain? Neuroreport. 2000;11:1025–1030. doi: 10.1097/00001756-200004070-00026. [DOI] [PubMed] [Google Scholar]
- 24.Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR, Cunha RA. Adenosine a3 receptors are located in neurons of the rat hippocampus. Neuroreport. 2003;14:1645–1648. doi: 10.1097/00001756-200308260-00021. [DOI] [PubMed] [Google Scholar]
- 25.Swanson TH, Drazba JA, Rivkees SA. Adenosine a1 receptors are located predominantly on axons in the rat hippocampal formation. J Comp Neurol. 1995;363:517–531. doi: 10.1002/cne.903630402. [DOI] [PubMed] [Google Scholar]
- 26.Rivkees SA. The ontogeny of cardiac and neural a1 adenosine receptor expression in rats. Brain Res Dev Brain Res. 1995;89:202–213. doi: 10.1016/0165-3806(95)00120-3. [DOI] [PubMed] [Google Scholar]
- 27.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine a(2a) and a(2b) receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H650–656. doi: 10.1152/ajpheart.2000.279.2.H650. [DOI] [PubMed] [Google Scholar]
- 28.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2b adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivkees SA, Chen M, Kulkarni J, Browne J, Zhao Z. Characterization of the murine a1 adenosine receptor promoter, potent regulation by gata-4 and nkx2.5. J Biol Chem. 1999;274:14204–14209. doi: 10.1074/jbc.274.20.14204. [DOI] [PubMed] [Google Scholar]
- 30.Ghatpande SK, Billington CJ, Jr, Rivkees SA, Wendler CC. Hypoxia induces cardiac malformations via a1 adenosine receptor activation in chicken embryos. Birth Defects Res A Clin Mol Teratol. 2008;82:121–130. doi: 10.1002/bdra.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner CP, Seli M, Ment L, Stewart W, Yan H, Johansson B, Fredholm BB, Blackburn M, Rivkees SA. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci U S A. 2003;100:11718–11722. doi: 10.1073/pnas.1931975100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendler CC, Poulsen RR, Ghatpande S, Greene RW, Rivkees SA. Identification of the heart as the critical site of adenosine mediated embryo protection. BMC Dev Biol. 2010;10:57. doi: 10.1186/1471-213X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendler CC, Amatya S, McClaskey C, Ghatpande S, Fredholm BB, Rivkees SA. A1 adenosine receptors play an essential role in protecting the embryo against hypoxia. Proc Natl Acad Sci U S A. 2007;104:9697–9702. doi: 10.1073/pnas.0703557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnstock G, Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68:1369–1394. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koos BJ. Adenosine aa receptors and o sensing in development. Am J Physiol Regul Integr Comp Physiol. 2011;301:R601–622. doi: 10.1152/ajpregu.00664.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momoi N, Tinney JP, Liu LJ, Elshershari H, Hoffmann PJ, Ralphe JC, Keller BB, Tobita K. Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am J Physiol Heart Circ Physiol. 2008;294:H2248–2256. doi: 10.1152/ajpheart.91469.2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Yaar R, Ladd D, Cataldo LM, Ravid K. Overexpression of a3 adenosine receptors in smooth, cardiac, and skeletal muscle is lethal to embryos. Microvasc Res. 2002;63:61–69. doi: 10.1006/mvre.2001.2366. [DOI] [PubMed] [Google Scholar]
- 38.Browne ML. Maternal exposure to caffeine and risk of congenital anomalies: A systematic review. Epidemiology. 2006;17:324–331. doi: 10.1097/01.ede.0000208476.36988.44. [DOI] [PubMed] [Google Scholar]
- 39.Fredholm BB. Astra award lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 40.Leon D, Albasanz JL, Ruiz MA, Fernandez M, Martin M. Adenosine a1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem. 2002;82:625–634. doi: 10.1046/j.1471-4159.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 41.Christian MS, Brent RL. Teratogen update: Evaluation of the reproductive and developmental risks of caffeine. Teratology. 2001;64:51–78. doi: 10.1002/tera.1047. [DOI] [PubMed] [Google Scholar]
- 42.Ross CP, Persaud TV. Cardiovascular primordium of the rat embryo following in utero exposure to alcohol and caffeine. Can J Cardiol. 1986;2:160–163. [PubMed] [Google Scholar]
- 43.Ross CP, Persaud TV. Early embryonic development in the rat following in utero exposure to alcohol and caffeine. Histol Histopathol. 1986;1:13–17. [PubMed] [Google Scholar]
- 44.Grosso LM, Triche EW, Belanger K, Benowitz NL, Holford TR, Bracken MB. Caffeine metabolites in umbilical cord blood, cytochrome p-450 1a2 activity, and intrauterine growth restriction. Am J Epidemiol. 2006;163:1035–1041. doi: 10.1093/aje/kwj125. [DOI] [PubMed] [Google Scholar]
- 45.Cnattingius S, Signorello LB, Anneren G, Clausson B, Ekbom A, Ljunger E, Blot WJ, McLaughlin JK, Petersson G, Rane A, Granath F. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med. 2000;343:1839–1845. doi: 10.1056/NEJM200012213432503. [DOI] [PubMed] [Google Scholar]
- 46.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: A prospective cohort study. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 47.Vik T, Bakketeig LS, Trygg KU, Lund-Larsen K, Jacobsen G. High caffeine consumption in the third trimester of pregnancy: Gender-specific effects on fetal growth. Paediatr Perinat Epidemiol. 2003;17:324–331. doi: 10.1046/j.1365-3016.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 48.Larroque B, Kaminski M, Lelong N, Subtil D, Dehaene P. Effects of birth weight of alcohol and caffeine consumption during pregnancy. Am J Epidemiol. 1993;137:941–950. doi: 10.1093/oxfordjournals.aje.a116764. [DOI] [PubMed] [Google Scholar]
- 49.Wendler CC, Busovsky-McNeal M, Ghatpande S, Kalinowski A, Russell KS, Rivkees SA. Embryonic caffeine exposure induces adverse effects in adulthood. Faseb J. 2009;23:1272–1278. doi: 10.1096/fj.08-124941. [DOI] [PMC free article] [PubMed] [Google Scholar]


