Abstract
Terpenoids represent a large and diverse class of naturally occurring compounds found in a variety of fruits, vegetables and medicinal plants. Structurally some of the terpenoids are similar to human hormones. A diet rich in terpenoids is inversely related with the risk of chronic diseases including cancers. Breast and prostate cancers are hormone-related diseases and the second leading cause of female and male cancer mortality. Diterpenoid paclitaxel, and its semi-synthetic analogue docetaxel, have entered clinical use against established breast and prostate cancers. Here we reviewed potential molecular targets and biological properties of natural terpenoids, including monoterpenoids, diterpenoids, triterpenoids and tetraterpenoids, and their applications in treatment of human breast and prostate cancers. These terpenoids are able to inhibit tumor cell proliferation and induce tumor cell death by inhibiting multiple cancer-specific targets including the proteasome, NF-κB, and antiapoptotic protein Bcl-2. The efficacy of these terpenoids against breast or prostate cancer cells, as demonstrated in pre-clinical studies supports clinical application of these naturally occurring terpenoids in treatment of hormone-related human cancers.
Keywords: Terpenoids, breast cancer, prostate cancer, proteasome inhibitors, apoptosis
1. INTRODUCTION
Terpenoids represent a large class of natural compounds. They are classified according to the number of containing cyclic structures such as hemiterpenoids (with 1 isoprene unit), monoterpenoids (with 2 isoprene units), sesquiterpenoids (with 3 isoprene units), diterpenoids (with 4 isoprene units), sesterterpenoids (with 5 isoprene units), triterpenoids (with 6 isoprene units), tetraterpenoids (with 8 isoprene units) and polyterpenoids (with a larger number of isoprene units). Terpenoids are extensively found in fruits, vegetables and medicinal plants. This category of compounds exhibits multiple properties including antioxidation, anti-inflammation and anticancer. In vitro and in vivo studies indicate that terpenoids caused inhibition on cell proliferation and tumor growth in a verity of human cancers. However, because of the space limitation of this review article, we only focus on human breast and prostate cancers. In addition, multiple molecular pathways were found to be involved in the anticancer actions of terpenoids, including activation of apoptosis triggered by inhibition of the ubiquitin-proteasome and NF-κB pathways. It has been well documented that suppression of the ubiquitin-proteasome and NF-κB pathways is essential for induction of tumor cell apoptosis. Therefore in this review, we first summarize the research on the effect of terpenoinds on apoptosis, proteasome and NF-κB pathways and then discuss the potential application of terpenoinds for treatment of human breast and prostate cancers.
1.1. Breast and Prostate Cancers
Breast cancer is the most common cancer expected to occur in women, and in 2009 it was the second cause of cancer death in women [1]. Steroid hormone estrogen, especially estradiol plays an important role in the carcinogenesis of breast cancer [2]. Estrogen such as estradiol binds to the estrogen receptor α or β (ERα or ERβ) in the nucleus of breast cancer cells and initiates conformational changes in ER structures. The conformational changes result in the dimerization and binding of ER at the appropriate estrogen response elements (EREs) in the promoter region of estrogen-responsive genes, recruiting coactivators and initiating the formation of transcriptional complex. In addition to estradiol binding, ER activity could be enhanced via phosphorylation at several specific sites of ERα [3].
Based on gene expression, breast cancer could be divided into three subtypes: (i) the luminal subtype, which expresses the human epidermal growth factor receptor 2 (HER2) and ER, (ii) hormone receptor-negative subtype, and (iii) the basal-like subtype which recently is of particular interest [4, 5]. Basal-like breast cancer is characterized by the absence of ER, progesterone receptor (PR) and HER2 overexpression [4] or referred as ER-, PR- and HER2-negative breast cancer, so often called triple-negative breast cancer [6]. The subtypes vary in prognosis, with worse outcomes observed in hormone receptor-negative patients compared with the luminal subgroups, while triple-negative subtype of breast cancer is a very aggressive tumor with poor prognosis [4, 7].
Prostate cancer is the most common cancer expected to occur in men and the major cause of cancer death, next to lung cancer in men, in 2009 [1]. Steroid hormone androgens contribute to the initiation and promotion of multistage carcinogenesis through binding to hormone receptors [2]. An androgen receptor (AR) is a physiological mediator of the development and function of male reproductive organs [8]. Upon androgen binding, inactive AR is activated through dimerization and nuclear translocation, where it functions as a transcription factor to alter the expression of androgen responsive genes [9]. AR plays an important role in the initiation and progression of prostate cancer by regulating cell proliferation, differentiation and apoptosis [8]. Early stages of prostate cancer can be effectively treated by androgen-ablation therapy through surgical and medical castration. However, most of these prostate cancer patients eventually relapse to a hormone-refractory state that no longer responds to androgen deprivation [10]. AR appears to be a dominant factor in the transition from hormone-sensitive to hormone-refractory disease [11].
There is well-established evidence to show that the AR gene undergoes alterations such as amplification or mutation in hormone-independent cancers. As a result, these hormone-independent cancer cells are very sensitive to low or no androgen environments, and are responsive to a broad range of ligands such as growth factors, other steroid hormones, anti-androgens, etc [12, 13]. It has also been reported that wild type AR can be activated by other signaling pathways in a ligand-independent manner [14, 15]. Furthermore, unliganded AR can bind the enhancer elements on the promoters of target genes and mediate their expression even in the absence of androgen, as seen with the prostate specific antigen (PSA) gene in androgen-independent prostate cancer cells [16]. Therefore, finding an effective means to eliminate AR from prostate cancer cells is essential for an effective treatment of prostate cancer.
1.2. Apoptosis
Programmed cell death (apoptosis) is a highly conserved form of cell death that plays crucial roles for embryonic development and tissue homeostasis. Dysregulated apoptosis leads to tumor formation or even development of cancer cell drug resistance [17]. Apoptosis is triggered through two main routes; the extrinsic (depending on triggering of death receptors, transmembrane proteins expressed on the cell surface) and intrinsic (mediated by molecules released from the mitochondria) pathways [18]. A set of cysteine proteases, caspases that cleave substrates at specific aspartyl residues, is regarded as the cascade of both apoptotic pathways. However, caspase-independent apoptosis was also observed [19]. Caspases are expressed in cells as inactive proenzyme forms that require oligomerization and/or cleavage for activation.
The extrinsic apoptosis pathway is initiated through the binding of ligand to death receptors that contain an intracellular death domain. Upon ligand binding, the death receptors trimerize and recruit the adaptor protein Fas-associated death domain (FADD) and procaspase-8 and/or caspase-10 to form a death-inducing signaling complexes (DISC) in the intracellular death domain [20]. In the DISC, caspase-8 or -10 oligomerization drives its activation through auto-proteolytic cleavage [18]. This leads to triggering of the enzymatic activity of downstream effector caspases, such as caspase-3 and -7 [18].
