Abstract
The health benefits of green tea and its main constituent (-)-epigallocatechin gallate [(-)-EGCG] have been widely supported by results from epidemiological, cell culture, animal and clinical studies. On the other hand, there are a number of issues, such as stability, bioavailability and metabolic transformations under physiological conditions, facing the development of green tea polyphenols into therapeutic agents. We previously reported that the synthetic peracetate of (-)-EGCG has improved stability and better bioavailability than (-)-EGCG itself and can act as pro-drug under both in vitro and in vivo conditions. Analogs of catechins have been synthesized and their structure activity relationship provides an understanding to the mechanism of proteasome inhibition. Metabolic methylation of catechins leading to methylated (-)-EGCG may alter the biological activities of these compounds.
Introduction
Green tea, produced from the unfermented dried leaves of the plant Camellia sinensis, has been consumed by humans for thousands of years. Regular drinking of green tea has been associated with many health benefits (Hara, 2001; Higdon, 2003). These include reducing the risk of cardiovascular diseases; reduced incidence and mortality due to cancer; decreasing fat absorption; anti-ageing; suppressing inflammation and inhibiting viral or bacterial infections. Many of these claims have been supported by in vitro cellular studies and some in vivo animal models. Since tea consumption is generally not associated with toxic effect, the attraction of using green tea extract as therapeutic agents is considerable. Yet, the U.S. Food and Drug Administration (FDA), after reviewing the human data, concluded recently that “there is no credible evidence to support qualified health claims for green tea or green tea extract reducing the risk of heart disease” and “it is highly unlikely that green tea reduces the risk of breast cancer or prostate cancer” (U.S. FDA, 2005, 2006). This article will discuss some of the issues facing the development of green tea polyphenols as therapeutic agents, based on the challenge of extrapolations from experiments in vitro to situation in vivo.
Separation and purification of catechins
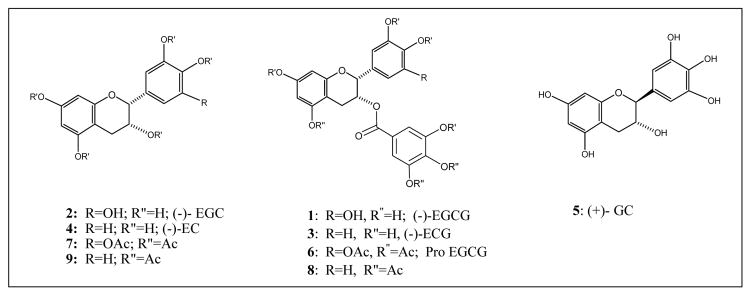
On brewing the green tea leaves with hot water, the aqueous solution contains tannic acid, caffeine (about 10–50 mg per average cup of green tea, half that of coffee) and polyphenolic catechins (about 50–100 mg polyphenols per cup) and a number of minor components (Haslam, 1989). The major catechins are: (-)-epigallocatechin-3-gallate (EGCG, 1), (-)-epigallocatechin (EGC, 2), (-)-epicatechin-3-gallate (ECG, 3), (-)-epicatechin (EC, 4) and (+)-gallocatechin (GC, 5) (Fig. 1). Of these, EGCG is by far the most abundant and has various biological activities which may account for the beneficial effects attributed to green tea. Green tea extract is thus a complex mixture, often with various proportions of different components depending on the origin, time of harvest, method of preparation and many other factors. In human clinical trial, pure active ingredient should be used instead of green tea extract.
Fig. 1.
Chemical structures of green tea polyphenols and synthetic analogs.
In a phase II clinical trial in the treatment of patients with androgen independent metastatic prostate carcinoma, patients were prescribed green tea powder at a dose of 6 grams per day for one to four months. At this dosage, thirty-one percent of patients reported no toxicity whatsoever directly attributed to the green tea, 28 percent of the patients dropped out of the study because of varying degree of toxicity such as nausea, emesis, insomnia, fatigue, diarrhea, abdominal pain and confusion (Common Toxicity Criteria Grade 1 to 4) presumably from the tea’s caffeine (Jatoi, 2003).
