Abstract
Adoptive immunotherapy with cytomegalovirus (CMV) specific cytotoxic T lymphocytes (CTL) is an effective strategy for preventing and treating viral reactivation following allogeneic stem cell transplantation (SCT). We have previously shown that CMV CTL can be generated in 1-2 weeks by stimulating donor lymphocytes with peptide mixes derived from full length pp65 and IE1. We conducted a multi-institutional study of CMV specific CTL for patients with persistent or anti-viral resistant CMV infections following allogeneic SCT, to determine the safety, feasibility, and immunologic effects of this approach. We were successful in stimulating CTL from 10/10 donors with pooled CMV overlapping peptide mixes. Five of the 7 subjects who met infusion criteria had new onset CMV specific CTL activity detected within 4-6 weeks post infusion. Of the two subjects who did not have immunologic responses post-infusion, one received CTL with a low viability post-thawing, and the other patient was receiving cyclosporine A and systemic corticosteroids at the time of the infusion, achieving only a low, transient increase (10%) in pp65 specific activity. There was no GVHD attributable to these infusions. These findings indicate that the infusion of CTL stimulated over 1-2 weeks with overlapping CMV peptides can result in virus specific immune reconstitution in SCT recipients, without exacerbations of GVHD.
Keywords: cytomegalovirus, pp65, IE-1, immunotherapy, CTL
Introduction
Infections with CMV continue to be a source of morbidity following allogeneic SCT, although the majority of patients are successfully treated with antiviral agents when they reactivate this virus. Nevertheless, these agents have significant toxicities, most notably myelosuppression and nephrotoxicity 1, 2. The incidence of neutropenia ranges from 40-60% in ganciclovir recipients3, 4, with an increased risk of bacterial and fungal infections 3, 5. Prolonged usage of this agent can be associated with lymphopenia, further delaying virus specific immune reconstitution2. Cidofovir and foscarnet can be associated with nephrotoxicity, requiring intravenous hydration and careful monitoring of electrolyte status6. Some patients who reactivate CMV develop resistance to these agents, or due to their profoundly immunosuppressed state are unable to clear the virus. Persistent CMV infections are particularly a problem in recipients of T cell depleted SCT, for whom adoptive immunotherapy with antigen specific T cells has been shown to be effective in preventing and treating viral reactivation7. Considering the long term risks of persistent CMV infection and the impact of prolonged use of anti-virals, individuals with persistent viremia are ideal candidates for an intervention that would restore antigen specific immunity to CMV.
While adoptive immunotherapy has been shown to be effective for the treatment and prevention of Epstein Barr virus (EBV), adenovirus, and CMV infections post-transplant8-12, many strategies for expanding CTL require genetic manipulation of antigen presenting cells or special expertise in cell separation. The fact that not all institutions have this capacity has largely precluded implementation of adoptive immunotherapy on a wider scale. We have previously demonstrated that CMV pp65 specific CTL can be cultured from the majority of normal healthy donors using autologous adherent cells pulsed with pooled, overlapping peptides derived from full length pp65 and IE113. Cellular immunotherapy for CMV could be used in more patients if it was possible to generate a relatively low cost CTL product, with a low risk of conferring GVHD and a high likelihood of restoring CMV specific immunity. To test the clinical and immunologic effects of CMV CTL that are expanded using monocytes pulsed with CMV pp65 peptide mixes, we conducted a multi-center, Phase 1 study of CMV CTL in allogeneic SCT recipients with persistent, therapy refractory infections.
Patients and Methods
Subject Eligibility
Subjects were enrolled at Penn State Hershey Medical Center (PSHMC), the University of California at San Francisco, Benioff Children’s Hospital, the University of Kansas Medical Center, and Levine Children’s Hospital (Charlotte, NC). These studies were approved by the US Food and Drug Administration under IND 13192, by each center’s institutional review board, as well as the National Marrow Donor Program. Subject entry criteria included any recipient of an allogeneic SCT > 1 month of age who had CMV antigenemia for > 2 weeks or CMV DNA levels > 600 copies/mcg DNA, despite anti-viral therapy targeting CMV. Subjects needed to have a CMV sero-positive donor, or a CMV sero-negative donor who was willing to receive a CMV vaccine. Details of administering the Towne strain CMV vaccine (from Dr. Stuart Adler, Medical College of Virginia) have been previously described14. At the time of infusion, the subjects could not have any sign of ongoing GVHD, and needed to be on < 1 mg/kg/day of prednisone, or its equivalent. Shortly after the study began accruing patients, a modification was made to permit stimulation with IE1 peptide mixes simultaneously with pp65, in the event some donors would preferentially respond to the former antigen. Depending upon the version of the protocol approved at each center, pp65 or pp65/IE1 CTL were infused.