Upon physical or chemical stimulations, such as hypoxia, growth factor deprivation, cell detachment, or stress signals, the intrinsic mitochondrial pathway is activated. The proapoptotic Bcl-2 family members, such as Bax or Bak play an important role in initiating this pathway. They oligomerize and integrate into the mitochondria outer membrane, which is thought to open a pore and cause subsequent mitochondria outer membrane permeabilization (MOMP) [21]. As a result, a component of the mitochondrial respiratory chain, cytochrome-c, present in the intermembrane space, is released from mitochondria into the cytosol [21]. In the cytosol, cytochrome c promotes the oligomerization of apoptotic peptidase activating factor 1 (Apaf-1), leading to recruitment and activation of caspase-9 in a large complex called the apoptosome. Caspase-9 is the initiator caspase associated with the intrinsic or mitochondrial pathway of apoptosis. Once activated, caspase-9 cleaves and activates downstream effector caspases-3 and -7, which then cleave the key regulatory and structural proteins to execute cell death.
1.3. Ubiquitin-Proteasome Pathway and Nuclear Factor kappa B (NFκB)
The ubiquitin-proteasome pathway is responsible for the degradation of most intracellular proteins involved in regulation of cell cycle and apoptosis. A targeted protein is marked with a chain of small polypeptides called ubiquitin. The ubiquitination process is mediated by a series of enzymes, called E1 ubiquitin activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin-ligating enzyme [22, 23]. Polyubiquitinated proteins can then be recognized and degraded by the proteasome.
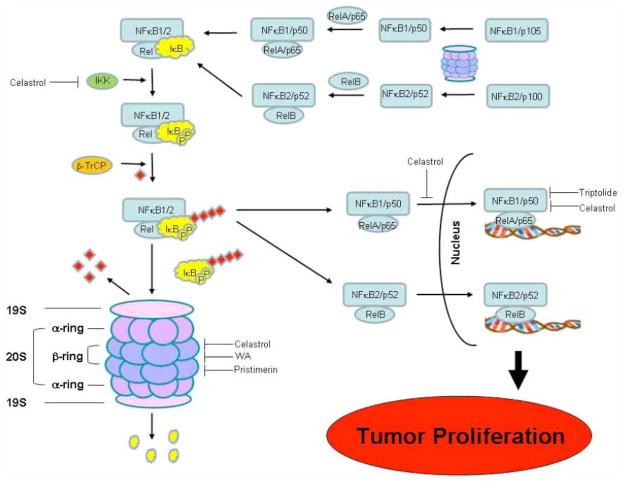
The proteasome is a large proteolytic complex which is referred as the 26S proteasome (Fig. 1), and expressed in both the nucleus and the cytoplasm in all eukaryotic cells. It consists of one or two 19S regulatory particles and a 20S catalytic core. The 20S proteasome consists of four stacked rings: two inner beta rings and two outer alpha rings, each of which consists of seven subunits [24] (Fig. 1). The 19S particle (700 kDa) consists of both a lid and a base, which is responsible for controlling the access of substrates into the 20S proteasome. Furthermore, the 19S particle is responsible for unfolding and threading the ubiquitinated proteins into the catalytic site of the 20S proteasome [25]. These diverse actions are fulfilled by multiple subcomplexes of the 19S regulatory particle [26].
Fig. 1. Inhibition of the proteasome and NF-κB pathways by selected terpenoids.
The proteasome contains a 20S multicatalytic core and one or two 19S regulatory caps. IκB–α, a proteasome substrate traps the active form of NF-κB (a dimer of RelA and p50, or of RelB and p52) in the cytosol. NFκB p50 or p52 are generated from the inactive precursor p105 or p100, respectively, through proteasome partial cleavage. After phosphorylation by IKK, a polyubiquitin chain (red square, ubiqutin) is attached to IκB-α with the help of β-TrCP E3 ligase. Then IκB-α is recognized and degraded by the proteasome, setting NF-κB free. The free, active NF-κB complex then enters into nucleus to transactivate target genes required for tumor proliferation. The selected terpenoids could target the ubiquitin-proteasome and NF-κB pathways at indicated specific steps, resulting in apoptosis induction and tumor growth inhibition.
The 20S proteasome hydrolyzes the target protein through three proteolytic activities, caspase or peptidyl-glutamyl peptide-hydrolyzing (PGPH)-like, trypsin-like and chymotrypsin-like activities. These activities are mediated through three β subunits, β1 (PGPH-like), β2 (trypsin-like), and β5 (chymotrypsin-like), respectively [27]. In accordance with enzymes with similar hydrolytic activities, the β1 subunit cuts peptides after acidic residues, the β2 subunit prefers to cleave after basic amino acids, and the β5 subunit hydrolyzes after hydrophobic residues. All three proteolytic activities depend on the presence of the threonine residue at the amino terminal (Thr1) [28].
Higher levels of proteasome activity were observed in tumor cells compared to normal cells [29, 30], suggesting the importance of the proteasome in the growth of tumor cells. Indeed, several antiapoptotic signaling pathways require proteasomal activity for their function. This is especially true for the pro-oncogenic nuclear factor kappa B (NF-κB) pathway. NF-κB plays an important role in tumorigenesis via transactivation of genes involved in cell proliferation, apoptosis, tumor cell invasion, metastasis, and angiogenesis [31]. Constitutive activation of NF-κB is commonly observed in many types of cancer [32].
Five members of the NF-κB family, that are p65 (RelA), RelB, c-Rel, NF-κB1 or p50, and NF-κB2 or p52 subunits, have been identified, all of which contain a REL-homology domain that is responsible for DNA binding, dimerization, nuclear translocation and endogeous inhibitor of NF-κB (IκB) binding [33]. They form homo- and heterodimers through combinatorial assembly, but p50 and p52 must dimerize with a transactivation domain-containing member such as p65, c-REL or REL-B to activate gene transcription [34] (Fig. 1). The classic form of NF-κB is p50/p65 heterodimer. IκB binds to the REL-homology domain of NF-κB through ankyrin repeats, covering the nuclear localization sequence of NF-κB [33]. Therefore NF-κB is normally sequestered in the cytoplasm through association with its endogenous inhibitor IκB. Upon stimulation, IκB-α is rapidly phosphorylated by kinase IKK (IκB kinase) composed of two catalytic subunits, IKK-α and IKK-β, and one regulatory subunit IKK-γ [35]. Phosphorylated IκB-α is specifically recognized by SCFβ-TrCP ubiquitin ligase at the 19-amino-acid destruction motif in residues 21-41 [36] and transferred to the 26S proteasome for degradation while NF-κB is allowed to be translocated to the nucleus and mediate transcription of anti-apoptotic genes (Fig. 1). However, accumulation of the IκB-α protein via proteasome inhibition prevents the activation of anti-apoptotic NF-κB [37], resulting in tumor cell apoptosis. The classical NF-κB activation is the major pathway through which NF-κB mediates anti-apoptotic effects. Alternatively, the p100 precursor is activated through limited ubiquitin-dependent proteasome degradation, generating active form p52 [38] (Fig. 1). p52 binds with RelB and transactivates target genes in the nucleus. Therefore, inhibition on the proteasome activity will prevent alternative NF-κB activation (Fig. 1).