Caffeine-free green tea extract, under the trademark of Polyphenon™, is obtained by treating tea leaves with water and then spray dried to powder. The powder is dissolved in water and washed with chloroform; then extracted with ethyl acetate. The ethyl acetate solution was then concentrated and freeze-dried to give Polyphenon™. It contains about: 1 % (+)-GC; 18 % (-)-EGC; 6 % (-)-EC; 54 % (-)-EGCG; 12 % (-)-ECG and 9 % other substances (Hara, 2001). Ointment of Polyphenon™ has recently been approved for topical application in the treatment of genital warts and marketed as Veregen™ by MediGene Company.
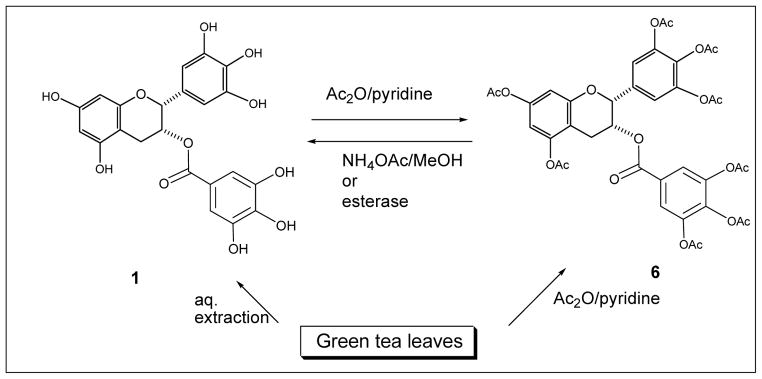
Further purification of individual catechins to high purity (>98 %) in large quantity has not been easy because of the ready water solubility and the structural similarities of the catechins. A US patent described a process involving three column chromatographic separations using expensive reverse phase column fillings to purify EGCG (Bailey, 2001). A more recent patent application described a process of separating catechins using chromatography on a macroporous polar resin with a polar elution solvent under pressure (Burdick, 2003). The lack of quantities of pure catechins of high purity at reasonable cost may well hamper the clinical development of using green tea polyphenols for possible therapeutic applications. We have recently devised an alternative method of purifying catechins to high purity by treating green tea leaves directly with acetic anhydride in pyridine. This acetylation reaction converted the mixture of catechins into fully acetylated catechins (Scheme 1) and rendered them less hydrophilic and separable by simple column chromatography over silica gel with ethyl acetate/hexane as eluent. In this way, EGCG octaacetate (6), EGC hexaacetate (7), ECG heptaacetate (8) and EC pentaacetate (9) (Fig. 1) were obtained as solids with >98 % purity (Huo, 2008). The amounts of the four acetates depended on the source of green tea. Selective removal of the acetate moiety by hydrolysis using ammonium acetate in aqueous methanol gives the original catechin back. In this way, for example, EGCG (1) can be obtained from EGCG octaacetate (6) (Chan, 2005) (Scheme 1).
Scheme 1.