CTL culture
60-80 mL of peripheral blood was collected from stem cell donors, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation. 50-100×106 PBMC were placed in 15ml of RPMI (Gibco, Chicago, IL) with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) for 1 hour in T150 flasks (Corning, NY). Non-adherent peripheral blood lymphocytes (PBL) were removed and resuspended at 2×106/mL in RPMI 1640 / 10% FBS, and adherent cells were removed with a cell scraper (Corning). Adherent cells were washed in RPMI 1640 and placed at 10×106 in 0.5 mL RPMI in 50 mL conical centrifuge tubes, and then pulsed with CMV pp65, IE-1, or both peptides simultaneously. The CMVpp65 and IE1 peptide mixes (JPT Peptide Technologies, Berlin) consisted of 138 and 120 overlapping 15mers, respectively. Each of these peptides was suspended in 40μL of DMSO (Sigma Aldrich, St. Louis, MO) per the manufacturer’s instructions. 3μL (0.7mg/ml) of the peptide suspension was added to 10-20×106 adherent cells and the capped centrifuge tubes were incubated at room temperature for 2 hours, and then washed/centrifuged in RPMI 1640 three times. PBL were plated with peptide pulsed adherent cells at a responder: stimulator ratio of 10:1 in 24 well plates (Corning), 2 mL per well. CMV CTL were assayed for CMV specific cytotoxicity at 10-12 days of culture. Target cells included donor B cell blasts (BB) infected with vaccinia encoding full length pp65 or IE1, (vacc-pp65 from Dr. William Britt, University of Alabama, Birmingham, and vacc-IE1 from Dr. Donald Diamond, City of Hope), BB pulsed with pp65 or IE1peptide mixes, as well as autologous and allogeneic BB, the latter as negative controls. If specific cytotoxicity against both antigens was < 25% at a responder:stimulator ratio of 10:1, then the cells were re-stimulated for one more week with peptide pulsed adherent cells, and then assayed. Infusion criteria included ≥ 25 % cytotoxicity against either BB expressing pp65 or BB-IE1, and testing negative for endotoxin, mycoplasma, bacteria, and fungus by culture. CTL were cryopreserved in a controlled rate freezer at PSHMC Cell Therapy laboratory and were shipped to participating institutions for patients meeting eligibility criteria for CTL infusion. Cells were thawed in the participating institution’s Cell Therapy Lab, viability was checked, and the cells were transferred to the patient care unit for infusion.
Cell function analysis
CMV specific T cell responses were assessed before and at bi-weekly intervals post-infusion by chromium release assays and using flow cytometry for CMV antigen specific interferon-γ (IFN-γ) production, as previously described13. Targets for chromium release assays (CRA) included autologous and allogeneic BB (used as a negative control) and BB pulsed with the pp65 or IE1 peptide mixes. To determine whether these effector cells recognized naturally processed and presented pp65 and IE1 epitopes, we also infected BB with vaccinia encoding pp65 and IE1. B cell blasts were cultured from donor PBMC as previously described15. Targets were labeled overnight with 51Cr (100 μCi/106 cells; from PerkinElmer Life and Analytical Science, Boston, MA), washed in PBS, and dispensed in triplicate into 96 well V-bottom plates (ICN, Costa Mesa, CA) at 4 × 103 cells/well, as previously described16. CTL were added at a responder: target ratio (of 10:1), and after pelleting and incubation for 4 hours, the supernatant was counted in a gamma-counter. Spontaneous and total release for each target was used to calculate percent specific release with the following formula: % specific release = (experimental cpm-spontaneous cpm)/(total cpm-spontaneous cpm).