2. MONOTERPENOIDS
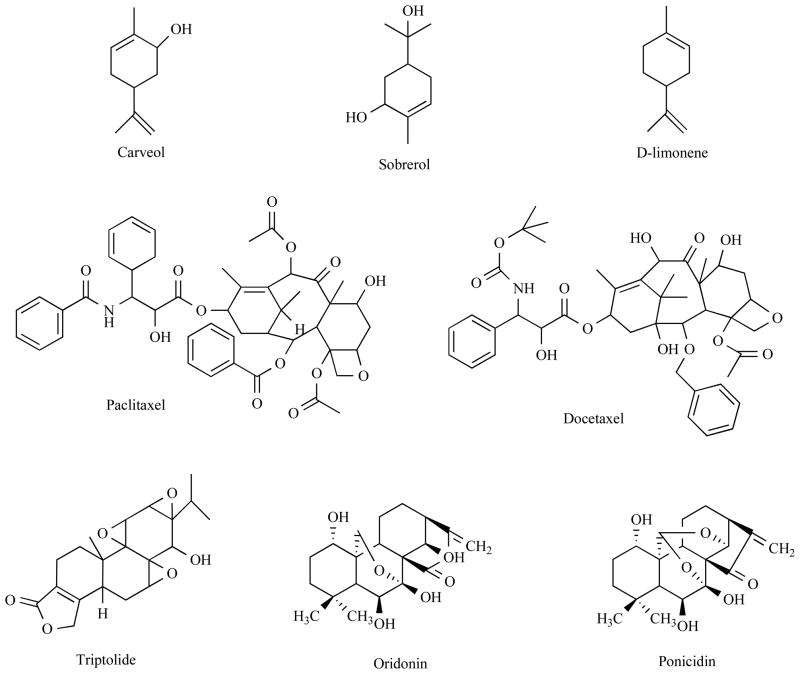
A number of monoterpenoids have chemopreventive activity against hormone-related cancers. For example, a Cinnamomum monoterpenoid subamone was cytotoxic against prostate cancer cell lines DU-145 and LNCaP [39]. Carveol, uroterpenol, and sobrerol (Fig. 2) were reported to have chemopreventive activity against mammary cancer [40].
Fig. 2. Chemical structure of selected terpenoids I.
Structures of monoterpenoids and diterpenoids are shown.
D-limonene (Fig. 2) is a major component of citrus peel oil that has been well investigated in both breast and prostate cancer. It demonstrated anti-carcinogenic effects when given during the initiation phase as well as the promotion/progression phase in mammary cancer in rats [41, 42]. Limonene-induced regression was also observed in established mammary carcinomas in rat models [43]. In a phase I clinical trial using d-limonene, partial response was observed in one breast cancer patient and that was maintained for 11 months before withdraw from the trial because of the progression of cancer in the bone [44].
Several cellular molecules in cancer cells were potentially targeted by d-limonene for mediating its chemopreventive and antitumor activities. Associated with its chemoprevitive effect, d-limonene induced phase I and phase II carcinogen-metabolizing enzymes, resulting in carcinogen detoxification [45]. Related to the antitumor activity, d-limonene induced apoptosis, tumor remodeling/re-differentiation to a more benign phenotype, and/or inhibition of the post-translational isoprenylation of cell growth-regulating proteins [40, 45, 46].
Dietary supplementation with limonene can not delay the development of prostate cancer in a rat model [47]. However, it was found that limonene supplement enhanced the antitumor effect of docetaxel against prostate cancer cells without being toxic to normal prostate epithelial cells [48]. Exposure of DU-145 cells to a combined d-limonene and docetaxel resulted in higher reactive oxygen species (ROS) generation and depletion of GSH, accompanied by increased caspase activity than docetaxel alone. The combined beneficial effect could be through the modulation of proteins involved in mitochondrial pathway of apoptosis. It triggered a series of effects involving cytochrome c release, caspase-9 and -3 activation and poly (ADP-ribose) polymerase (PARP) cleavage, as well as a shift in Bad:Bcl-XL ratio in favor of apoptosis [48].
3. DITERPENOIDS
A well practiced diterpenoid in cancer patients is paclitaxel (Fig. 2). Paclitaxel and second generation docetaxel (Fig. 2) are regarded as first-line or second line therapy as single treatment or in combination with other chemotherapeutics for both early stage or metastatic breast or prostate cancer. Since this area has been extensively reviewed recently [49, 50], we only briefly refer it here.
3.1 Triptolide
Triptolide (PG490) (Fig. 2) is a diterpenoid triepoxide, which is highly oxygenated at the 7,8; 9,11; and 12,13 positions of epoxides. Triptolide is mainly extracted from the tender root of Chinese herb Tripterygium wilfordii Hook F (lei gong teng) [51]. The hydrogen-bonded 9,11-epoxy-14beta-hydroxy system was reported to be involved in triptolide’s antineoplastic activity [52]. However, recent structure-activity relationship (SAR) studies showed that substitution of the C14 β-hydroxyl group of triptolide, with a chiral epoxy group, resulted in retain of anticancer activity, which challenge the traditional viewpoint on the necessity of C14 β-hydroxyl group of triptolide [53].
Triptolide inhibited cell growth at low concentrations and induced apoptosis at high concentration [54]. In vivo, treatment of mice with triptolide inhibited the growth of xenografts formed by different human tumor cell lines including MDA-MB-435 breast cancer line, indicating that triptolide has a broad spectrum of activity against tumors [55]. The antiproliferaotry activity of triptolide is associated with inhibition of proteasome-mediated NF-κB pathway (Fig. 1). Triptolide inhibited transactivation of NF-κB induced by tumor necrosis factor alpha (TNF-α), and further blocked NF-κB-mediated induction of the inhibitor of apoptosis c-IAP1 and c-IAP2 (IAP, inhibitor of apoptosis protein) [56]. Triptolide inhibited NF-κB transactivation without inhibiting nuclear NF-κB DNA binding activity [56, 57] or even with increased nuclear NF-κB DNA binding activity [58]. Further study demonstrated that triptolide inhibited the binding of p65 to transcriptional coactivator CBP/p300 [59]. Triptolide also suppressed the phorbol myristyl acetate (PMA)-induced activation of NF-κB, in turn, inhibited the overexpression of urokinase-type plasminogen activator receptor (uPAR), which is required for tumor cell invasion [60].
In addition, triptolide inhibited up to 80% mRNA de novo synthesis by inhibiting RNA polymerase I and II [61], indicating that it hits a wide spectrum of targets. Indeed, a disintegrin and metalloproteinase 10 (ADAM10) has been identified as a novel molecular target of triptolide in breast cancer MCF 7 cells. ADAM10 is a type 1 transmembrane glycoprotein that cleaves several plasma membrane proteins and involved in malignant cell growth and cancer progression [62]. Triptolide treatment at nM concentrations resulted in a significantly decreased expression of ADAM10 with a concomitant increase in ADAM10 cleaved products in MCF-7 breast cancer cells [62]. This might be correlated with triptolide cellular location. A study using a 3H-labeled triptolide showed that triptolide binding was saturable, reversible, and primarily localized to cell membranes [63]. Triptolide induced suppression of phospholipase D expression, resulting in inhibition of cell in MDA-MB-231 breast cancer cells [64]. Up-regulation of programmed death-1-ligand 1 (PD-L1) in cancer cells is an important mechanism of tumor immune evasion. Triptolide was able to inhibit interferon-gamma-induced PD-L1 surface expression in human breast cancer cells [65]. Therefore, by down-regulating PD-1/PD-L1 pathway, triptolide may also serve as a modulator to promote cancer cell-reactive immune responses [65]. Triptolide could also down-regulate the expression of ERα in MCF-7 cells, which was associated with greater anti-proliferation and apoptotic effects in estrogen-sensitive MCF-7 than in estrogen-insensitive MDA-MB-231 human breast cancer cells [66]. Triptolide was also able to decrease Bcl-2 expression and increase caspase-3 expression, leading to apoptosis induction in prostate carcinoma cells [67].