Bioavailability issues
A major challenge in extrapolating the biological activities of green tea polyphenols in vitro to possible effects in vivo is bioavailability. In this respect, it is known that EGCG has poor bioavailability (Lambert, 2003). The poor bio-availability of EGCG can be attributed to several factors: (a) the instability of EGCG in alkaline or neutral conditions (Chen, 2001), (b) low cellular uptake due to high aqueous solubility and poor hydrophobicity to cross cell membrane; (c) metabolic transformations such as methylation, glucuronidation and sulfation (Lu, 2003) and (d) active efflux of many polyphenolic compounds by the multidrug resistance-associated protein 2 (MRP2) (Hong, 2003). Following i. g. administration of decaffeinated green tea, to the rats the absolute plasma bioavailability of EGCG, EGC and EC was 0.1 %, 14 % and 31 % respectively. For mice, by comparison, the absolute plasma bioavailability of EGCG was 26.5 % but with greater than half of the EGCG present as the glucuronide conjugates. Several studies on the pharmacokinetics of tea polyphenols in humans have been reported (Chow, 2001, 2003, 2005; Yang, 1998). For example, oral administration of green tea at a dose of 20 mg/kg body weight resulted in plasma Cmax for EGCG at 78 ng/mL, a concentration far below the micromolar concentration usually required for in vitro activity. The extent of bioavailability and thus therapeutic efficacy depends on the route of administration as well as the organ site to be considered. Ultraviolet-induced skin tumor incidence in BALB/cAnNHsd mice was significantly reduced by topical, but not by oral, administration of purified EGCG (Gensler, 1996). This is in line with the success of topical treatment of genital warts with Polyphenon™ ointment referred to earlier. For oral administration of tea polyphenols, one would expect the oral cavity and the digestive tract to have the highest bioavailability (Lee, 2004; Suganuma, 1998). On the other hand, because of their hydrophilic nature, the catechins are not expected to cross the blood-brain barrier to reach the brain to any significant extent (Suganuma, 1998). This will have an impact on any in vivo study of the effect of green tea polyphenols on neurodegenerative conditions.
An effective way to improve the bioavailability of a drug is to use the pro-drug approach (Ionescu, 2005). In 2004, we proposed the use of (-)-EGCG octaacetate (6, Pro-EGCG) as a pro-drug of (-)-EGCG (1) (Lam, 2004). Compound 6 is much more stable than EGCG (1) in solution of pH = 8. When cultured human breast cancer MDA-MB-231 cells were treated with Pro-EGCG (6), accumulation of both Pro-EGCG (6) and EGCG (1) were found inside the cells (Landis-Piwowar, 2007). This proved that Pro-EGCG was converted intracellularly into EGCG, presumably by cellular esterases (Scheme 1). Furthermore, Pro-EGCG (6) was better absorbed into the cells, giving higher accumulation of EGCG (1) by at least 2.4 fold than when the cells were treated with similar levels of EGCG. Similarly, treatment of HCT116 human colon cancer cells with Pro-EGCG (6) resulted in a 2.8 to 30 fold greater intracellular concentration of EGCG as compared with treatment with equivalent amount of EGCG. Intragastric administration of Pro-EGCG (6) to CF-1 mice led to higher bioavailability in plasma, small intestinal and colonic tissues compared with administration of equimolar doses of EGCG (Lambert, 2006). This improved bioavailability is reflected in enhanced bioactivity. Even though it is not an inhibitor of proteasome in cell-free system, Pro-EGCG (6) is more potent than EGCG at inhibiting the proteasomal chymotrypsin-like activity in MDA-MB-231 cells (Landis-Piwowar, 2007). More importantly, the enhanced bioactivity also manifested in vivo. In animal xenograft models, Pro-EGCG (6) was found to be more effective than EGCG (1) at equivalent dosages in inhibiting tumor growth for MBA-MB-231 breast tumors (Landis-Piwowar, 2007a) and for CWR22R androgen-independent prostate cancer (Lee, 2008). It is obviously of interest to see if such improved bioavailability and enhanced bioactivity by using a pro-drug are also true in humans.