Intracellular cytokine staining (ICS)
Flow cytometry for IFN-γ production was performed on a FACScan (Becton Dickinson) to detect pp65 and IE1 specific T cells. Multiple-color staining of immunophenotypic markers, both surface and intracellular, was performed as previously described17. In brief, T cells were incubated with equal numbers of stimulators, including autologous BB pulsed with pp65 and IE1 overlapping peptides or B blasts infected with either vaccinia encoding pp65 or IE1, and autologous and allogeneic BB, in RPMI 1640 with 10% FCS and in the presence of 10 μg/ml brefeldin A (Sigma) at 37°C for 5 hours. After stimulation, EDTA was added at a final concentration of 2.5 mM, and the cells were incubated at room temperature for 10 minutes. 10 vol Lysing Solution (BD PharMingen) was added, and the cells were incubated for 10 minutes. The cells were washed with 3% fetal calf serum and 0.1% NaN3 in PBS, incubated with permeabilization buffer (BD PharMingen) for 10 minutes, aliquoted, and stained with the following labeled antibodies (BD PharMingen): CD4-FITC, CD8-peridinin chlorophyll protein and IFN γ-APC. Control antibodies were the respective isotype antibodies conjugated with relevant fluoresceins.
RESULTS
We were able to culture CMV pp65 CTL from all 10 stem cell donors stimulated with pp65 and 6/6 stimulated with IE1 peptides. Specific cytotoxicity was measured at day 10 of culture using B cell blasts (BB) infected with either vaccinia encoding pp65 or IE-1. IFN-γ producing T cells specific for CMV were analyzed by intracellular staining for pp65 and IE1. Autologous and allogeneic BB were used as negative controls. CTL which showed < 10% of cytotoxicity to negative control cell lines and > 25% pp65 or IE1 specific cytotoxicity were considered CMV specific. CMV pp65 or IE1 specific cytotoxicity and IFN-γ production was present in each of these donors, and data from donor CTL that were infused are presented in Figure 1. This is consistent with our previous findings in which CMV pp65 or IE1 specific CTL could be cultured from the majority of healthy donors17. The average pp65 specific cytotoxicity for the products was 37 % (range 21-58 %) and 30% for IE1 (range 21%-38%). Seven products were cultured for 9-11 days (one stimulation), and 3 products were cultured for an additional week to meet infusion criteria (lack of allo-reactivity and > 25% specific cytotoxicity). All CTL products met potency and sterility infusion criteria.
Figure 1.
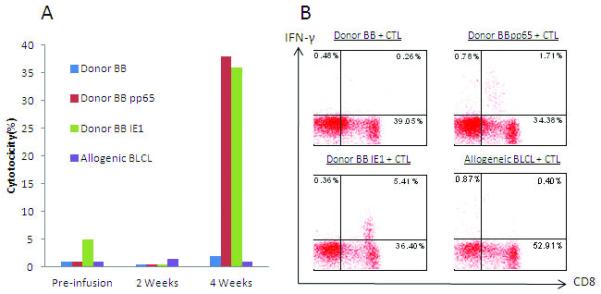
CMV specific CTL from stem cell donors. Adherent cells from donor peripheral blood mononuclear cells were pulsed with pooled CMV pp65 (donor 1 and 2); pp65 and IE1 peptides (donor 3, 4, 5, 6, and 7) and were incubated with donor peripheral blood lymphocytes. Specific cytotoxicity was measured at day 10 of culture using B cell blasts (BB) infected with either vaccinia encoding pp65 (A) or IE1 (B). IFN-γ producing T cells specific for CMV were analyzed by intracellular staining for pp65 and IE1 (panels C and D). Autologous and allogeneic BB were used as negative controls. All CTL showed < 10% cytotoxicity and < 0.1% IFN-γ production to autologous and allogeneic BB. Note: donor 1 and 2 were not stimulated with IE1.
Patient characteristics are presented in Table 1. Seven of the 10 subjects for whom CTL were cultured met eligibility criteria for infusion. Three of the subjects did not meet eligibility criteria after the cells were prepared, generally due to control of CMV DNA levels with anti-viral medications, and therefore did not receive CTL. 5/7 CTL recipients were transplanted with a T cell depleted, haplo-identical SCT from a parent, and two received unmodified SCT from unrelated donors. 6 subjects initially reactivated CMV within 4 weeks post-transplant and one subject reactivated the virus 8 months post-transplant, with the majority having persistent CMV infection despite months of antiviral therapy with ganciclovir or foscarnet.