In brief, triptolide is able to inhibit NF-κB activation and multiple other targets in human breast and prostate cancer cells, which is responsible for its apoptosis-inducing and anti-tumor activities.
3.2 Oridonin and Ponicidin
Recently, a formula PC SPES (PC for prostate cancer and SPES in Latin for “hope”) composed of one North American plant extract and seven Chinese herbs extracts has been approved as an alternative therapy for advanced prostate cancer. Rabdosia rubescens is one of the constituents of PC SPES that contains two diterpenoids, oridonin and ponicidin (Fig. 2). Oridonin exhibited antiproliferative activity toward prostate cancer cells LNCaP, DU145 and PC3 as well as other type of cancer cells [68, 69]. Flow cytometric analysis demonstrated that oridonin induced a G1 phase arrest in androgen receptor-positive LNCaP cells containing wt p53 through induction of p21 [69], while it blocked the cell cycle at G2 and M phases in androgen receptor-negative DU-145 cells with mutated p53 [68]. Oridonin inhibits cancer cell growth in a cell cycle-specific manner and shifts the balance between pro- and anti-apoptotic proteins in favor of apoptosis induction in prostate cancer cells. It up-regulated p53 and Bax and down-regulated Bcl-2 expression in a dose-dependent manner [68].
Effects of ponicidin and oridonin on cell cycle distribution and apoptosis were also investigated in human breast cancer MCF 7 and MDA-MB-231 cells along with untransformed MCF-10A cells [70]. Induction of apoptosis by either diterpenoid was observed in MCF-10A cells. MCF 7 is lack of caspase-3 [71]. Oridonin was shown to induce apoptotic nucleosomal DNA fragmentation in MCF-7 cells via a postmitochondrial caspase-9-dependent pathway. Moreover, PARP was partially cleaved by calpain rather than by caspase-3 [72]. Oridonin was reported to induce MCF-7 cells and undergo both apoptosis and autophagy simultaneously. Combined treatment with oridonin and 3-MA (the specific inhibitor of autophagy) decreased apoptosis induction compared with treatment of oridonin, indicating that autophagy facilitated apoptosis in oridonin-treated MCF-7 cells [73]. Within the dose range effective for MCF-7 or MCF-10A cells, neither oridonin nor ponicidin affected the growth of MDA-MB-231 cells, suggesting that they are not effective against highly aggressive breast cancer cells [70].
4. TRITERPENOIDS
Triterpenoid is a class of compounds that are close to steroids in structure. Therefore they are extensively studied in hormone-related cancers although they are also effective against other type of cancers. A number of triterpenoids have demonstrated antitumor properties. For example, betulinic acid was reported to inhibit growth of breast cancer cells [74].
4.1 Celastrol
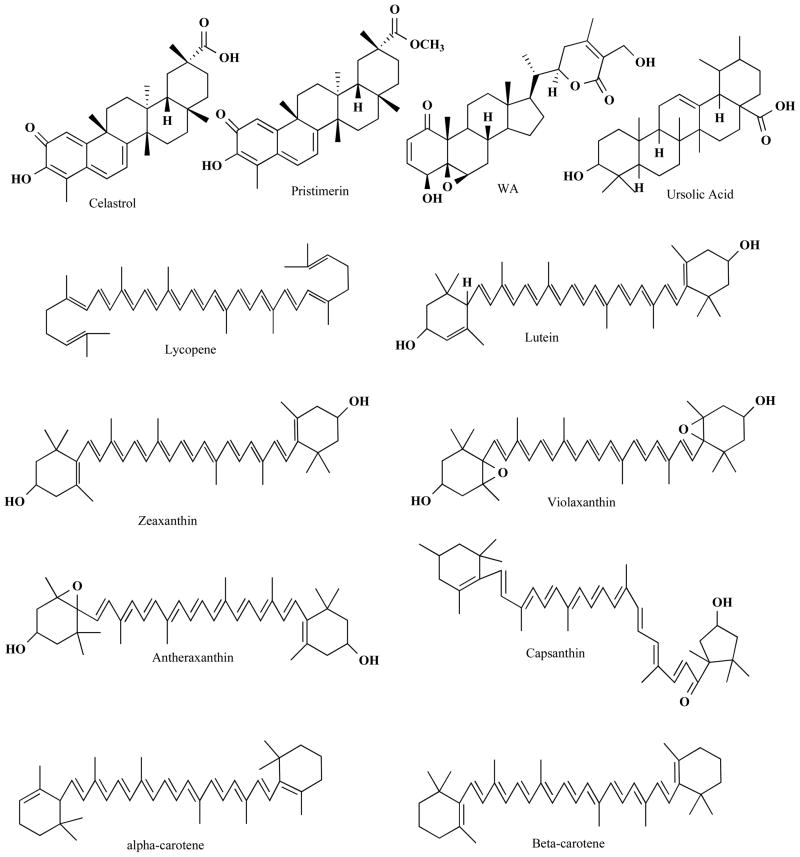
Celastrol is a quinone methide triterpene (Fig. 3) isolated from lei gong teng (Tripterygium wilfordii Hook F). It is also found in Tripterygium regeli, a substitute of lei gong teng in northeast of China [75]. Celastrol was predicted as a proteasome inhibitor by molecular modeling, and was confirmed by in vitro and in vivo experiments [76]. Analysis of atomic orbital energy on celastrol showed that the ketone carbons on B-ring of celastrol had a high susceptibility toward a nucleophilic attack (Fig. 4A). Celastrol could be docked at 92% possibility into the S1 pocket of β5 subunit of the proteasome in a conformation in which it interacts with Thr1, the active site of β5 subunit (Fig. 4B). Celastrol potently and preferentially inhibits β5-mediated chymotrypsin-like activity of purified 20S proteasome with an IC50 value 2.5 μmol/L [76]. In intact human prostate cancer cells, cellular 26S proteasome was inhibited by celastrol at 1–5 μmol/L. Inhibition of the proteasome activity by celastrol results in accumulation of ubiquitinated proteins and three natural proteasome substrates (IκB-α, Bax and p27), and induction of apoptosis in androgen receptor-positive or androgen receptor-negative prostate cancer cells (Fig. 1). Treatment of human prostate tumor-bearing nude mice with celastrol (1–3 mg/kg/day, i.p., for 1 to 31 days) resulted in significant inhibition (65–93%) of the tumor growth. Multiple assays using the animal tumor tissue samples from both early and end time-points demonstrated in vivo inhibition of the proteasomal activity and induction of apoptosis after celastrol treatment [76].
Fig. 3. Chemical structure of selected terpenoids II.
Structures of tritenpenoids and tetraterpenoids are shown.
Fig. 4. Molecilar docking of celastrol and pristimerin.