Chemical synthesis of analogs and structure activity relationships
In light of the wide range of biological activities attributed to green tea polyphenols, it is believed that green tea polyphenols affect a number of biological pathways and molecular targets (Chen, 2008). Structure-activity relationships, using both natural compounds and synthetic analogs, is helpful to understand the mechanism of interaction of the green tea polyphenols with the potential molecular targets. This has been applied in the case of proteasome inhibition (Dou, 2008). In 2001, we reported the first chemical synthesis of epigallocatechin gallate (1) in an enantioselective manner providing separately the natural (-)-EGCG as well as its enantiomer (Li, 2001). This was followed by the syntheses of EC, EGC (Wan, 2004) and a number of analogs (Smith, 2002; Kazi, 2004; Wan, 2005). Structure-activity studies using the natural green tea polyphenols and the synthetic analogs on proteasome inhibition revealed a number of interesting features: (a) the carbonyl function of EGCG and analogs is essential for inhibitory activity (Nam, 2001); (b) synthetic (+)-EGCG, the enantiomer of the natural (-)-EGCG, showed nearly equal potency (Smith, 2002); (c) the ester oxygen at C-3 can be replaced by the NH isostere with little reduced activity to purified proteasome but improved potency to cellular proteasome, probably due to increased stability (Smith, 2004) and (d) decreasing the number of –OH groups from either the A-, B- or D- ring of EGCG leads to diminished proteasome inhibitory activity in vitro (Osanai, 2008; Wan, 2004, 2005). On the basis of the structure activity relationships, a rational model has been proposed with in silico docking studies (Smith, 2004). The model suggests that (-)-EGCG and the active analogs predictably bind to the N-terminal threonine (Thr) of the proteasomal chymotrypsin β-5 subunit active site (Dou, 2008). This orientation is suitable for nucleophilic attack by the hydroxyl group of Thr 1 to the carbonyl carbon of (-)-EGCG, thus deactivating the proteasomal chymotrypsin-like activity. Similar structure-activity studies can be profitably applied to other molecular targets to gain further understanding on the potential of green tea polyphenols as therapeutic agents.
Metabolic transformations of green tea polyphenols
In vivo activity of the green tea polyphenols may also be affected by metabolic transformations. EGCG and the other tea catechins undergo biotransformations including methylation (Lu, 2003a), glucuronidation (Lu, 2003b), sulfation (Vaidyanathan, 2002) as well as oxidative degradation products (Li, 2000; Lambert, 2003). In a case-control study of Asian-American women in Los Angeles, the relationship between intake of green tea and risk of breast cancer was examined according to catechol-O-methyltransferase (COMT) genotype (Wu, 2003). Among women who carried at least one low activity COMT allele, inverse association between tea intake and breast cancer risk was observed; but for women who were homozygous for the high activity COMT allele, risk of breast cancer did not differ between tea drinkers and non-tea drinkers. To explain these results, it was suggested that O-methylation of the catechins by COMT, an enzyme ubiquitously present in humans, may reduce the cancer preventive effect of the catechins (Wu, 2003). Indeed, catechins are known to be substrates of human COMT (Zhu, 2000). In humans, O-methylated EGCG derivatives were detected after consumption of green tea and catechin (Meng, 2002). Some methylated catechins have been found as minor components in tea infusions (Sano, 1999). Recently, we completed the syntheses of 9 different methylated catechins which are metabolites or potential metabolites of tea catechins in biomethylation (Wan, 2006). We found that the addition of a methyl group on the B- or D- ring of (-)-EGCG or (-)-ECG led to decreased proteasome inhibition and, as the number of methyl groups increased, the inhibitory potencies further decreased (Dou, 2008). Metabolic O-methylation of EGCG may indeed reduce the effectiveness of EGCG in its anti-cancer activity (Landis-Piwowar, 2007b), in support of the human study (Wu, 2003).
On the other hand, metabolic O-methylation of EGCG may not always lead to reduction of biological activities. For example, methylated EGCG has been shown to be more potent than EGCG in the inhibition of type I allergic reactions in mice (Tachibana, 2000). Metabolic biotransformations also affect the physiochemical properties of the green tea polyphenols and therefore their bioavailability. How these metabolites affect in vivo biological activity deserves greater examination.
Conclusions
Many beneficial effects have been attributed to green tea and the polyphenolic catechins are implicated as the active ingredients. The most abundant catechin, (-)-epigallocate-chin gallate (EGCG, 1), has been found to have a number of biological activities, potentially applicable for the prevention and treatment of cancer, heart diseases, diabetes, neurodegenerative diseases and other conditions. However, there are a number of challenges in developing green tea polyphenols into therapeutic agents. Pure active ingredients with better stability should be used. The poor bioavailability of EGCG and other catechins needs to be overcomed. Structure-activity relationships, using both natural compounds and synthetic analogs, need to be conducted to understand the mechanism of interaction of the green tea polyphenols with the potential molecular targets. Finally, metabolic biotransformation of the green tea polyphenols and their effects on biological activity in vivo will need to be understood better.