Table 1. Patient Characteristics and Outcome.
| Subject Number |
Age/Diagnosis | Transplant type/Center |
Time post-SCT for CMV reactivation |
Cell dose of CMV specific CTL( CD3+CMV CTL/kg) |
Number of infusions |
Immuno- suppression |
CMV CTL response/timing |
Changes in CMV DNA Levels Post- infusion over tIme |
|---|---|---|---|---|---|---|---|---|
| 1 | 19 years /MDS | Haplo-TCD UCSF |
4weeks | 5×105 | 2 | No | Yes/4 weeks | 2000 to 100 3 weeks |
| 2 | 4 years/Hyper IgM syndrome |
Haplo-TCD UCSF |
2 weeks | 5×105 | 1 | No | No* | persistent viremia |
| 3 | 14 years/AML | MUD-unmod PSHMC |
4 weeks | 5×105 | 1 | CSA/prednis one |
Transient/3 weeks |
2200 to 0 3 weeks |
| 4 | 9 mo/SCID | Haplo-TCD UCSF |
8 months | 5×105 | 1 | No | Yes/6 weeks | 2054 to 0 2 weeks |
| 5 | 11 years/T cell lymphoblastic lymphoma |
Haplo-TCD LCH |
3 weeks | 2.5×105 | 2 | Plaquenil/ Prednisone |
Yes/4 weeks | Intermittent viremia post- infusion; CMV retinitis |
| 6 | 2 years/CID | Haplo-TCD UCSF |
1 week | 2.5×105 | 1 | No | Yes/4 weeks | 1500 to 0 1 week |
| 7 | 19/HLH | MUD-unmod KUMC |
1 week | 5×105 | 1 | Prednisone | Transient/3 weeks |
Viremia recurred after steroids used for presumed HLH |
AML: acute myelogenous leukemia; MDS: myelodysplastic syndrome; HLH: Hemophagocytic lymphohistiocytosis; (S)CID: (severe) combined immunodeficiency; Haplo: human leukocyte antigen haplo-identical, related donor; MUD: matched unrelated donor; TCD: T cell depleted; unmod: unmodified bone marrow. Centers: University of California at San Francisco-UCSF; Penn State Hershey Medical Center-PSHMC; Levine Children’s Hospital-LCH; University of Kansas Medical Center-KUMC.
The first subject, who received CMV CTL from a vaccinated, CMV sero-negative donor, was previously shown to have had resolution of CMV viremia and reconstitution of pp65 specific T cell responses post-infusion14. Of all 7 patients with persistent CMV receiving CMV CTL infusions, four developed pp65 or IE1 specific cytotoxicity by 4 weeks post-infusion, and one subject developed pp65 specific cytotoxicity 6 weeks post-infusion. Four of seven CTL recipients had DNA levels decrease within three weeks of CTL to 0-100 copies CMV DNA. Three individuals who were receiving immunosuppressive medications at the time of CTL infusion and afterward had reactivation and needed further antivirals. Subject 3 was receiving cyclosporine A and 0.3 mg/kg/day of prednisone at the time of the CTL infusion, and this subject had only a transient increase (10%) in CMV pp65 specific cytotoxicity 3 weeks post-infusion, correlating with control of CMV pp65 DNA levels. Subject 5 was treated for chronic skin GVHD with prednisone and plaquenil at the time of the CTL infusion. Despite developing CMV specific T cell function post-infusion, this patient had intermittent CMV viremia and developed CMV retinitis 4 months later, which resolved with intra-ocular ganciclovir and intravenous foscarnet. Subject 7, a recipient of CMVpp65 and IE1 CTL, developed IE1 specific cytotoxicity (17%) 3 weeks post-infusion, but at this time was thought to have progression of his primary illness (hemophagocytic lymphohistiocytosis), and was placed on systemic corticosteroids. The use of steroids eradicated CMV specific CTL activity from all subsequent time points and resulted in recurrence of CMV viremia. Subject 3 and 7 subsequently died from systemic fungal infections. Two subjects not receiving systemic immunosuppression failed to achieve CMV specific immune reconstitution within 4 weeks of the infusion. Subject 2 received 5 × 105 CMV specific CD3+ cells/kg but unfortunately the viability of the CTL was 60%, which could have compromised the efficacy of this product. This subject had no CMV specific T cell function after the CTL infusion, and his primary transplant physician gave him an unmodified donor lymphocyte infusion 6 weeks later, which was associated with an increase in CMV pp65 cytotoxicity 3 weeks post-infusion. Subject 4 received 5 × 105 CD3+ cells/kg and achieved CMV pp65 specific cytotoxicity 6 weeks post-infusion.