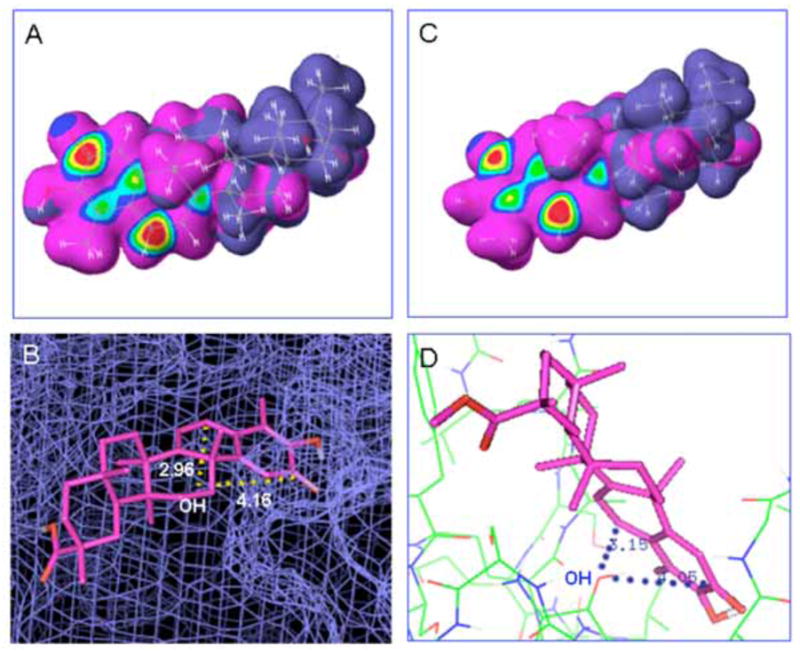
A, High susceptibility towards nuceophilic attack of celastrol molecule was indicated by red center. B, Celastrol was docked the S1 pocket of β5 subunit of the proteasome as shown by blue smesh. The distances from ketone carbons to OH group of Thr1 were 2.96 or 4.16 Å. C, Similarly, high electronic density was obtained at the ketone carbons surface of the pristimerin molecule. D, Pristimerin was shown in a confirmation that is suitable to interact with the OH group of Thr 1 of β5 subunit as shown by rainbow lines. The distances from ketone carbons to OH group of Thr1 were 3.15 or 4.05 Å. The celastrol and pristimerin molecules were shown in pink sticks.
Antitumor activity of celastrol was also observed in other types of cancer. Celastrol inhibited ~60% tumor growth in breast cancer xenograft through NF-κB inactivation including inhibition of its DNA-binding activity and inhibition of IκB-α degradation which was induced by TNF-α or phorbol myristyl acetate (PMA) [77] (Fig. 1). Further investigation showed that celastrol suppressed the NF-κB activation by targeting cysteine 179 in the IκB-α kinase (IKK) [77] (Fig. 1). Celastrol was able to potentiate apoptotic effects induced by TNF and chemotherapeutic agents. Celastrol was able to inhibit the TNF-induced activation of IκB-α kinase, IκB-α phosphorylation, IκB-α degradation, p65 nuclear translocation and phosphorylation, and NF-κB-mediated reporter gene expression [78] (Fig. 1). Finally, celastrol treatment suppressed expression of several TNF-induced genes that are involved in anti-apoptosis, proliferation, invasion, and angiogenesis [78].
Inhibition of NF-κB was observed in concurrence with of activation of heat shock response, indicating a potential link between the two pathways [79]. Indeed, celastrol induced activation of Hsp70, Hsp40 and Hsp27, which was similar as observed in heat shock treatment [79]. Up-regulation of Hsp70A is a well established cell response to Hsp90 inhibition [80]. Two research groups reported that celastrol is an inhibitor of Hsp90 [81, 82]. Both groups confirmed that celastrol is different from classic Hsp90 inhibitor such as geldanamycin. However, Hieronymus et al. reported that celastrol inhibits ATP-binding activity of Hsp90 through mechanisms outside the N-terminal ATP-binding pocket of Hsp90 [82] while Zhang et al. reported that celastrol did not interfere with ATP binding to Hsp90 [81]. Celastrol disrupted the interaction between Hsp90 and its cochaperone such as Cdc37 or p23 [81, 82]. Inhibition of Hsp90 by celastrol resulted in suppression of its client proteins that play an important role in tumor initiation and progression. For example, celastrol depleted AR protein in LNCaP cells [82]. The aryl hydrocarbon receptor (AhR) is another client protein of Hsp 90. It plays a significant role in polycyclic aromatic hydrocarbon (PAH)-induced carcinogenesis. Celastrol suppressed AhR expression, resulting in decreased transactivation of CYP1A1 and CYP1B1, both of which encode proteins that convert PAH to genotoxic metabolites [83].
In conclusion from the literature available, celastrol mainly targets three pathways that link to each other, the ubiquitin-proteasome, the NF-κB, and the heat shock protein pathways to mediating apoptosis in human breast or prostate cancers.
4.2 Pristimerin
Pristimerin is a natural analog of celastrol (Fig. 3). Not surprisingly, it targets the proteasome [84] (Fig. 4C–D). Nucleophilic susceptibility and in silico docking studies show that one ketone carbon of pristimerin is highly susceptible towards a nucleophilic attack by the hydroxyl group of Thr1 of the proteasomal chymotrypsin subunit (Fig. 4C–D). The distance from that carbon to OH group of Thr1 was 3.05 Å, within the range of possible interaction between each other. Consistently, pristimerin potently inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50 2.2 μmol/L) and human prostate cancer 26S proteasome (IC50 3.0 μmol/L) [84] (Fig. 1). The accumulation of ubiquitinated proteins and three proteasome target proteins, Bax, p27 and IκB-α, in AR-negative PC-3 prostate cancer cells supports the conclusion that proteasome inhibition by pristimerin is physiologically functional. This observed proteasome inhibition subsequently led to the induction of apoptotic cell death in a dose- and kinetic-dependent manner. Furthermore, in AR-positive, androgen-dependent LNCaP and AR-positive, androgen-independent C4-2B prostate cancer cells, proteasome inhibition by pristimerin results in suppression of AR protein prior to apoptosis [84]. The data demonstrates that the proteasome is a target of pristimerin in prostate cancer cells and inhibition of the proteasomal chymotrypsin-like activity by pristimerin is responsible for its cancer cell death-inducing properties (Fig. 1). Inhibition of the chymotryptic activity of the proteasome was confirmed by another group [85].
Pristimerin-triggered caspase activation was also observed in human breast cancer cells [86]. MDA-MB-231 cells treated with pristimerin showed rapid induction of apoptosis through caspase activation, which could be completely prevented by pretreatment with a pancaspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk). Treatment of breast tumor cells with pristimerin resulted in a rapid release of cytochrome c from mitochondria, which preceded caspase activation and the decrease of mitochondrial membrane potential. This process did not depend on Bcl-2 family (Bcl-2, Bcl-XL and Bax) protein levels and does not require translocation of Bax to the mitochondria. Moreover, the generation of reactive oxygen species (ROS) in MDA-MB-231 cells was also not affected by pristimerin [86].
Therefore, pristimerin can inhibit the proteasome activity, induce cytochrome c release, and activate caspase pathway in human breast or prostate cancer cells.
4.3 Withaferin A
Withaferin A (WA) (Fig. 3) is derived from the medicinal plant Withania somnifera Dunal (ashwagandha), which has been used for over centuries in Indian Ayurvedic medicine and as a dietary supplement in the United States recently [87]. WA suppressed human breast cancer growth, which is correlated with apoptosis induction characterized by DNA condensation, cytoplasmic histone-associated DNA fragmentation, and cleavage of PARP. WA-mediated DNA fragmentation was significantly attenuated by knockdown of protein levels of Bim and its transcriptional regulator forkhead box O3 (FOXO3a) in breast cancer MCF7 and MDA-MB-231 cells, suggesting an important role of FOXO3a and Bim in regulation of WA-mediated apoptosis in human breast cancer cells [88]. Interleukin-6 (IL-6) is involved in angiogenesis and metastasis. WA was found to repress IL-6 gene transcription upon dual inhibition of NF-κB and activator protein 1 (AP-1) and silencing of IL-6 promoter chromatin accessibility in highly metastatic breast cancer cells [89].