Acknowledgments
This work was supported in part by research grants from the National Cancer Institute-National Institutes of Health (to Q. P. D.; 1R01CA120009; 5R03CA112625) and the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Administrative Region, China (Project No. AoE/P-10/01, to T. H. C.) and NSERC of Canada (to T.H.C). We also thank American Diagnostic Inc. for financial support.
References
- Bailey DT, Yuhasz RL, Zheng B. Method for isolation of caffeine-free catechins from green tea. 6210679. US Patent. 2001 issued on April 3, 2001.
- Burdick DC, Egger H, Gum AG, Koschinski I, Muelchi E, Prevot-Halter I. Process for the production of (-)-epigallocatechin gallate. 20030083270. US Patent application. 2003 May 1;
- Chan T, Lam WH. Methods of Separating Catechins from Green Tea Leaves. PCT/CN2005/001644. International Patent Application. 2005 October;
- Chen D, Milacic V, Chen MS, Wan SB, et al. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol. 2008;23:487–96. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–82. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- Chow HH, Cai Y, Alberts DS, Hakim I, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- Chow HH, Cai Y, Hakim I, Crowell JA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, et al. Effect of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- Dou QP, Landis-Piwowar KR, Chen D, et al. Green tea polyphenols as a natural tumour cell proteasome inhibitor. 2008. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensler HL, Timmermann BN, Valcic S, Wachter GA, Dorr R, Dvorakova K, et al. Prevention of photocarcinogenesis by topical administration of pure epigallocatechin gallate isolated from green tea. Nutr Cancer. 1996;26:325–35. doi: 10.1080/01635589609514488. [DOI] [PubMed] [Google Scholar]
- Haslam E. Plant Polyphenols: Vegetable Tannins Revisited. Cambridge University Press; New York: 1989. [Google Scholar]
- Hara Y. Green Tea: Health Benefits and Applications. Marcel Dekker Inc; New York: 2001. [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hong J, Lambert JD, Lee SH, Sinko PJ, et al. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–7. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Huo C, Shi GQ, Lam W, et al. Semi-synthesis and proteasome inhibition of D-ring deoxy analogs of (-)epigallocatechin gallate (EGCG), the active ingredient of green tea extract. Can J Chem. 2008;86:495–502. [Google Scholar]
- Ionescu C, Caira MR, editors. Drug metabolism and current concepts. Springer; Dordrecht, Netherlands: 2005. [Google Scholar]
- Jatoi A, Ellison N, Burch PA, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- Kazi A, Wang ZG, Kumar N, et al. Structure-activity relationships of synthetic analogs of (-)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–54. [PubMed] [Google Scholar]
- Lam WH, Kazi A, Kuhn DJ, et al. A potential prodrug for a green tea polyphenol: proteasome inhibitor evaluation of the peracetate ester of (-)-epigallocatechin gallate. Bioorg Med Chem. 2004;12:5587–93. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:727–47. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Rice JE, Hong J, Hou Z, et al. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy derivatives. Bioorg Med Chem Lett. 2005;15:873–6. doi: 10.1016/j.bmcl.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Sang S, Hong J, Kwon SJ, Lee MJ, Ho CT, et al. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispo. 2006;34:2111–6. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Huo CD, Chen D, Cui QC, Minic V, Shi GQ, et al. A Novel Pro-drug of the Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate as a Potential Anti-Cancer Agent. Cancer Res. 2007a;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, et al. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007b;213(1):252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Lambert JD, Prabhu S, Meng X, et al. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract, Cancer Epidemiol. Biomarkers Prev. 2004;13:132–7. doi: 10.1158/1055-9965.epi-03-0040. [DOI] [PubMed] [Google Scholar]