Figure 2A shows CMV pp65 and IE1 specific cytotoxicity over time post-infusion in these subjects. The overall trend was for these individuals to have an increase in CMV pp65 and IE1 specific CTL activity post-infusion, although there were variations in the extent of T cell responses. CMV pp65 and IE1 specific IFN-γ production was measured between 1 and 2 months post-infusion, and these data are presented in Figure 2B. Of four subjects for whom there were sufficient cells to perform this analysis, each had specific IFN-γ production to either CMV pp65 or IE1. A detailed depiction of the timing of CMV specific cytotoxicity and IFN-γ production for subject 5 is depicted in figure 3. This individual received CMV pp65/IE1 CTL, and specific cytotoxicity against both antigens was detected at 4 weeks post-infusion, with CD8+ cells producing IFN-γ in response to both of these antigens at this time. There was a relatively low number of CD4+ cells making IFN-γ in response to pp65 and IE-1 (0.35% and 0.19%, respectively, data not shown). This subject continued off anti-virals but had persistent, low level (0-1550) CMV DNA detected from the peripheral blood, and anti-virals were resumed. A second CTL infusion was given 4 weeks after the first dose of CTL, and despite sustained CMV CTL activity, he developed CMV retinitis 3 months later.
Figure 2.
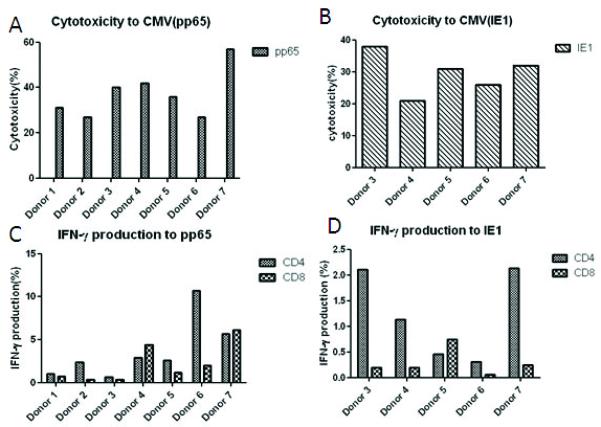
Patient T-cell responses after CMV specific CTL infusions. Patient peripheral blood monocytes were pulsed with pooled CMV pp65 and IE1 peptides and incubated with peripheral blood lymphocytes. Specific cytotoxicity to pp65 (A) and IE1(B) was measured at day 10 of culture using B cell blasts (BB) infected with either vaccinia encoding pp65 or IE-1 (vvp65; vvIE1). IFN-γ producing T cells specific for CMV were analyzed at 1-2 months post-infusion by intracellular staining to pp65 (C) and IE1 (D). Autologous and allogeneic BB were used as negative controls.
Figure 3.
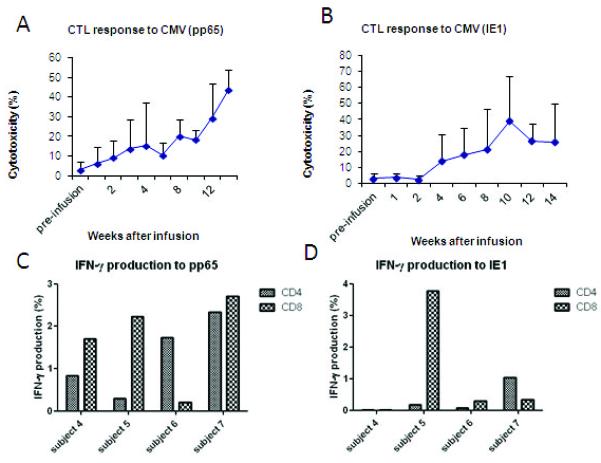
T-cell responses of patient 5 after a CMV specific CTL infusion. (A) CMVpp65 and IE1 specific cytoxicity was detected at 4 weeks post infusion. (B) IFN-γ production by CD8+ cells was also detected at 4 weeks post infusion.