We have reported that WA potently inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50=4.5 μM) and 26S proteasome in human prostate cancer cultures (at 5–10 μM) and xenografts (4–8 mg/kg/day) [90] (Fig. 1). Inhibition of prostate tumor cellular proteasome activity in cultures and in vivo by WA results in accumulation of ubiquitinated proteins and three proteasome target proteins (Bax, p27, and IκB-α) accompanied by androgen receptor protein suppression (in androgen-dependent LNCaP cells) and apoptosis induction. Treatment of WA under conditions of the aromatic ketone reduction had significantly decreased the proteasome-inhibitory and apoptosis-inducing activities. Treatment of human prostate PC-3 xenografts with WA for 24 days resulted in 70% inhibition of tumor growth in nude mice, associated with 56% inhibition of the tumor tissue proteasomal chymotrypsinlike activity [90]. WA was also able to induce prostate apoptosis response-4 (Par-4)-dependent apoptosis in androgen-refractory prostate cancer cells and regression of PC-3 xenografts in nude mice [91].
Therefore, at least the proteasome, IL-6, FOX3a and Par-4 are involved in WA-mediated apoptosis process in human breast or prostate cancer cells.
4.4 Ursolic Acid
Ursolic acid (UA) (Fig. 3) is an apentacyclic triterpene acid found in Ocimum leaves and also in berries, leaves, flowers and fruits of Rosemarinus officinalis, Eriobotrya japonica, Calluna vulgaris and Eugenia jumbolana [92]. Several studies suggest that UA has potent cancer-preventive activity and great therapeutic potential. Breast cancer MCF-7 cells exhibited typical apoptotic features, including chromatin clumps and aggregation and DNA fragmentation after UA treatment, which was in correlation with the down-regulation of Bcl-2 and up-regulation of caspase-3 [93, 94]. An aqueous extract of Ocimum gratissimum (OG) leaves containing UA was investigated on tumor progression and angiogenesis. Results showed that the OG leaf extract inhibits proliferation, migration, anchorage-independent growth, 3D growth and morphogenesis and induction of cyclooxygenase-2 (COX-2) protein in breast cancer cells [95]. In addition, the OG extract reduced tumor size and neoangiogenesis in a MCF10 DCIS.com xenograft model [95].
UA inhibited significantly the cell viability and induced apoptosis in PC-3 cells and LNCaP cells associated with down-regulation of Bcl-2 protein [95], suggesting its potential therapeutic use for the treatment of hormone refractory and androgen-sensitive prostate cancer. Bcl-2 down-regulation may be one of the molecular mechanisms by which UA induces apoptosis. This was confirmed in another human hormone-refractory prostate cancer cell line, DU145 [96]. UA caused the activation of c-Jun N-terminal kinase (JNK), resulting in Bcl-2 phosphorylation (Ser70) and degradation and consequent caspase 3 activation in DU145 cells [96]. In conclusion, downregulation of Bcl-2 may be one of the mechanisms responsible for the apoptosis-inducing activity of UA in human prostate cancer cells.
5. TETRATERPENOIDS
One representative of the tetraterpenes family is carotenoids and more than 600 known natural structural variants are present in this family [97]. Higher concentrations of carotenoids such as lycopene or beta-carotene (Fig. 3) was associated with lower risk of breast cancer [98, 99]. An inverse association has been observed between dietary intake of lycopene and the risk of prostate cancer [100]. On the contrary, it was also reported that β-carotene supplementation had no effect on the incidence of breast, prostate and other types of cancers [101].
5.1 Acyclic Tetraterpenoids
Lycopene is an open chain hydrocarbon containing 11 conjugated and 2 non-conjugated double bonds arranged in a linear array (Fig. 3). It is derived predominantly from tomatoes and tomato products. Lycopene modified breast cancer gene expression involved in various molecular pathways, such as apoptosis, cell communication, mitogen-activated protein kinase (MAPK) pathway and cell cycle [102]. Similarly, it has been reported to trigger G2/M arrest and suppress Bcl-2 expression in MCF 7 cells [103]. In addition, lycopene inhibited the function of the multidrug resistance (MDR) proteins in MDA-MB-231 cells [104]. It was found that lycopene treatment induced apoptosis in human MDR1-transfected breast cancer MDA-MB-231 cell line [104].
In a phase II clinical trial, the efficacy of lycopene alone or in combination with soy isoflavones on serum PSA levels in men with prostate cancer was investigated. Lycopene and soy isoflavones have activity in prostate cancer patients with PSA relapse disease and may delay progression of both hormone-refractory and hormone-sensitive prostate cancer [105]. However, there may not be an additive effect between these two compounds when taken together [105]. It has also been reported that the combination of tomato and broccoli was more effective at slowing prostate tumor growth than alone in a rat model [106]. Results from a small, randomized clinical trial, showed that lycopene supplementation may increase prostate tissue levels of lycopene, modulate biomarkers of growth and differentiation, and decrease clinical parameters of disease aggressiveness of clinically localized prostate cancer [107].
5.2 Bicyclic Tetraterpenoids
Lutein and zeaxanthin (Fig. 3) are xanthophyll carotenoids found particularly in dark-green leafy vegetables such as kale, spinach, turnip greens, collard, and in egg yolks. Alpha- and beta-carotenes are enriched in dark green-yellow vegetables. Bicyclic tetraterpenoids were shown to modulate drug resistance. For example, lutein, antheraxanthin and violaxanthin (Fig. 3) had moderate effects on the reversal of MDR in the tumor cells. Apoptosis was found in human MDR1-transfected mouse lymphoma cells and human breast cancer MDA-MB-231 (HTB-26) cell lines in the presence of zeaxanthin and capsanthin [104]. Beta-carotene-induced apoptosis has also been investigated in breast cancer model. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) and ROS production have been implicated in β-carotene-regulated apoptosis [108]. Beta-carotene significantly increased PPAR-gamma mRNA and protein levels in MCF 7 cells. Moreover, it induced ROS generation, resulting in mitochondrial dysfunction and cytochrome c release [108].
6. FUTURE DIRECTIONS
Breast and prostate cancers are two major cancers in women and men. It is estimated that 67,970 people died of these two type of cancers in 2009 [1]. It is therefore very urgent to find a powerful strategy to control both breast and prostate cancer progression. Nature has already provided a great source for anticancer drug discovery. Statistics on approved anticancer drugs from 1940 to 2006 reveal that 73% drugs are discovered from nature either directly or semi-synthesized based on the scaffolds of natural products. Paclitatel and decetaxel are plant-derived or semi-synthetic; both are currently used as first or second line treatment of breast or prostate cancer. New terpenoid compounds have also been isolated from nature. Currently, most of the research is in preclinical study phase. Although water-soluble analog of triptolide has been developed, many other drugs are not water soluble, which limits their clinical application. Furthermore, the pharmacokenetic characteristics of most of the terpenoids need to be determined. Future investigation should focus on how to translate the bench work into clinical application, including treatment and prevention of human breast and prostate cancers.