- Lee SK, Chan WK, Lee TW, Lam WH, Wang X, Chan TH, et al. Effect of a pro-drug of the green tea polyphenol (-)-epigallocatechin-3-gallate on the growth of androgen independent prostate cancer in vivo, Nutri. Cancer. 2008;60:483–91. doi: 10.1080/01635580801947674. [DOI] [PubMed] [Google Scholar]
- Li C, Lee MJ, Sheng S, Meng X, et al. Structural identification of two metabolites of catechins and their kinetics in human uring and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–84. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- Li L, Chan TH. Enantioselective synthesis of epigallocatechin-3-gallate (EGCG), the active polyphenol component from green tea. Org Lett. 2001;3:739–41. doi: 10.1021/ol000394z. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos. 2003a;31:572–9. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Li C, Sang S, et al. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003b;31:452–61. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- Meng X, Sang S, Zhu N, Lu H, et al. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice and rats. Chem Res Toxicol. 2002;15:1042–50. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Osanai K, Milacic V, Dou QP, Chan TH. Enantioselective synthesis and proteasome inhibition of A ring analogs of (-)-epigallocatechin gallate (EGCG), the active ingredient of green tea extract. Heterocycles. 2008 in press. [Google Scholar]
- Sano M, Suzuki M, Miyase T, Yoshino K, Maeda-Yamamoto M. Novel antiallergic catechin derivatives isolated from oolong tea. J Agric Food Chem. 1999;47:1906–10. doi: 10.1021/jf981114l. [DOI] [PubMed] [Google Scholar]
- Smith DM, Wang ZG, Kazi A, Li LH, Chan TH, Dou QP. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–92. [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Daniel KG, Wang ZG, Guida WC, Chan TH, Dou QP. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins: Structure, Function, and Bioinformatics. 2004;54:58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Oniyama M, tade Y, Ito H, Fujiki H. Wide distribution of [3H]-(-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–6. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Sunada Y, Miyase T, Sano M, Maeda-Yamamoto M, Yamada K. Identification of a methylated tea catechin as an inhibitor of degranulation in human basophilic KU812 cells. Biosci Biotechnol Biochem. 2000;64:452–4. doi: 10.1271/bbb.64.452. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Letter Responding to Health Claim Petition dated January 27, 2004: Green Tea and Reduced Risk of Cancer Health Claim, Docket number 2004Q-0083. 2005. Jun 30, [Google Scholar]
- U.S. Food and Drug Administration. Qualified Health Claims: Letter of Denial – Green Tea and Reduced Risk of Cardiovascular Disease, Docket number 2005Q-0297. 2006. May 9, [Google Scholar]
- Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (-)-epicatechin by the human and rat enzymes. Drug Metab Dispos. 2002;30:897–903. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- Wan SB, Chen D, Dou QP, Chan TH. Study of the green tea polyphenols catechin-3-gallate (CG) and epicatechin-3-gallate (ECG) as proteasome inhibitors. Bioorg Med Chem. 2004;12:3521–7. doi: 10.1016/j.bmc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Wan SB, Landis-Piwowar KR, Kuhn DJ, Chen D, Dou QP, Chan TH. Structure-activity study of epi-gallocatechin gallate (EGCG) analogs as proteasome inhibitors. Bioorg Med Chem. 2005;13:2177–85. doi: 10.1016/j.bmc.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Wan SB, Dou QP, Chan TH. Regiospecific and enantioselective synthesis of methylated metabolites of tea catechins. Tetrahedron. 2006;62:5897–904. [Google Scholar]
- Wu AH, Tseng C-C, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in asian-american women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]
- Yang CS, Chen L, Lee MJ, Balentine D, et al. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–4. [PubMed] [Google Scholar]
- Zhu BT, Patel UK, Cia MX, Conney AH. O-Methylation of tea polyphenols catalyzed by human placental cytosolic cat-echol-O-methyltransferase. Drug Metab Dispos. 2000;28:1024–30. [PubMed] [Google Scholar]