DISCUSSION
Several previous studies have demonstrated the efficacy of infusing CMV specific CTL for the treatment and prevention of CMV reactivation post-transplant8-12. Riddell and group initially reported the safety and efficacy of infusing stem cell donor derived CTL clones8, but this approach was not applied on a larger scale due to the technical challenges associated with cell cloning. Einsele and group generated CMV CTL by repetitive stimulation with CMV lysate, and 5/7 subjects had sustained anti-CMV immune responses9. As seen in our study, subjects receiving immune suppression at the time of the CTL infusion had only transient reductions in viral load. Despite these limitations, 5/7 CTL recipients developed CMV specific immune responses, all of whom had persistent CMV infections on anti-viral therapy. Our culture system is novel in that CD4+ and CD8+, CMV specific CTL can be generated is a cost-effective, timely manner (2 weeks), using technology that can be applied at most transplant centers17. There were no exacerbations of GVHD associated with these infusions even though these CTL were only stimulated 1-2 times with CMV antigen.
Two subjects (subject 1 and 5) required repeat CTL infusions to treat CMV viremia. Although these were transient increases in CMV specific T cell activity post-infusion, CTL recipients on systemic immunosuppressive medications were less likely to respond to cellular immunotherapy. Three subjects in our series were on systemic immunosuppression on or shortly after receiving CTL, impairing viral clearance and reducing the likelihood of an observable immune effect. One of the challenges to applying prophylactic anti-viral immunotherapy is the fact that some SCT patients will reactivate CMV at a time point during which they are at risk for GVHD, and may be receiving systemic immunosuppression. It is unclear whether the presence of these medications is an absolute contraindication to giving donor T cells for viral reactivation. It is likely that the concomitant use of immunosuppressive medications will likely subvert the function of these CTL, although there may be a role for CTL infusions in recipients on calcineurin inhibitors, in which case some CTL function may be retained post-infusion. One group demonstrated that EBV CTL activity was retained in vitro when CTL were exposed to lower dose (250 ng/ml) cyclosporine A18. Subject 5 achieved CMV specific T cell function post-infusion, but later developed CMV retinitis while on immunosuppressive therapy for chronic GVHD. These findings highlight the deleterious effects of prolonged systemic immunosupression on viral clearance. While some effector functions might be preserved in patients who are on chronic immunosuppression, these medications clearly have a negative impact on the ability of infused CTL to function in vivo.
The optimal cell dose of CMV CTL for adoptive immunotherapy is unknown. MacKinnon and group reported the use 1 × 105 CD3+ CMV specific T cells/kg, with no GVHD and the majority of recipients having no subsequent CMV reactivation19. This group also described the selection of CTL based on IFN–γ capture, with a mean CMV reactive T cell dose of 3.4 × 106 total cells per patient20. CTL infused on this study were stimulated with antigen only 1-2 times, and therefore had a greater potential to contain allo-reactive cells. CTL cultured in this manner would also be expected to have a lower CMV specific CTL precursor frequencies compared to CTL that had been cloned or selected for antigenic specificity. While there were no exacerbations of GVHD attributable to the CMV CTL used in this study, the infusion of CTL that have been stimulated more than once might be of benefit, enriching for CMV CTL activity and reducing the risk of conveying cells that could trigger GVHD. Two patients required more than one CTL infusion due to persistent viremia, but this might have been avoided if a higher cell dose was used initially, or if a product with a higher proportion of CMV CTL is used.