7. CONCLUSION
Plants provide a broad spectrum of sources for modern anticancer drug discovery. Plant-derived diterpenoid paclitaxel and its semi-synthetic analog docetaxel are used as standard therapy in breast or prostate cancer patients. Other terpenoids, for example tritepenoids celastrol and withaferin A, have been isolated. Terpenoids evoke apoptosis in cultured breast or prostate cancer cells or xenografts, resulting in inhibition on tumor cell proliferation and growth. Multiple mechanisms are involved in the action of apoptosis induction by various terpenoids. Inhibition of ubiquitin-prteasome and NF-κB pathways are often observed in terpenoids-treated breast or prostate cancer cells, which is linked with up-regulation of proapoptotic protein Bax and down-regulation of anti-apoptotic protein Bcl-2, resulting in cytochrome c release, caspase activation and apoptotic cell death.
The efficacy of terpenoids as s single or combination strategy against breast or prostate cancers as demonstrated in pre-clinical studies provides strong support for their clinical use in treatment and even prevention of human hormone-related cancers.
Acknowledgments
The authors thank Ms. Carol Maconochie for critical reading of the manuscript. This research is partially supported by Karmanos Cancer Institute of Wayne State University (to Q.P. Dou) and National Cancer Institute/NIH (1R01CA120009; 3R01CA120009-04S1 to Q.P. Dou).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–33. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Tharakan R, Lepont P, Singleton D, Kumar R, Khan S. Phosphorylation of estrogen receptor alpha, serine residue 305 enhances activity. Mol Cell Endocrinol. 2008;295:70–8. doi: 10.1016/j.mce.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Irvin WJ, Jr, Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44:2799–805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 7.Bouchalova K, Cizkova M, Cwiertka K, Trojanec R, Hajduch M. Triple negative breast cancer--current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:13–7. doi: 10.5507/bp.2009.002. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, Saltzman A, Yeh S, et al. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 9.Schaufele F, Carbonell X, Guerbadot M, et al. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA. 2005;102:9802–7. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 11.Tamura K, Furihata M, Tsunoda T, et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67:5117–25. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 13.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 14.Culig Z. Androgen receptor cross-talk with cell signalling pathways. Growth Factors. 2004;22:179–84. doi: 10.1080/08977190412331279908. [DOI] [PubMed] [Google Scholar]
- 15.Unni E, Sun S, Nan B, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–68. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 16.Jia L, Shen HC, Wantroba M, et al. Locus-wide chromatin remodeling and enhanced androgen receptor-mediated transcription in recurrent prostate tumor cells. Mol Cell Biol. 2006;26:7331–41. doi: 10.1128/MCB.00581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 18.Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 19.Anichini A, Mortarini R, Sensi M, Zanon M. APAF-1 signaling in human melanoma. Cancer Lett. 2006;238:168–79. doi: 10.1016/j.canlet.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 21.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–35. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 23.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. Embo J. 1998;17:7151–60. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 25.Dou QP, Smith DM, Daniel KG, Kazi A. Interruption of tumor cell cycle progression through proteasome inhibition: implications for cancer therapy. Prog Cell Cycle Res. 2003;5:441–6. [PubMed] [Google Scholar]
- 26.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–87. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 27.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–57. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 28.Groll M, Ditzel L, Lowe J, et al. Structure of 20S proteasome from yeast at 2. 4 A resolution. Nature. 1997;386:463–71. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 29.Masdehors P, Merle-Beral H, Maloum K, Omura S, Magdelenat H, Delic J. Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood. 2000;96:269–74. [PubMed] [Google Scholar]
- 30.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA. 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 32.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–7. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 35.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–83. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645–52. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- 38.Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–44. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- 39.Lin RJ, Lo WL, Wang YD, Chen CY. A novel cytotoxic monoterpenoid from the leaves of Cinnamomum subavenium. Nat Prod Res. 2008;22:1055–9. doi: 10.1080/14786410802228637. [DOI] [PubMed] [Google Scholar]
- 40.Crowell PL. Monoterpenes in breast cancer chemoprevention. Breast Cancer Res Treat. 1997;46:191–7. doi: 10.1023/a:1005939806591. [DOI] [PubMed] [Google Scholar]
- 41.Maltzman TH, Hurt LM, Elson CE, Tanner MA, Gould MN. The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis. 1989;10:781–3. doi: 10.1093/carcin/10.4.781. [DOI] [PubMed] [Google Scholar]
- 42.Elson CE, Maltzman TH, Boston JL, Tanner MA, Gould MN. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis. 1988;9:331–2. doi: 10.1093/carcin/9.2.331. [DOI] [PubMed] [Google Scholar]
- 43.Haag JD, Lindstrom MJ, Gould MN. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992;52:4021–6. [PubMed] [Google Scholar]
- 44.Vigushin DM, Poon GK, Boddy A, et al. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol. 1998;42:111–7. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 45.Sun J. D-Limonene: safety and clinical applications. Altern Med Rev. 2007;12:259–64. [PubMed] [Google Scholar]
- 46.Crowell PL, Siar Ayoubi A, Burke YD. Antitumorigenic effects of limonene and perillyl alcohol against pancreatic and breast cancer. Adv Exp Med Biol. 1996;401:131–6. doi: 10.1007/978-1-4613-0399-2_10. [DOI] [PubMed] [Google Scholar]
- 47.Wilson MJ, Lindgren BR, Sinha AA. The effect of dietary supplementation with limonene or myo-inositol on the induction of neoplasia and matrix metalloproteinase and plasminogen activator activities in accessory sex organs of male Lobund-Wistar rats. Exp Mol Pathol. 2008;85:83–9. doi: 10.1016/j.yexmp.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Rabi T, Bishayee A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J Carcinog. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saloustros E, Mavroudis D, Georgoulias V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother. 2008;9:2603–16. doi: 10.1517/14656566.9.15.2603. [DOI] [PubMed] [Google Scholar]
- 50.Mancuso A, Oudard S, Sternberg CN. Effective chemotherapy for hormone-refractory prostate cancer (HRPC): present status and perspectives with taxane-based treatments. Crit Rev Oncol Hematol. 2007;61:176–85. doi: 10.1016/j.critrevonc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Luo XL, Shao Q, Qu HB, Cheng YY. Simple method for determination of five terpenoids from different parts of Tripterygium wilfordii and its preparations by HPLC coupled with evaporative light scattering detection. J Sep Sci. 2007;30:1284–91. doi: 10.1002/jssc.200600450. [DOI] [PubMed] [Google Scholar]
- 52.Kupchan SM, Schubert RM. Selective alkylation: a biomimetic reaction of the antileukemic triptolides? Science. 1974;185:791–3. doi: 10.1126/science.185.4153.791. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Zhou ZL, Miao ZH, et al. Design and Synthesis of Novel C14-Hydroxyl Substituted Triptolide Derivatives as Potential Selective Antitumor Agents. J Med Chem. 2009 doi: 10.1021/jm900342g. [DOI] [PubMed] [Google Scholar]
- 54.Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–74. [PubMed] [Google Scholar]
- 55.Yang S, Chen J, Guo Z, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 56.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–5. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 57.Jiang XH, Wong BC, Lin MC, et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001;20:8009–18. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- 58.Qiu D, Zhao G, Aoki Y, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–50. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 59.Zhu W, Ou Y, Li Y, et al. A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-kappa B pathway. Mol Pharmacol. 2009;75:812–9. doi: 10.1124/mol.108.052605. [DOI] [PubMed] [Google Scholar]
- 60.Chang HJ, Kim MH, Baek MK, et al. Triptolide inhibits tumor promoter-induced uPAR expression via blocking NF-kappaB signaling in human gastric AGS cells. Anticancer Res. 2007;27:3411–7. [PubMed] [Google Scholar]
- 61.Vispe S, Devries L, Creancier L, et al. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-09-0549. [DOI] [PubMed] [Google Scholar]
- 62.Soundararajan R, Sayat R, Robertson GS, Marignani PA. Triptolide: An inhibitor of a disintegrin and metalloproteinase 10 (ADAM10) in cancer cells. Cancer Biol Ther. 2009:8. doi: 10.4161/cbt.8.21.9803. [DOI] [PubMed] [Google Scholar]
- 63.Leuenroth SJ, Crews CM. Studies on calcium dependence reveal multiple modes of action for triptolide. Chem Biol. 2005;12:1259–68. doi: 10.1016/j.chembiol.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang DW, Lee JY, Oh DH, et al. Triptolide-induced suppression of phospholipase D expression inhibits proliferation of MDA-MB-231 breast cancer cells. Exp Mol Med. 2009;41:678–85. doi: 10.3858/emm.2009.41.9.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang M, Fu J. Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett. 2008;270:337–41. doi: 10.1016/j.canlet.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Jiang Z, Xiao J, et al. Effects of triptolide from Tripterygium wilfordii on ERalpha and p53 expression in two human breast cancer cell lines. Phytomedicine. 2009;16:1006–13. doi: 10.1016/j.phymed.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Zhang R, Zhang PY, Guo J, et al. Effects of triptolide on prostate carcinoma in mouse RM-1 cells. Zhonghua Nan Ke Xue. 2007;13:237–41. [PubMed] [Google Scholar]
- 68.Chen S, Gao J, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. The cytostatic and cytotoxic effects of oridonin (Rubescenin), a diterpenoid from Rabdosia rubescens, on tumor cells of different lineage. Int J Oncol. 2005;26:579–88. [PubMed] [Google Scholar]
- 69.Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H, Koeffler HP. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int J Oncol. 2003;23:1187–93. [PubMed] [Google Scholar]
- 70.Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem Biophys Res Commun. 2005;337:224–31. doi: 10.1016/j.bbrc.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 71.Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001;61:348–54. [PubMed] [Google Scholar]
- 72.Cui Q, Yu JH, Wu JN, et al. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol Sin. 2007;28:1057–66. doi: 10.1111/j.1745-7254.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 73.Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–64. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- 74.Rzeski W, Stepulak A, Szymanski M, et al. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:11–20. doi: 10.1007/s00210-006-0090-1. [DOI] [PubMed] [Google Scholar]
- 75.Zhou BN. Some progress on the chemistry of natural bioactive terpenoids from Chinese medicinal plants. Mem Inst Oswaldo Cruz. 1991;86(Suppl 2):219–26. doi: 10.1590/s0074-02761991000600049. [DOI] [PubMed] [Google Scholar]
- 76.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 77.Lee JH, Koo TH, Yoon H, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–21. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–35. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 79.Westerheide SD, Bosman JD, Mbadugha BN, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–60. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 80.Cullinan SB, Whitesell L. Heat shock protein 90: a unique chemotherapeutic target. Semin Oncol. 2006;33:457–65. doi: 10.1053/j.seminoncol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Hieronymus H, Lamb J, Ross KN, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–30. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Zhang T, Hamza A, Cao X, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7:162–70. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 83.Hughes D, Guttenplan JB, Marcus CB, Subbaramaiah K, Dannenberg AJ. Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev Res (Phila Pa) 2008;1:485–93. doi: 10.1158/1940-6207.CAPR-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Yang H, Landis-Piwowar KR, Lu D, et al. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J Cell Biochem. 2008;103:234–44. doi: 10.1002/jcb.21399. [DOI] [PubMed] [Google Scholar]
- 85.Tiedemann RE, Schmidt J, Keats JJ, et al. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood. 2009;113:4027–37. doi: 10.1182/blood-2008-09-179796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR, Wu YC. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–85. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- 87.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 88.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–9. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ndlovu N, Van Lint C, Van Wesemael K, et al. Hyperactivated NF-{kappa}B and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol Cell Biol. 2009;29:5488–504. doi: 10.1128/MCB.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–37. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 91.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–53. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 92.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 93.Zhang GP, Lu YY, Lv JC, Ou HJ. Effect of ursolic acid on caspase-3 and PARP expression of human MCF-7 cells. Zhongguo Zhong Yao Za Zhi. 2006;31:141–4. [PubMed] [Google Scholar]
- 94.Kassi E, Sourlingas TG, Spiliotaki M, et al. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Invest. 2009;27:723–33. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 95.Nangia-Makker P, Tait L, Shekhar MP, et al. Inhibition of breast tumor growth and angiogenesis by a medicinal herb: Ocimum gratissimum. Int J Cancer. 2007;121:884–94. doi: 10.1002/ijc.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang YX, Kong CZ, Wang HQ, Wang LH, Xu CL, Sun YH. Phosphorylation of Bcl-2 and activation of caspase-3 via the c-Jun N-terminal kinase pathway in ursolic acid-induced DU145 cells apoptosis. Biochimie. 2009;91:1173–9. doi: 10.1016/j.biochi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–10. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato R, Helzlsouer KJ, Alberg AJ, Hoffman SC, Norkus EP, Comstock GW. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:451–7. [PubMed] [Google Scholar]
- 99.Dahan K, Fennal M, Kumar NB. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol. 2008;6:29–36. [PubMed] [Google Scholar]
- 100.Wu K, Erdman JW, Jr, Schwartz SJ, et al. Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:260–9. doi: 10.1158/1055-9965.epi-03-0012. [DOI] [PubMed] [Google Scholar]
- 101.Druesne-Pecollo N, Latino-Martel P, Norat T, et al. Beta-carotene supplementation and cancer risk: A systematic review and meta-analysis of randomized controlled trials. Int J Cancer. 2009 doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 102.Chalabi N, Satih S, Delort L, Bignon YJ, Bernard-Gallon DJ. Expression profiling by whole-genome microarray hybridization reveals differential gene expression in breast cancer cell lines after lycopene exposure. Biochim Biophys Acta. 2007;1769:124–30. doi: 10.1016/j.bbaexp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Wang Y, Mo B. The effects of carotenoids on the proliferation of human breast cancer cell and gene expression of bcl-2. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:254–7. [PubMed] [Google Scholar]
- 104.Molnar J, Gyemant N, Mucsi I, et al. Modulation of multidrug resistance and apoptosis of cancer cells by selected carotenoids. In Vivo. 2004;18:237–44. [PubMed] [Google Scholar]
- 105.Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 106.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–43. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 107.Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–8. [PubMed] [Google Scholar]
- 108.Cui Y, Lu Z, Bai L, Shi Z, Zhao WE, Zhao B. beta-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007;43:2590–601. doi: 10.1016/j.ejca.2007.08.015. [DOI] [PubMed] [Google Scholar]