A decision to administer antigen specific CTL must be based on the risks associated with anti-viral medications and with prolonged CMV infection. Previous studies have shown that the restoration of CMV specific CD4 cell function is critical for long term immune reconstitution following adoptive immunotherapy21, providing helper functions as well as exerting direct control in vivo of viral replication 22. Our results show that IFN-γ producing, CD4 and CD8 CMV (pp65 and IE1) T cells increase after the infusion of peptide mix stimulated cells. The method used to generate CMV CTL for these patients are simple to carry out and could be done at centers without the infrastructure to carry out more complex cell manipulations or gene therapy. We have previously reported that a single cell infusion for a typical 70 kg adult was comparably priced to less expensive than 28 day courses of ganciclovir and foscarnet17, with the added benefit of restoring cellular immunity to CMV. It is possible that a similar culture method could also be used to generate EBV or adenovirus specific CTL. Since many patients with these infections can be stabilized for a few weeks with pharmacologic agents, it is possible that antigen specific CTL could be cultured on an “as needed” basis, reserved for those patients with refractory infections. Alternatively, these CTL could be infused into patients at high risk for prolonged viral reactivation, such as recipients of T cell depleted transplants. Further work needs to be done to optimize the timing and dose of these CTL, as well as determining whether there is any role for cellular immunotherapy in patients who are receiving immunosuppressive agents.
Acknowledgements
This work was supported by grants NIH R01CA106319-2 and the Four Diamonds Foundation for Cancer Research
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Conflicts of Interest: All authors have declared there are no conflicts of interest with regards to this study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Salzberger B, Bowden RA, Hackman RC, et al. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997;90:2502–8. [PubMed] [Google Scholar]
- 2.Battiwalla M, Wu Y, Bajwa RP, et al. Ganciclovir inhibits lymphocyte proliferation by impairing DNA synthesis. Biol Blood Marrow Transplant. 2007;13:765–70. doi: 10.1016/j.bbmt.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Goodrich JM, Bowden RA, Fisher L, et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173–8. doi: 10.7326/0003-4819-118-3-199302010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Riddell SR, Greenberg PD. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–86. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 5.Boeckh M, Gooley TA, Myerson D, et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–71. [PubMed] [Google Scholar]
- 6.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71:154–63. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–41. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 9.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–22. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 10.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 11.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–86. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:707–14. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Bao L, Sun Q, Lucas KG. Rapid generation of CMV pp65-specific T cells for immunotherapy. J Immunother. 2007;30:557–61. doi: 10.1097/CJI.0b013e31803b945b. [DOI] [PubMed] [Google Scholar]
- 14.Horn B, Bao L, Dunham K, et al. Infusion of Cytomegalovirus Specific Cytotoxic T Lymphocytes from a Sero-Negative Donor Can Facilitate Resolution of Infection and Immune Reconstitution. Pediatric Infectious Disease Journal. 2009;28:65–67. doi: 10.1097/INF.0b013e318182026f. [DOI] [PubMed] [Google Scholar]
- 15.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, Burton RL, Dai LJ, et al. B lymphoblastoid cell lines as efficient APC to elicit CD8+ T cell responses against a cytomegalovirus antigen. J Immunol. 2000;165:4105–11. doi: 10.4049/jimmunol.165.7.4105. [DOI] [PubMed] [Google Scholar]
- 17.Bao L, Dunham K, Stamer M, et al. Expansion of cytomegalovirus pp65 and IE-1 specific cytotoxic T lymphocytes for cytomegalovirus-specific immunotherapy following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:1156–62. doi: 10.1016/j.bbmt.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan X, Brown B, Slobod KS, et al. Inhibition of ex vivo-expanded cytotoxic T-lymphocyte function by high-dose cyclosporine. Transplantation. 2003;76:739–40. doi: 10.1097/01.TP.0000078623.64968.E5. [DOI] [PubMed] [Google Scholar]
- 19.Peggs KS, Mackinnon S. Augmentation of virus-specific immunity after hematopoietic stem cell transplantation by adoptive T-cell therapy. Hum Immunol. 2004;65:550–7. doi: 10.1016/j.humimm.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Thomson K, Julie M, Pang K, et al. Direct isolation of donor-derived antigen-specific T cells and their adoptive transfer for treatment or prophylaxis of CMV infection following allogeneic transplantation. Blood. 2006;108:177a. [Google Scholar]
- 21.Waldrop SL, Pitcher CJ, Peterson DM, et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–50. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DM. Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


